Elevated Mitochondrial Reactive Oxygen Species within Cerebrospinal Fluid as New Index in the Early Detection of Lumbar Spinal Stenosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Rat LSS Model
2.2. Locomotor Function Assays
2.3. CSF Collection
2.4. Flow Cytometry Analysis
2.5. DNA Extraction
2.6. PCR-Based Analysis of mtDNA/nDNA Ratio
2.7. Statistical Analysis
3. Results
3.1. LSS Surgical Procedure and Technical Collection of CSF
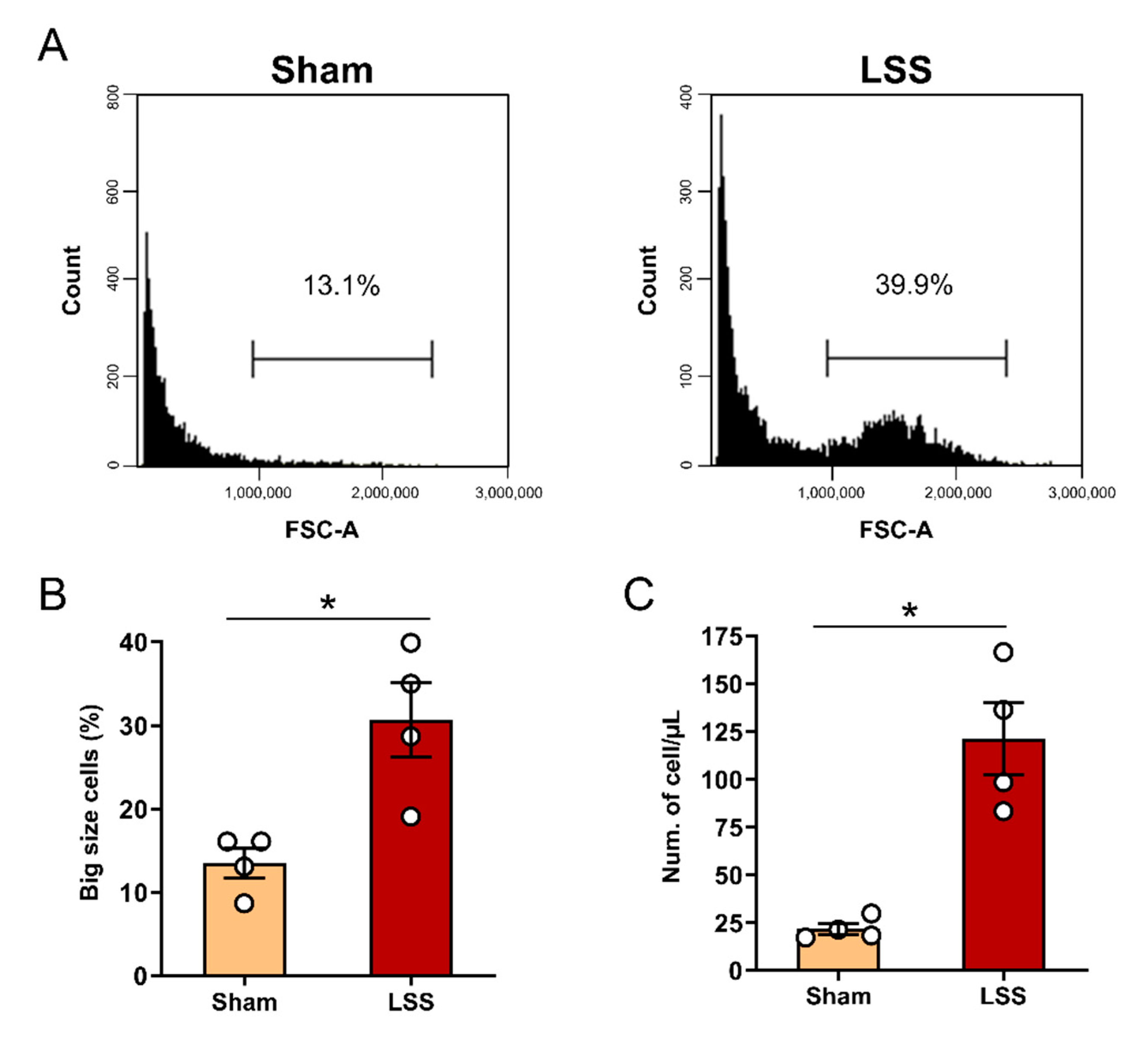
3.2. Flow Cytometric Cell Size and Number Analysis in CSF after LSS
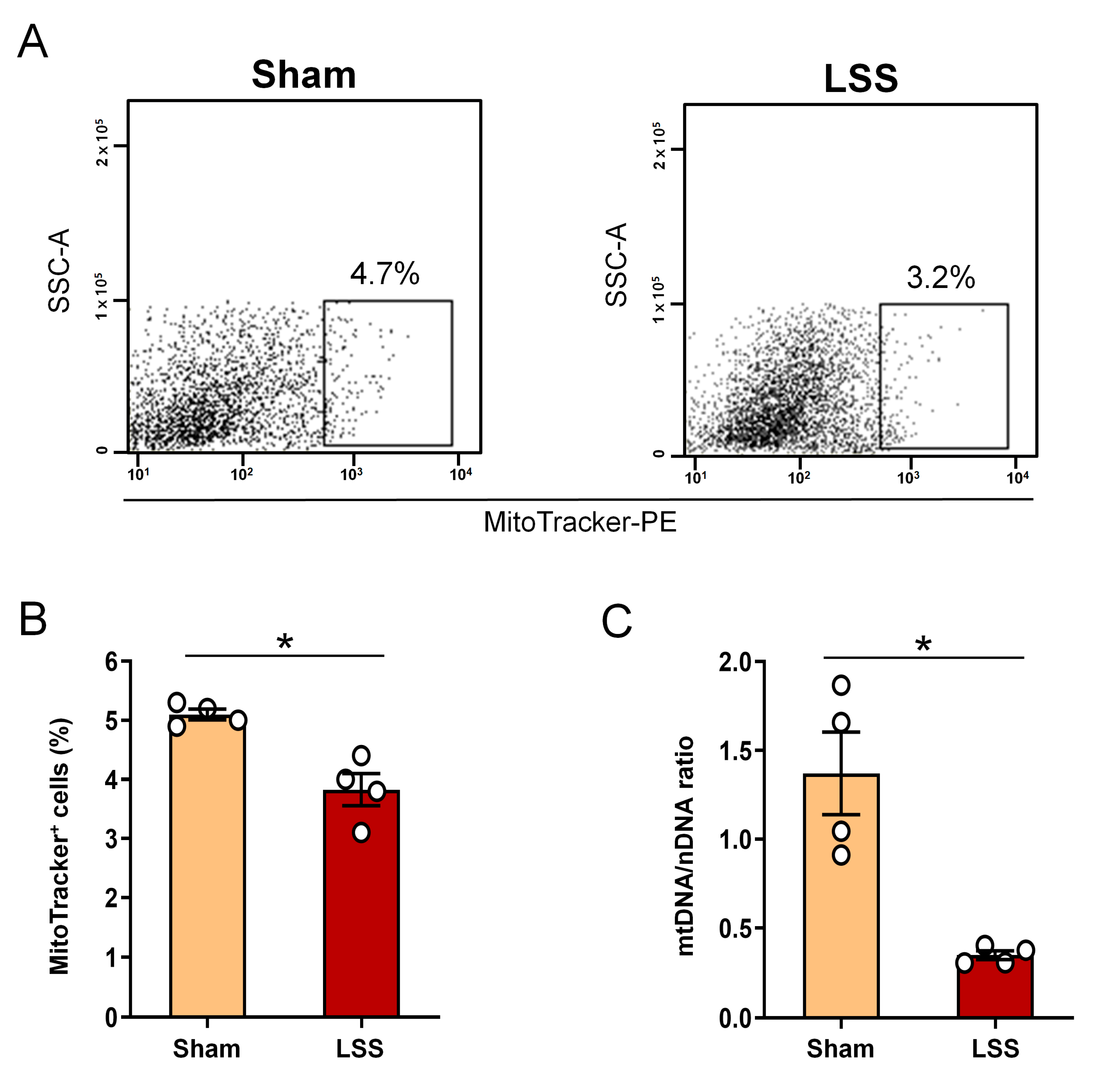
3.3. Analysis of Extracellular Mitochondrial Mass within CSF after Given LSS
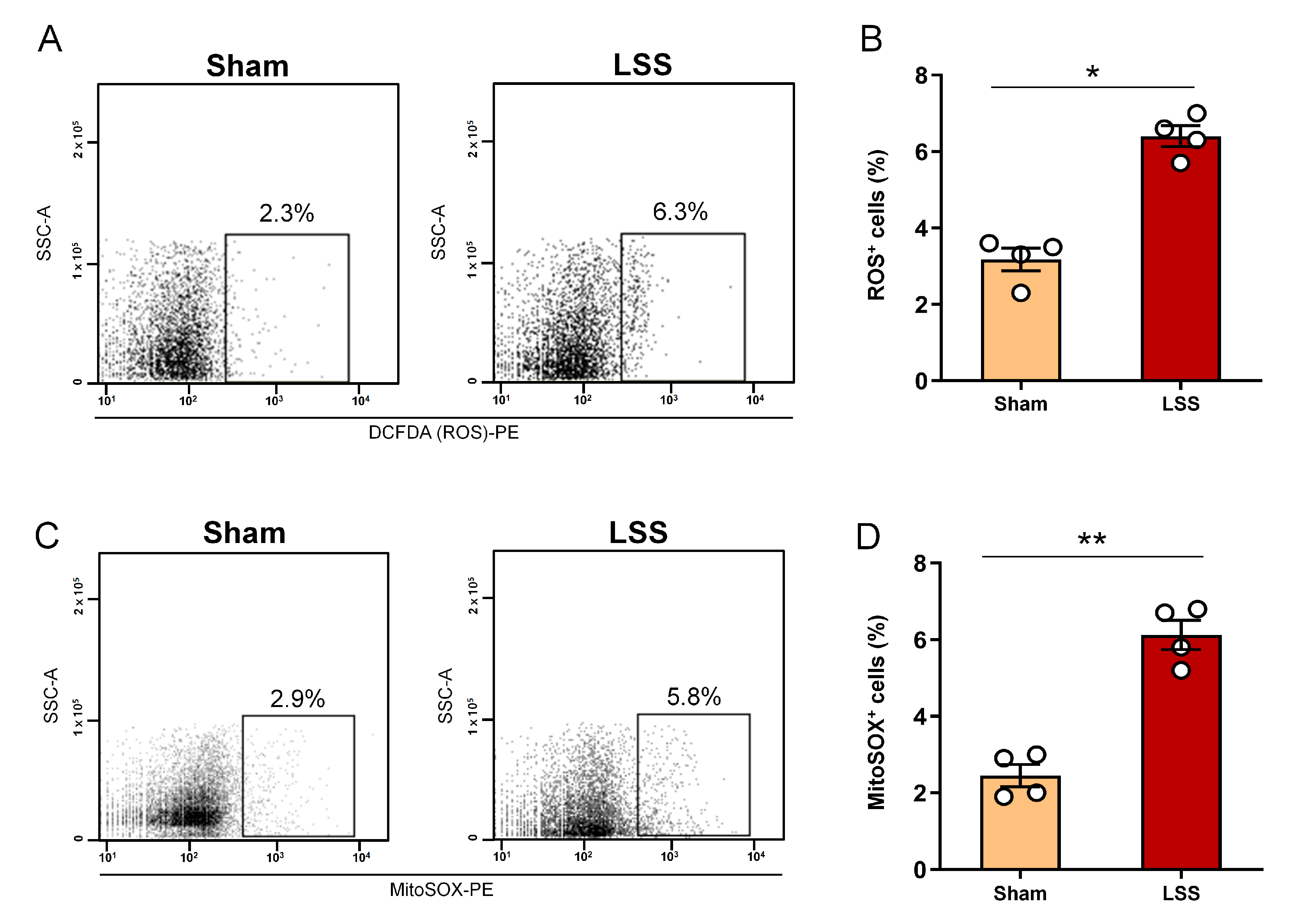
3.4. Analysis of Cellular ROS and Mitochondrial ROS Production within CSF after LSS
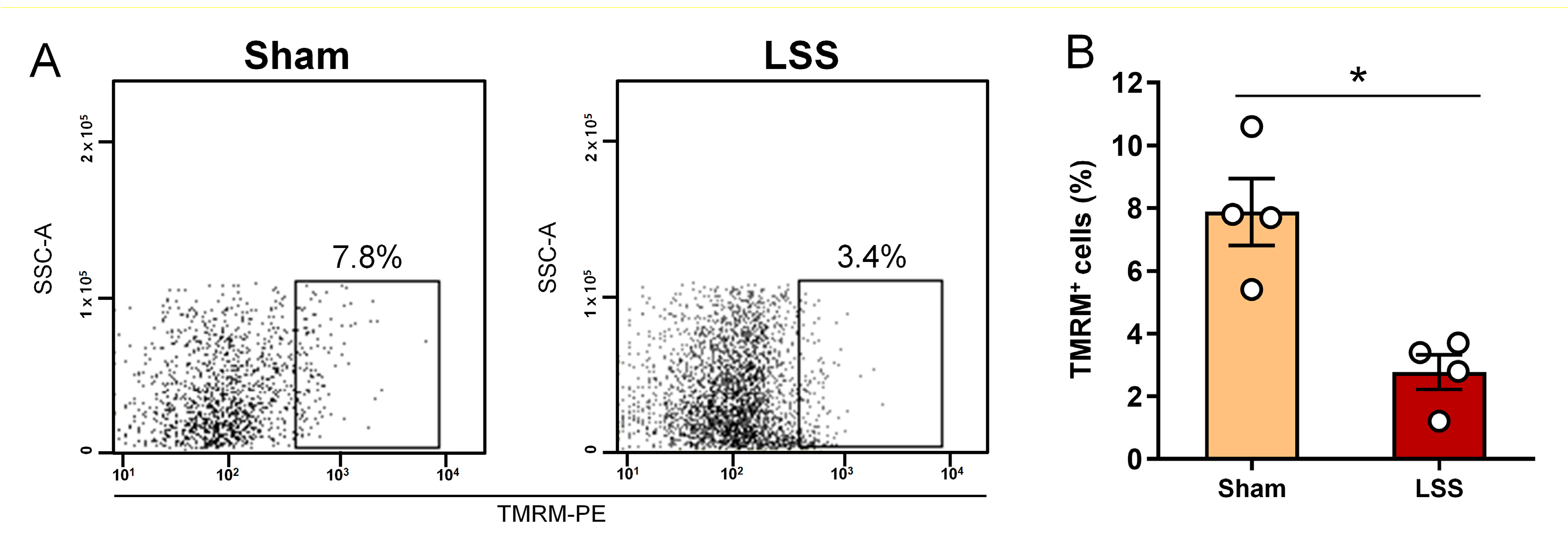
3.5. Analysis of MMP within CSF after Given LSS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seehusen, D.A.; Reeves, M.M.; Fomin, D.A. Cerebrospinal fluid analysis. Am. Fam. Physician 2003, 68, 1103–1108. [Google Scholar]
- Akiba, C.; Bandai, H.; Ito, Y.; Maeda, T.; Yamaguchi, K.; Nakajima, M.; Miyajima, M. Cerebrospinal fluid leak presented with the C1-C2 sign caused by spinal canal stenosis: A case report. BMC Neurol. 2020, 20, 151. [Google Scholar] [CrossRef]
- Chun, S.W.; Lee, H.J.; Nam, K.H.; Sohn, C.H.; Kim, K.D.; Jeong, E.J.; Chung, S.G.; Kim, K.; Kim, D.J. Cerebrospinal fluid dynamics at the lumbosacral level in patients with spinal stenosis: A pilot study. J. Orthop. Res. 2017, 35, 104–112. [Google Scholar] [CrossRef]
- Khachatryan, T.; Robinson, J.S. The possible impact of cervical stenosis on cephalad neuronal dysfunction. Med. Hypotheses 2018, 118, 13–18. [Google Scholar] [CrossRef]
- Chou, S.H.; Lan, J.; Esposito, E.; Ning, M.; Balaj, L.; Ji, X.; Lo, E.H.; Hayakawa, K. Extracellular Mitochondria in Cerebrospinal Fluid and Neurological Recovery After Subarachnoid Hemorrhage. Stroke 2017, 48, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Leurs, C.E.; Podlesniy, P.; Trullas, R.; Balk, L.; Steenwijk, M.D.; Malekzadeh, A.; Piehl, F.; Uitdehaag, B.M.; Killestein, J.; van Horssen, J.; et al. Cerebrospinal fluid mtDNA concentration is elevated in multiple sclerosis disease and responds to treatment. Mult. Scler. 2018, 24, 472–480. [Google Scholar] [CrossRef]
- Pyle, A.; Brennan, R.; Kurzawa-Akanbi, M.; Yarnall, A.; Thouin, A.; Mollenhauer, B.; Burn, D.; Chinnery, P.F.; Hudson, G. Reduced cerebrospinal fluid mitochondrial DNA is a biomarker for early-stage Parkinson’s disease. Ann. Neurol. 2015, 78, 1000–1004. [Google Scholar] [CrossRef]
- Regenold, W.T.; Phatak, P.; Makley, M.J.; Stone, R.D.; Kling, M.A. Cerebrospinal fluid evidence of increased extra-mitochondrial glucose metabolism implicates mitochondrial dysfunction in multiple sclerosis disease progression. J. Neurol. Sci. 2008, 275, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Varhaug, K.N.; Vedeler, C.A.; Myhr, K.M.; Aarseth, J.H.; Tzoulis, C.; Bindoff, L.A. Increased levels of cell-free mitochondrial DNA in the cerebrospinal fluid of patients with multiple sclerosis. Mitochondrion 2017, 34, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Walko, T.D., 3rd; Bola, R.A.; Hong, J.D.; Au, A.K.; Bell, M.J.; Kochanek, P.M.; Clark, R.S.; Aneja, R.K. Cerebrospinal fluid mitochondrial DNA: A novel DAMP in pediatric traumatic brain injury. Shock 2014, 41, 499–503. [Google Scholar] [CrossRef]
- Gmitterova, K.; Heinemann, U.; Gawinecka, J.; Varges, D.; Ciesielczyk, B.; Valkovic, P.; Benetin, J.; Zerr, I. 8-OHdG in cerebrospinal fluid as a marker of oxidative stress in various neurodegenerative diseases. Neurodegener. Dis. 2009, 6, 263–269. [Google Scholar] [CrossRef]
- Podlesniy, P.; Llorens, F.; Puigros, M.; Serra, N.; Sepulveda-Falla, D.; Schmidt, C.; Hermann, P.; Zerr, I.; Trullas, R. Cerebrospinal Fluid Mitochondrial DNA in Rapid and Slow Progressive Forms of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6298. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.Y.; Lee, J.; Jeon, W.J.; Ha, I.H. IL-1beta promotes disk degeneration and inflammation through direct injection of intervertebral disk in a rat lumbar disk herniation model. Spine J. 2021. [Google Scholar] [CrossRef]
- Nirogi, R.; Kandikere, V.; Mudigonda, K.; Bhyrapuneni, G.; Muddana, N.; Saralaya, R.; Benade, V. A simple and rapid method to collect the cerebrospinal fluid of rats and its application for the assessment of drug penetration into the central nervous system. J. Neurosci. Methods 2009, 178, 116–119. [Google Scholar] [CrossRef]
- Palomo, F.S.; Rivero, M.G.C.; Quiles, M.G.; Pinto, F.P.; Machado, A.M.O.; Carlos Campos Pignatari, A. Comparison of DNA Extraction Protocols and Molecular Targets to Diagnose Tuberculous Meningitis. Tuberc. Res. Treat. 2017, 2017, 5089046. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Dechsupa, S.; Yingsakmongkol, W.; Limthongkul, W.; Singhatanadgige, W.; Honsawek, S. Relative telomere length and oxidative DNA damage in hypertrophic ligamentum flavum of lumbar spinal stenosis. PeerJ 2018, 6, e5381. [Google Scholar] [CrossRef]
- Chuang, H.C.; Tsai, K.L.; Tsai, K.J.; Tu, T.Y.; Shyong, Y.J.; Jou, I.M.; Hsu, C.C.; Shih, S.S.; Liu, Y.F.; Lin, C.L. Oxidative stress mediates age-related hypertrophy of ligamentum flavum by inducing inflammation, fibrosis, and apoptosis through activating Akt and MAPK pathways. Aging 2020, 12, 24168–24183. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid. Med. Cell Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef] [PubMed]
- Rabchevsky, A.G.; Michael, F.M.; Patel, S.P. Mitochondria focused neurotherapeutics for spinal cord injury. Exp. Neurol. 2020, 330, 113332. [Google Scholar] [CrossRef]
- Henchcliffe, C.; Beal, M.F. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 2008, 4, 600–609. [Google Scholar] [CrossRef]
- Barcelos, I.P.; Troxell, R.M.; Graves, J.S. Mitochondrial Dysfunction and Multiple Sclerosis. Biology 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Wentling, M.; Lopez-Gomez, C.; Park, H.J.; Amatruda, M.; Ntranos, A.; Aramini, J.; Petracca, M.; Rusielewicz, T.; Chen, E.; Tolstikov, V.; et al. A metabolic perspective on CSF-mediated neurodegeneration in multiple sclerosis. Brain 2019, 142, 2756–2774. [Google Scholar] [CrossRef]
- Podlesniy, P.; Trullas, R. Biomarkers in Cerebrospinal Fluid: Analysis of Cell-Free Circulating Mitochondrial DNA by Digital PCR. Methods Mol. Biol. 2018, 1768, 111–126. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.Y.; Kim, H.; Jeon, W.-J.; Lee, J.; Ha, I.-H. Elevated Mitochondrial Reactive Oxygen Species within Cerebrospinal Fluid as New Index in the Early Detection of Lumbar Spinal Stenosis. Diagnostics 2021, 11, 748. https://doi.org/10.3390/diagnostics11050748
Hong JY, Kim H, Jeon W-J, Lee J, Ha I-H. Elevated Mitochondrial Reactive Oxygen Species within Cerebrospinal Fluid as New Index in the Early Detection of Lumbar Spinal Stenosis. Diagnostics. 2021; 11(5):748. https://doi.org/10.3390/diagnostics11050748
Chicago/Turabian StyleHong, Jin Young, Hyunseong Kim, Wan-Jin Jeon, Junseon Lee, and In-Hyuk Ha. 2021. "Elevated Mitochondrial Reactive Oxygen Species within Cerebrospinal Fluid as New Index in the Early Detection of Lumbar Spinal Stenosis" Diagnostics 11, no. 5: 748. https://doi.org/10.3390/diagnostics11050748
APA StyleHong, J. Y., Kim, H., Jeon, W.-J., Lee, J., & Ha, I.-H. (2021). Elevated Mitochondrial Reactive Oxygen Species within Cerebrospinal Fluid as New Index in the Early Detection of Lumbar Spinal Stenosis. Diagnostics, 11(5), 748. https://doi.org/10.3390/diagnostics11050748





