Diagnostic Performance and Usability of the Genedrive® HCV ID Kit in Two Decentralized Settings in Cameroon and Georgia
Abstract
1. Background
2. Methods
2.1. Study Design
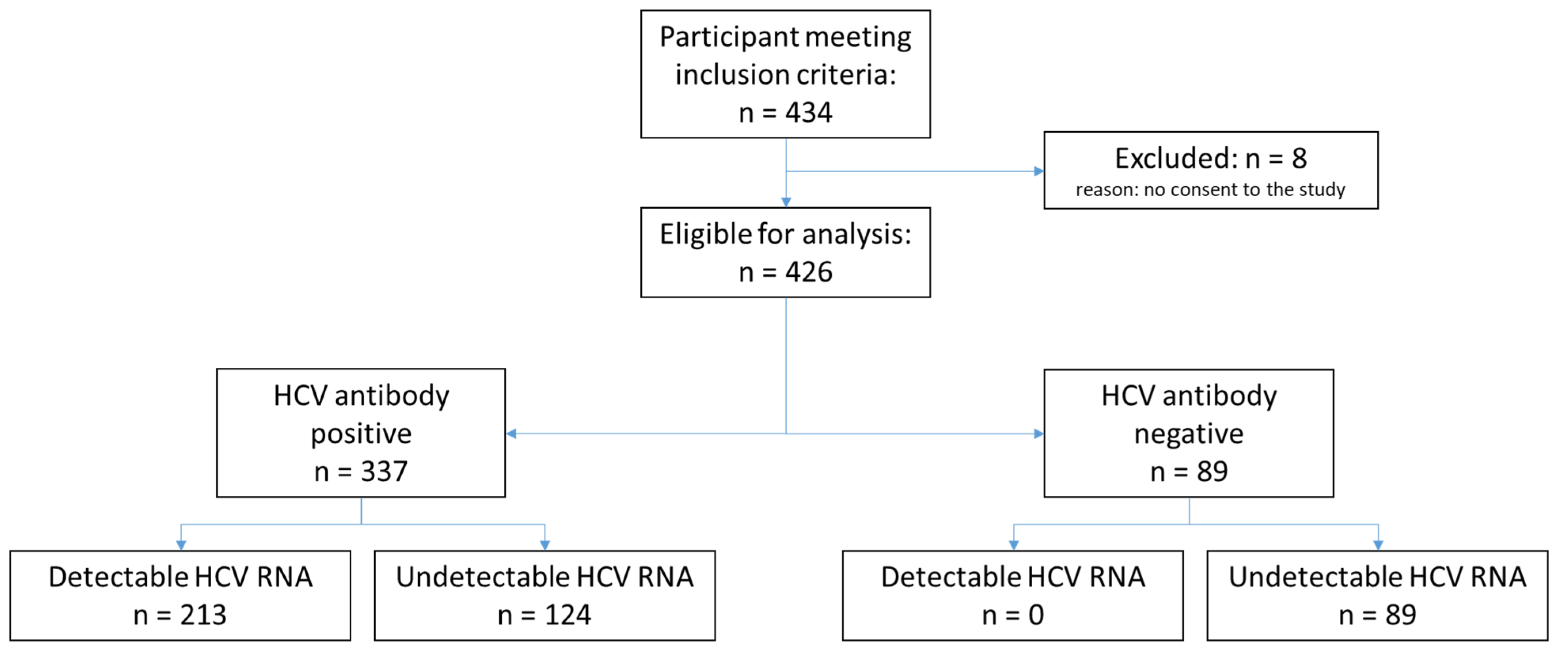
2.2. Study Population and Study Settings
2.3. Testing Methods
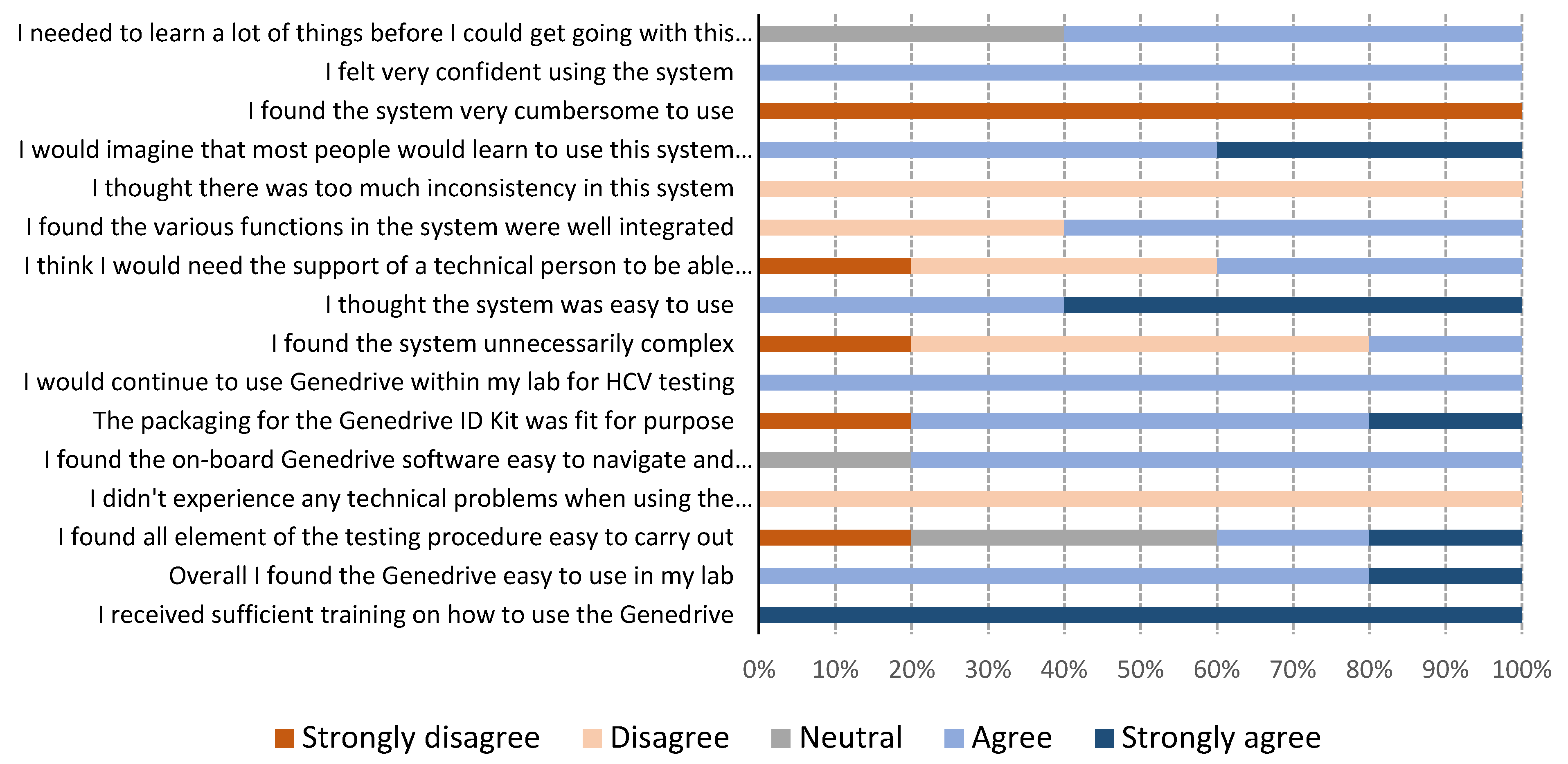
2.4. Usability of Genedrive
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Genedrive Diagnostic Performance
3.3. Genedrive Usability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gower, E.; Estes, C.; Blach, S.; Razavi-Shearer, K.; Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61, S45–S57. [Google Scholar] [CrossRef]
- World Health Organization. Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ (accessed on 14 August 2020).
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis, 2016–2021; World Health Organization: Geneva, Switzerland, 2016; Available online: https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ (accessed on 14 August 2020).
- Feld, J.J. Direct-Acting Antivirals for Hepatitis C Virus (HCV): The Progress Continues. Curr. Drug Targets 2017, 18, 851–862. [Google Scholar] [CrossRef]
- Hill, A.; Khoo, S.; Fortunak, J.; Simmons, B.; Ford, N. Minimum Costs for Producing Hepatitis C Direct-Acting Antivirals for Use in Large-Scale Treatment Access Programs in Developing Countries. Clin. Infect. Dis. 2014, 58, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Simmons, B.; Gotham, D.; Fortunak, J. Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C. J. Virus Erad. 2016, 2, 28–31. [Google Scholar] [CrossRef]
- Reipold, E.I.; Trianni, A.; Krakower, D.; Ongarello, S.; Roberts, T.; Easterbrook, P.; Denkinger, C. Values, preferences and current hepatitis B and C testing practices in low- and middle-income countries: Results of a survey of end users and implementers. BMC Infect. Dis. 2017, 17 (Suppl. S1), 702. [Google Scholar] [CrossRef]
- Ivanova Reipold, E.; Easterbrook, P.; Trianni, A.; Panneer, N.; Krakower, D.; Ongarello, S.; Roberts, T.; Miller, V.; Denkinger, C. Optimising diagnosis of viraemic hepatitis C infection: The development of a target product profile. BMC Infect. Dis. 2017, 17 (Suppl. S1), 707. [Google Scholar] [CrossRef]
- Lemoine, M.; Tillmann, H.L. What is required from HCV point-of-care tests to reduce the burden of hepatitis C infection? ’De-velopment and clinical validation of the genedrive point-of-care test for qualitative detection of hepatitis C virus’. Gut 2018, 67, 1916–1917. [Google Scholar] [CrossRef]
- Genedrive plc. Hepatitis C Point-of-Care Diagnostic Launched. 2018. Available online: http://www.genedriveplc.com/company-reports/Hardman.pdf (accessed on 20 September 2020).
- Genedrive plc. Press Release 11 September 2017. Genedrive HCV ID Kit Received CE-IVD Certification. Available online: http://www.genedriveplc.com/press-releases/genedrive_hcv_id_test_received_ce_certification_final.pdf (accessed on 20 September 2020).
- Genedrive plc. Press Release 4 May 2020. Genedrive HCV-ID Test Receives WHO Prequalification. Available online: http://www.genedriveplc.com/press-releases/gdr_who_pq.pdf (accessed on 20 September 2020).
- Llibre, A.; Shimakawa, Y.; Mottez, E.; Ainsworth, S.; Buivan, T.-P.; Firth, R.; Harrison, E.; Rosenberg, A.R.; Meritet, J.-F.; Fontanet, A.; et al. Development and clinical validation of the Genedrive point-of-care test for qualitative detection of hepatitis C virus. Gut 2018, 67, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; De Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Screening Care and Treatment of Persons with Chronic Hepatitis C Infection: Updated Version; World Health Organization: Geneva, Switzerland, 2016; Available online: https://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/ (accessed on 20 September 2020).
- Campos-Outcalt, D. Hepatitis C: New CDC screening recommendations. J. Fam. Pract. 2012, 61, 744–746. [Google Scholar] [PubMed]
- Mokhtari, C.; Ebel, A.; Reinhardt, B.; Merlin, S.; Proust, S.; Roque-Afonso, A.-M. Characterization of Samples Identified as Hepatitis C Virus Genotype 1 without Subtype by Abbott RealTime HCV Genotype II Assay Using the New Abbott HCV GenotypePlusRUO Test. J. Clin. Microbiol. 2015, 54, 296–299. [Google Scholar] [CrossRef]
- Wiesmann, F.; Naeth, G.; Sarrazin, C.; Berger, A.; Kaiser, R.; Ehret, R.; Knechten, H.; Braun, P. Variation analysis of six HCV viral load assays using low viremic HCV samples in the range of the clinical decision points for HCV protease inhibitors. Med. Microbiol. Immunol. 2014, 204, 515–525. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Prequalification of In Vitro Diagnostics. Public Report May 2020. Product: Genedrive HCV ID Kit. WHO Reference Number: PQDx 0380-133-00; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/diagnostics_laboratory/evaluations/pq-list/hcv/200501_final_pqpr_pqdx_0380_133_00_genedrive_hcv_id_v1.pdf?ua=1 (accessed on 20 September 2020).
- Padhi, A.; Gupta, E.; Singh, G.; Agarwal, R.; Sharma, M.K.; Sarin, S.K. Evaluation of the Point of Care Molecular Diagnostic Genedrive HCV ID Kit for the detection of HCV RNA in clinical samples. Epidemiol. Infect. 2020, 2020, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, W.S.; Obaje, E.A.; Bachmann, T.T.; Kersaudy-Kerhoas, M. Microfluidic blood plasma separation for medical diagnostics: Is it worth it? Lab Chip 2016, 16, 3441–3448. [Google Scholar] [CrossRef]
- Llibre, A.; Shimakawa, Y.; Duffy, D. Potential utility of the Genedrive point-of-care test for HCV RNA detection. Gut 2019, 68, 1903–1904. [Google Scholar] [CrossRef]
- McHugh, M.P.; Wu, A.H.B.; Chevaliez, S.; Pawlotsky, J.M.; Hallin, M.; Templeton, K.E. Multicenter Evaluation of the Cepheid Xpert Hepatitis C Virus Viral Load Assay. J. Clin. Microbiol. 2017, 55, 1550–1556. [Google Scholar] [CrossRef]
- Ndlovu, Z.; Fajardo, E.; Mbofana, E.; Maparo, T.; Garone, D.; Metcalf, C.; Bygrave, H.; Kao, K.; Zinyowera, S. Multidisease testing for HIV and TB using the GeneXpert platform: A feasibility study in rural Zimbabwe. PLoS ONE 2018, 13, e0193577. [Google Scholar] [CrossRef]
- Cazabon., D.; Pande, T.; Kik, S.; Van Gemert, W.; Sohn, H.; Denkinger, C.; Qin, Z.Z.; Waning, B.; Pai, M. Market penetration of Xpert MTB/RIF in high tuberculosis burden countries: A trend analysis from 2014–2016. Gates Open Res. 2018, 2, 35. [Google Scholar] [CrossRef]
- Médecins Sans Frontières. MSF Access Campaign. Putting HIV and HCV to the Test: A Product Guide for Point-of-Care CD4 and Laboratory-Based and Point-of-Care Virological HIV and HCV Tests. 2017. Available online: https://msfaccess.org/putting-hiv-and-hcv-test-3rd-ed-2017 (accessed on 20 September 2020).
- Clinton Health Access Initiative (CHAI). Hepatitis C Market Report. Issue 1 May 2020. Available online: https://3cdmh310dov3470e6x160esb-wpengine.netdna-ssl.com/wp-content/uploads/2020/05/Hepatitis-C-Market-Report_Issue-1_Web.pdf (accessed on 20 September 2020).
- Lamoury, F.M.J.; Bajis, S.; Hajarizadeh, B.; Marshall, A.D.; Martinello, M.; Ivanova, E.; Catlett, B.; Mowat, Y.; Marks, P.; Amin, J.; et al. Evaluation of the Xpert HCV Viral Load Finger-Stick Point-of-Care Assay. J. Infect. Dis. 2018, 217, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Freiman, J.M.; Wang, J.; Easterbrook, P.J.; Horsburgh, C.R.; Marinucci, F.; White, L.F.; Kamkamidze, G.; Krajden, M.; Loarec, A.; Njouom, R.; et al. Deriving the optimal limit of detection for an HCV point-of-care test for viraemic infection: Analysis of a global dataset. J. Hepatol. 2019, 71, 62–70. [Google Scholar] [CrossRef]
- Bajis, S.; Maher, L.; Treloar, C.; Hajarizadeh, B.; Lamoury, F.M.; Mowat, Y.; Schulz, M.; Marshall, A.D.; Cunningham, E.B.; Cock, V.; et al. Acceptability and preferences of point-of-care finger-stick whole-blood and venepuncture hepatitis C virus testing among people who inject drugs in Australia. Int. J. Drug Policy 2018, 61, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.; Al-Kurdi, D.; Nelson, M.; Shimakawa, Y.; Selvapatt, N.; Lacey, J.; Thursz, M.R.; Lemoine, M.; Brown, A.S. Time matters: Point of care screening and streamlined linkage to care dramatically improves hepatitis C treatment uptake in prisoners in England. Int. J. Drug Policy 2020, 75, 102608. [Google Scholar] [CrossRef] [PubMed]
- Hutin, Y.; Luhmann, N.; Easterbrook, P. Evaluating the impact of Georgia’s hepatitis C elimination plan: Lessons learned for the global initiative. Lancet Glob Health 2020, 8, e163–e164. [Google Scholar] [CrossRef]
| Characteristics | Cameroon (n = 156) | Georgia (n = 270) | Total (n = 426) |
|---|---|---|---|
| Female | 93 (59.6) | 18 (6.7) | 111 (26.1) |
| Male | 63 (40.4) | 252 (93.3) | 315 (73.9) |
| Median age (range), years | 63 (21–83) | 43 (18–69) | 47 (18–83) |
| Positive HCV antibody | 156 (100) | 181 (67.0) | 337 (79.1) |
| Positive HIV antibody | 2 (1.3) | 3 (1.1) | 5 (1.2) |
| HCV-positive mother | 3 (1.9) | 0 (0.0) | 3 (0.7) |
| Injects non-prescription drugs | 0 (0.0) | 269 (99.6) | 269 (63.1) |
| Treated in past 12 months | 47 (30.1) | 0 (0.0) | 47 (11.0) |
| Abbott HCV RNA undetectable | 64 (41.0) | 149 (55.0) | 213 (50.0) |
| Abbott HCV RNA detectable | 92 (59.0) | 121 (45.0) | 213 (50.0) |
| HCV genotype determined * | 85 (41.5) | 120 (58.5) | 205 (96.2) |
| Genotype 1 † | 33 (38.8) | 75 (62.5) | 108 (52.7) |
| Genotype 2 † | 13 (15.3) | 18 (15.0) | 31 (15.1) |
| Genotype 3 † | 0 (0.0) | 21 (17.5) | 21 (10.2) |
| Genotype 4 † | 38 (44.7) | 0 (0.0) | 38 (18.5) |
| Genotype undetermined † | 1 (1.2) | 6 (5.0) | 7 (3.4) |
| Abbott: 12–30 IU/mL Threshold | Total | Diagnostic Accuracy (95% CI) | |||
|---|---|---|---|---|---|
| Target Detected | Target Undetected | ||||
| Genedrive® HCV ID assay | Positive | 205 | 1 | 206 | Sensitivity: 96.2% (92.7–98.4) Specificity: 99.5% (97.4–100) |
| Negative | 8 | 212 | 220 | ||
| Total | 213 | 213 | 426 | ||
| Abbott: 1000 IU/mL threshold | Total | ||||
| Target detected | Target undetected | ||||
| Genedrive® HCV ID assay | Positive | 205 | 1 | 206 | Sensitivity: 100% (98.2–100) Specificity: 99.5% (97.5–100) |
| Negative | 0 | 220 | 220 | ||
| Total | 205 | 221 | 426 | ||
| Abbott: 2362 IU/mL threshold | Total | ||||
| Target detected | Target undetected | ||||
| Genedrive® HCV ID assay | Positive | 203 | 3 | 206 | Sensitivity: 100% (98.2–100) Specificity: 98.7 (96.1–99.7) |
| Negative | 0 | 220 | 220 | ||
| Total | 203 | 223 | 426 | ||
| Genedrive Result | Cameroon | Georgia | Total |
|---|---|---|---|
| Negative | 69 (44.2) | 146 (54.1) | 215 (50.5) |
| Positive | 84 (53.8) | 120 (44.4) | 204 (47.9) |
| Indeterminate | 2 (1.3) | 0 (0.0) | 2 (0.5) |
| Control failed | 1 (0.6) | 4 (1.5) | 5 (1.2) |
| Total tests | 156 (36.6) | 270 (63.4) | 426 (100) |
| Invalid tests repeated | 3 (1.9) | 4 (1.5) | 7 (1.7) |
| Negative | 2 (66.7) | 3 (75.0) | 5 (71.4) |
| Positive | 1 (33.3) | 1 (25.0) | 2 (28.6) |
| Total tests performed | 159 (36.7) | 274 (63.3) | 433 (100) |
| Total invalid rate | 3 (1.9) | 4 (1.5) | 7 (1.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamoury, F.M.J.; Njouom, R.; Amougou-Atsama, M.; Yiagnigni Mfopou, E.; Berishvili, N.; Sologashvili, M.; Fajardo, E.; Malobela, A.; Macé, A.; Chirehwa, M.; et al. Diagnostic Performance and Usability of the Genedrive® HCV ID Kit in Two Decentralized Settings in Cameroon and Georgia. Diagnostics 2021, 11, 746. https://doi.org/10.3390/diagnostics11050746
Lamoury FMJ, Njouom R, Amougou-Atsama M, Yiagnigni Mfopou E, Berishvili N, Sologashvili M, Fajardo E, Malobela A, Macé A, Chirehwa M, et al. Diagnostic Performance and Usability of the Genedrive® HCV ID Kit in Two Decentralized Settings in Cameroon and Georgia. Diagnostics. 2021; 11(5):746. https://doi.org/10.3390/diagnostics11050746
Chicago/Turabian StyleLamoury, Francois M. J., Richard Njouom, Marie Amougou-Atsama, Euloge Yiagnigni Mfopou, Nino Berishvili, Manana Sologashvili, Emmanuel Fajardo, Agnes Malobela, Aurélien Macé, Maxwell Chirehwa, and et al. 2021. "Diagnostic Performance and Usability of the Genedrive® HCV ID Kit in Two Decentralized Settings in Cameroon and Georgia" Diagnostics 11, no. 5: 746. https://doi.org/10.3390/diagnostics11050746
APA StyleLamoury, F. M. J., Njouom, R., Amougou-Atsama, M., Yiagnigni Mfopou, E., Berishvili, N., Sologashvili, M., Fajardo, E., Malobela, A., Macé, A., Chirehwa, M., Alkhazashvili, M., & Ivanova Reipold, E. (2021). Diagnostic Performance and Usability of the Genedrive® HCV ID Kit in Two Decentralized Settings in Cameroon and Georgia. Diagnostics, 11(5), 746. https://doi.org/10.3390/diagnostics11050746






