HCV Diagnosis and Sequencing Using Dried Blood Spots from Patients in Kinshasa (DRC): A Tool to Achieve WHO 2030 Targets
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Collection
2.3. Serological Tests
2.3.1. Vidas® Anti-HCV
2.3.2. Elecsys Anti-HCV II
2.3.3. INNO-LIA HCV Score
2.4. HCV RNA Detection: COBAS® AmpliPrep/COBAS® TaqMan® HCV Test
2.5. Molecular HCV Characterization
2.5.1. Primers Design
2.5.2. RNA Extraction and Amplification
2.5.3. Sequencing and Interpretation
2.6. Ethical Aspects
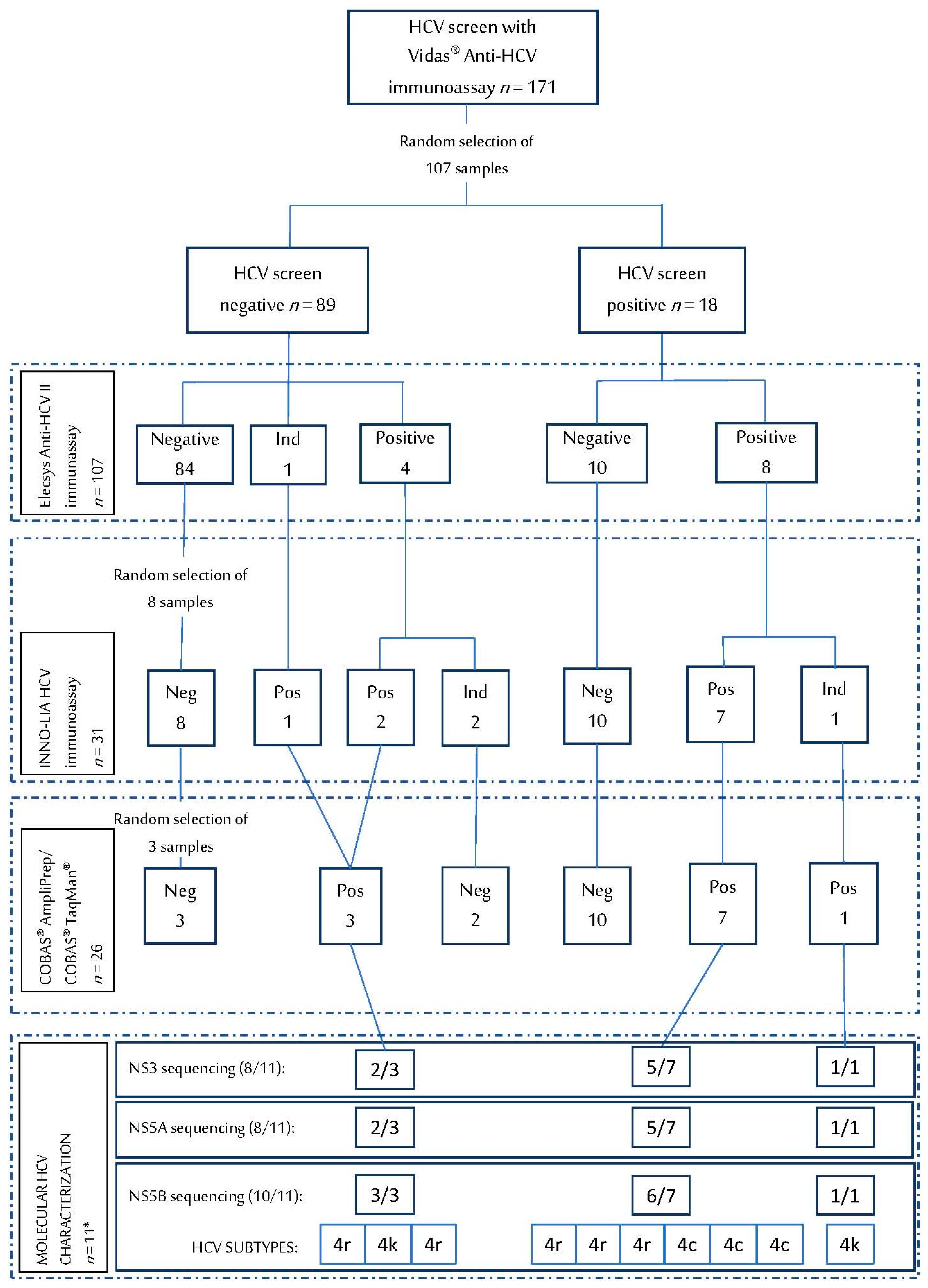
3. Results
3.1. HCV Serological Assays on DBS
3.2. HCV RNA Detection on DBS
3.3. HCV Genotype and Resistance Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Heffernan, A.; Cooke, G.S.; Nayagam, S.; Thursz, M.; Hallett, T.B. Scaling up prevention and treatment towards the elimination of hepatitis C: A global mathematical model. Lancet 2019, 393, 1319–1329. [Google Scholar] [CrossRef]
- Gower, E.; Estes, C.; Blach, S.; Razavi-Shearer, K.; Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61, S45–S57. [Google Scholar] [CrossRef] [PubMed]
- Sonderup, M.W.; Afihene, M.; Ally, R.; Apica, B.; Awuku, Y.; Cunha, L.; Dusheiko, G.; Gogela, N.; Lohouès-Kouacou, M.-J.; Lam, P.; et al. Hepatitis C in sub-Saharan Africa: The current status and recommendations for achieving elimination by 2030. Lancet Gastroenterol. Hepatol. 2017, 2, 910–919. [Google Scholar] [CrossRef]
- Blach, S.; Zeuzem, S.; Manns, M.; Altraif, I.; Duberg, A.-S.; Muljono, D.H.; Waked, I.; Alavian, S.M.; Lee, M.-H.; Negro, F.; et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef]
- Gupta, N.; Kateera, F.; Desalegn, H.; Ocama, P.; Njouom, R.; Lacombe, K. Is resistance to direct-acting antivirals in sub-Saharan Africa a threat to HCV elimination? Recommendations for action. J. Hepatol. 2020, 72, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Mgomella, G.S.; Filipe, A.D.S.; Frost, E.H.; Giroux, G.; Hughes, J.; Hogan, C.; Kaleebu, P.; Asiki, G.; Mclauchlan, J.; et al. Highly Diverse Hepatitis C Strains Detected in Sub-Saharan Africa Have Unknown Susceptibility to Direct-Acting Antiviral Treatments. Hepatology 2018, 69, 1426–1441. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.; Bane, A.; Braimoh, G.; Debes, J.D. Treatment of Hepatitis C Genotypes 1 to 5 in Sub-Saharan Africa Using Direct-Acting Antivirals. Am. J. Trop. Med. Hyg. 2020, 103, 2083–2084. [Google Scholar] [CrossRef]
- Nsanzimana, S.; Penkunas, M.J.; Liu, C.Y.; Sebuhoro, D.; Ngwije, A.; Remera, E.; Umutesi, J.; Ntirenganya, C.; Mugeni, S.D.; Serumondo, J. Effectiveness of Direct-Acting Antivirals for the treatment of chronic hepatitis C in Rwanda: A retrospective study. Clin. Infect. Dis. 2020, 6. [Google Scholar] [CrossRef]
- Duchesne, L.; Hejblum, G.; Njouom, R.; Touré Kane, C.; Toni, T.D.; Moh, R.; Sylla, B.; Rouveau, N.; Attia, A.; Lacombe, K. Model-based cost-effectiveness estimates of testing strategies for diagnosing hepatitis C virus infection in Central and Western Africa. PLoS ONE 2020, 15, e0238035. [Google Scholar] [CrossRef]
- Polaris Observatory. Available online: https://cdafound.org/dashboard/polaris/dashboard.html (accessed on 22 January 2021).
- Abergel, A.; Metivier, S.; Samuel, D.; Jiang, D.; Kersey, K.; Pang, P.S.; Svarovskaia, E.; Knox, S.J.; Loustaud-Ratti, V.; Asselah, T. Ledipasvir plus sofosbuvir for 12 weeks in patients with hepatitis C genotype 4 infection. Hepatology 2016, 64, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Mbituyumuremyi, A.; Kabahizi, J.; Ntaganda, F.; Muvunyi, C.M.; Shumbusho, F.; Musabeyezu, E.; Mukabatsinda, C.; Ntirenganya, C.; Van Nuil, J.I.; et al. Treatment of chronic hepatitis C virus infection in Rwanda with ledipasvir-sofosbuvir (SHARED): A single-arm trial. Lancet Gastroenterol. Hepatol. 2019, 4, 119–126. [Google Scholar] [CrossRef]
- Filipe, A.D.S.; Sreenu, V.; Hughes, J.; Aranday-Cortés, E.; Irving, W.L.; Foster, G.R.; Agarwal, K.; Rosenberg, W.; Macdonald, U.; Richardson, P.; et al. Reply to: “Reply to: ‘Response to DAA therapy in the NHS England Early Access Programme for rare HCV subtypes from low and middle income countries’”. J. Hepatol. 2018, 68, 864–866. [Google Scholar] [CrossRef] [PubMed]
- Carlos, S.; López del Burgo, C.; Ndarabu, A.; Osorio, A.; Rico-Campà, A.; Reina, G.; Burgueño, E.; De Irala, J. Heterosexual oral and anal sex in Kinshasa (D.R.Congo): Data from OKAPI prospective cohort. PLoS ONE 2019, 14, e0210398. [Google Scholar] [CrossRef]
- Holguín, A.; Norman, F.; Martín, L.; Mateos, M.L.; Chacón, J.; López-Vélez, R.; Pérez-Molina, J.A. Dried blood as an alternative to plasma or serum for Trypanosoma cruzi IgG detection in screening programs. Clin. Vaccine Immunol. 2013, 20, 1197–1202. [Google Scholar] [CrossRef][Green Version]
- Martínez-Campreciós, J.; Rando-Segura, A.; Buti, M.; Rodrigo-Velásquez, F.; Riveiro-Barciela, M.; Barreira-Díaz, A.; Álvarez-López, P.; Salmerón, P.; Palom, A.; Tabernero, D.; et al. Reflex viral load testing in dried blood spots generated by plasma separation card allows the screening and diagnosis of chronic viral hepatitis. J. Virol. Methods 2020, 289, 114039. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Garrido, M.; Ndarabu, A.; Reina, G.; Barquín, D.; Fernández-Alonso, M.; Carlos, S.; Holguín, A. Utility Of POC Xpert HIV-1 Tests For Detection-Quantification Of Complex HIV Recombinants Using Dried Blood Spots From Kinshasa, D. R. Congo. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Weck, K.E. Hepatitis C Virus Genotyping: Interrogation of the 5’ Untranslated Region Cannot Accurately Distinguish Genotypes 1a and 1b. J. Clin. Microbiol. 2002, 40, 3127–3134. [Google Scholar] [CrossRef]
- Chueca, N.; Rivadulla, I.; Lovatti, R.; Reina, G.; Blanco, A.; Fernandez-Caballero, J.A.; Cardeñoso, L.; Rodriguez-Granjer, J.; Fernández-Alonso, M.; Aguilera, A.; et al. Using NS5B Sequencing for Hepatitis C Virus Genotyping Reveals Discordances with Commercial Platforms. PLoS ONE 2016, 11, e0153754. [Google Scholar] [CrossRef]
- Peiffer, K.H.; Sommer, L.; Susser, S.; Vermehren, J.; Herrmann, E.; Döring, M.; Dietz, J.; Perner, D.; Berkowski, C.; Zeuzem, S.; et al. Interferon lambda 4 genotypes and resistance-associated variants in patients infected with hepatitis C virus genotypes 1 and 3. Hepatology 2016, 63, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kalaghatgi, P.; Sikorski, A.M.; Knops, E.; Rupp, D.; Sierra, S.; Heger, E.; Neumann-Fraune, M.; Beggel, B.; Walker, A.; Timm, J.; et al. Geno2pheno [HCV]–A web-based interpretation system to support hepatitis C treatment decisions in the era of direct-acting antiviral agents. PLoS ONE 2016, 11, e0155869. [Google Scholar] [CrossRef]
- Sorbo, M.C.; Cento, V.; Di Maio, V.C.; Howe, A.Y.; Garcia, F.; Perno, C.F.; Ceccherini-Silberstein, F. Hepatitis C virus drug resistance associated substitutions and their clinical relevance: Update 2018. Drug Resist. Updat. 2018, 37, 17–39. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines on Hepatitis B and C Testing; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Chevaliez, S.; Pawlotsky, J.M. New virological tools for screening, diagnosis and monitoring of hepatitis B and C in resource-limited settings. J. Hepatol. 2018, 69, 916–926. [Google Scholar] [CrossRef]
- Lange, B.; Cohn, J.; Roberts, T.; Camp, J.; Chauffour, J.; Gummadi, N.; Ishizaki, A.; Nagarathnam, A.; Tuaillon, E.; Van De Perre, P.; et al. Diagnostic accuracy of serological diagnosis of hepatitis C and B using dried blood spot samples (DBS): Two systematic reviews and meta-analyses. BMC Infect. Dis. 2017, 17, 87–106. [Google Scholar] [CrossRef]
- Soulier, A.; Poiteau, L.; Rosa, I.; Hézode, C.; Roudot-Thoraval, F.; Pawlotsky, J.-M.; Chevaliez, S. Dried Blood Spots: A Tool to Ensure Broad Access to Hepatitis C Screening, Diagnosis, and Treatment Monitoring. J. Infect. Dis. 2015, 213, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Candotti, D.; Sauvage, V.; Cappy, P.; Boullahi, M.A.; Bizimana, P.; Mbensa, G.O.; Coulibaly, S.O.; Alson, A.O.R.; Soumana, H.; Tagny-Tayou, C.; et al. High rate of hepatitis C virus and human immunodeficiency virus false-positive results in serologic screening in sub-Saharan Africa: Adverse impact on the blood supply. Transfusion 2019, 60, 106–116. [Google Scholar] [CrossRef]
- Mullis, C.E.; Laeyendecker, O.; Reynolds, S.J.; Ocama, P.; Quinn, J.; Boaz, I.; Gray, R.H.; Kirk, G.D.; Thomas, D.L.; Quinn, T.C.; et al. High Frequency of False-Positive Hepatitis C Virus Enzyme-Linked Immunosorbent Assay in Rakai, Uganda. Clin. Infect. Dis. 2013, 57, 1747–1750. [Google Scholar] [CrossRef] [PubMed]
- Seremba, E.; Ocama, P.; Opio, C.; Kagimu, M.; Thomas, D.; Yuan, H.; Attar, N.; Lee, W. Poor performance of hepatitis C antibody tests in hospital patients in Uganda. J. Med. Virol. 2010, 82, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Scheiblauer, H.; El-Nageh, M.; Nick, S.; Fields, H.; Prince, A.; Diaz, S. Evaluation of the performance of 44 assays used in countries with limited resources for the detection of antibodies to hepatitis C virus. Transfusion 2006, 46, 708–718. [Google Scholar] [CrossRef]
- Layden, J.E.; Phillips, R.O.; Owusu-Ofori, S.; Sarfo, F.S.; Kliethermes, S.; Mora, N.; Owusu, D.; E Nelson, K.; Opare-Sem, O.; Dugas, L.R.; et al. High Frequency of Active HCV Infection Among Seropositive Cases in West Africa and Evidence for Multiple Transmission Pathways. Clin. Infect. Dis. 2015, 60, 1033–1041. [Google Scholar] [CrossRef]
- Lontok, E.; Harrington, P.R.; Howe, A.; Kieffer, T.L.; Lennerstrand, J.; Lenz, O.; McPhee, F.; Mo, H.; Parkin, N.T.; Pilot-Matias, T.; et al. Hepatitis C virus drug resistance-associated substitutions: State of the art summary. Hepatology 2015, 62, 1623–1632. [Google Scholar] [CrossRef]
- Pawlotsky, J.-M. Hepatitis C Virus Resistance to Direct-Acting Antiviral Drugs in Interferon-Free Regimens. Gastroenterology 2016, 151, 70–86. [Google Scholar] [CrossRef]
- Fourati, S.; Rodriguez, C.; Hézode, C.; Soulier, A.; Ruiz, I.; Poiteau, L.; Chevaliez, S.; Pawlotsky, J. Frequent Antiviral Treatment Failures in Patients Infected With Hepatitis C Virus Genotype 4, Subtype 4r. Hepatology 2019, 69, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Smith, D.; Vaughan-Jackson, A.; Magri, A.; Barnes, E.; Simmonds, P. Efficacy of NS5A inhibitors against unusual and potentially difficult-to-treat HCV subtypes commonly found in sub-Saharan Africa and South East Asia. J. Hepatol. 2020, 73, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M. DAA failures in African patients with “unusual” HCV subtypes: Hey! Didn’t you know there was another world? J. Hepatol. 2019, 71, 1070–1072. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL). EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Shumbusho, F.; Liu, A.F.; Kateera, F.; Kabahizi, J.; Nsanzaimana, S.; Serumondo, J.; Makuza, J.D.; Grant, P.M.; Musabeyezu, E.; Muvunyi, C.; et al. Risk factors for difficult-to-treat hepatitis C virus genotype 4r in Rwanda and implications for elimination in sub-Saharan Africa. J. Viral Hepat. 2021. [Google Scholar] [CrossRef]
- The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance 2019 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Bradshaw, D.; Mbisa, J.L.; Geretti, A.M.; Healy, B.J.; Cooke, G.S.; Foster, G.R.; Thomson, E.C.; Mclauchlan, J.; Agarwal, K.; Sabin, C.; et al. Consensus recommendations for resistance testing in the management of chronic hepatitis C virus infection: Public Health England HCV Resistance Group. J. Infect. 2019, 79, 503–512. [Google Scholar] [CrossRef]
- Di Biagio, A.; Taramasso, L.; Cenderello, G. Treatment of hepatitis C virus genotype 4 in the DAA era. Virol. J. 2018, 15, 180. [Google Scholar] [CrossRef]
| PRIMER | SEQUENCE | Position * |
|---|---|---|
| NS5A-4ckr_F | GVCTCCAYAAGTGGATCAAYGA | 6146–6167 |
| NS5A-4ckr_R | GAACCTGRCAGGGRCACTTGAT | 6598–6619 |
| VHC-NS5A2F | TGGGSTTYKCVTATGAYACCCG | 8174–8195 |
| VHC-NS5A2R | GARTACCTRGTCATAGCCTCCG | 8549–8570 |
| VHC-NS5B2F | GAYACCCGCTGYTTYGACTC | 8188–8207 |
| VHC-NS5B3R | CATAGCCTCCGTGAAGRCTC | 8540–8559 |
| NS3-4ckr_F1 | AGGYTRGGCAATGARATAYTGCT | 3283–3305 |
| NS3-4ckr_R1 | GARGGGTTRAGCACBAGCAC | 4021–4040 |
| NS3-4ckr_F2 | GGAGRCTYCTTGCYCCCAT | 3339–3357 |
| NS3-4ckr_R2 | GGRACYTTGGTGCTYTTGCC | 3973–3992 |
| Vidas® Anti-HCV (n = 107) | Elecsys Anti-HCV II (n = 107) | INNO-LIA HCV (n = 31) | |
|---|---|---|---|
| Sensitivity (n/N) | 8/13 | 13/13 | 13/13 |
| % (95% CI) | 61.5 (31.6–86.1) | 100 (75.3–100) | 100 (75.3–100) |
| Specificity (n/N) | 84/94 | 94/94 | 18/18 |
| % (95% CI) | 89.4 (81.3–94.8) | 100 (96.1–100) | 100 (81.5–100) |
| Positive Predictive Value (n/N) | 8/18 | 13/13 | 13/13 |
| % (95% CI) | 44.4 (21.5–69.2) | 100 (75.3–100) | 100 (75.3–100) |
| Negative Predictive Value (n/N) | 84/89 | 94/94 | 18/18 |
| % (95% CI) | 94.4 (87.4–98.1) | 100 (96.1–100) | 100 (81.5–100) |
| Subtype | Sample ID | NS3 RAS Codons | |||||
|---|---|---|---|---|---|---|---|
| 41 | 56 | 80 | 155 | 156 | 168 | ||
| 4r | CUN-8 | Q | Y | Q | R | A | D |
| 4r | CUN-112 | Q | Y | Q | R | A | D |
| 4r | CUN-319 | Q | Y | Q | R | A | D |
| 4r | CUN-369 | Q | Y | Q | R | A | D |
| 4c | CUN-26 | Q | Y | Q | R | A | D |
| 4c | CUN-66 | Q | Y | Q | R | A | D |
| 4c | CUN-285 | Q | Y | Q | R | A | D |
| 4k | CUN-71 | Q | Y | Q | R | A | D |
| Subtype | Sample ID | NS5A RAS Codons | ||||
|---|---|---|---|---|---|---|
| 28 | 29 | 30 | 31 | 32 | ||
| 4r | CUN-8 | V | P | R | L | P |
| 4r | CUN-112 | I | P | R | L | P |
| 4r | CUN-319 | I | P | R | L | P |
| 4r | CUN-369 | F | P | S | L | P |
| 4c | CUN-26 | L | P | R | M | P |
| 4c | CUN-66 | L | P | R | M | P |
| 4c | CUN-285 | L | P | R | M | P |
| 4k | CUN-71 | L | P | R | L | P |
| Subtype | Sample ID | NS3 Mutations | NS5A Mutations | NS5B Mutations |
|---|---|---|---|---|
| 4r | CUN-8 | R26K, V134T, A181S | V8I, E10D, I28V, V56I, L108F | None |
| 4r | CUN-112 | R26K, M147L, V151A, A181S | L108F | C316H, V321I |
| 4r | CUN-319 | R26K, R130K, I132L, S133A, A181S | T17A, P58S, L108F, Y127F | H267Y, Y276H, C316H, V321I, E327G |
| 4r | CUN-369 | R26K, T95A, V107I, H110Q, A181S | E10D, T17A, I28F, R30S, V56T, N62S, K68R, L108V, R123Q | K270R, E327A |
| 4r | CUN-157 | N/A | N/A | None |
| 4c | CUN-26 | T122N | H6W, R44K, T56R, I74M, T75A, T83M, V121I, I130V | A252V, R309K |
| 4c | CUN-66 | L14F, T122N | H6W, R44K, T56R, T75A, T83M, V121I, I130V | A252V, R309K |
| 4c | CUN-285 | L14F, K92S, F105Y, H110R, M147K | H6R, R44K, T75V, E117D, V121I, I130V | H267Y, K333Q |
| 4k | CUN-71 | G15S, I71V, E95A, R98T, I107V, L127I, S181F | D10E, T17S, R44K, T83I, V101I, D117E, V121I, S127Y | S312T, T324A |
| 4k | CUN-202 | N/A | N/A | A252V, L262K, Y276L, V300T, T303A, K309R, C316N, T324A, E327D, R335A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco, T.; Barquín, D.; Ndarabu, A.; Fernández-Alonso, M.; Rubio-Garrido, M.; Carlos, S.; Makonda, B.; Holguín, Á.; Reina, G. HCV Diagnosis and Sequencing Using Dried Blood Spots from Patients in Kinshasa (DRC): A Tool to Achieve WHO 2030 Targets. Diagnostics 2021, 11, 522. https://doi.org/10.3390/diagnostics11030522
Carrasco T, Barquín D, Ndarabu A, Fernández-Alonso M, Rubio-Garrido M, Carlos S, Makonda B, Holguín Á, Reina G. HCV Diagnosis and Sequencing Using Dried Blood Spots from Patients in Kinshasa (DRC): A Tool to Achieve WHO 2030 Targets. Diagnostics. 2021; 11(3):522. https://doi.org/10.3390/diagnostics11030522
Chicago/Turabian StyleCarrasco, Teresa, David Barquín, Adolphe Ndarabu, Mirian Fernández-Alonso, Marina Rubio-Garrido, Silvia Carlos, Benit Makonda, África Holguín, and Gabriel Reina. 2021. "HCV Diagnosis and Sequencing Using Dried Blood Spots from Patients in Kinshasa (DRC): A Tool to Achieve WHO 2030 Targets" Diagnostics 11, no. 3: 522. https://doi.org/10.3390/diagnostics11030522
APA StyleCarrasco, T., Barquín, D., Ndarabu, A., Fernández-Alonso, M., Rubio-Garrido, M., Carlos, S., Makonda, B., Holguín, Á., & Reina, G. (2021). HCV Diagnosis and Sequencing Using Dried Blood Spots from Patients in Kinshasa (DRC): A Tool to Achieve WHO 2030 Targets. Diagnostics, 11(3), 522. https://doi.org/10.3390/diagnostics11030522








