Advantages of 18F-FDG PET/CT Imaging over Modified Duke Criteria and Clinical Presumption in Patients with Challenging Suspicion of Infective Endocarditis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. 18F-FDG PET/CT: Technical Features and Interpretation Criteria
2.3. Statistical Analysis
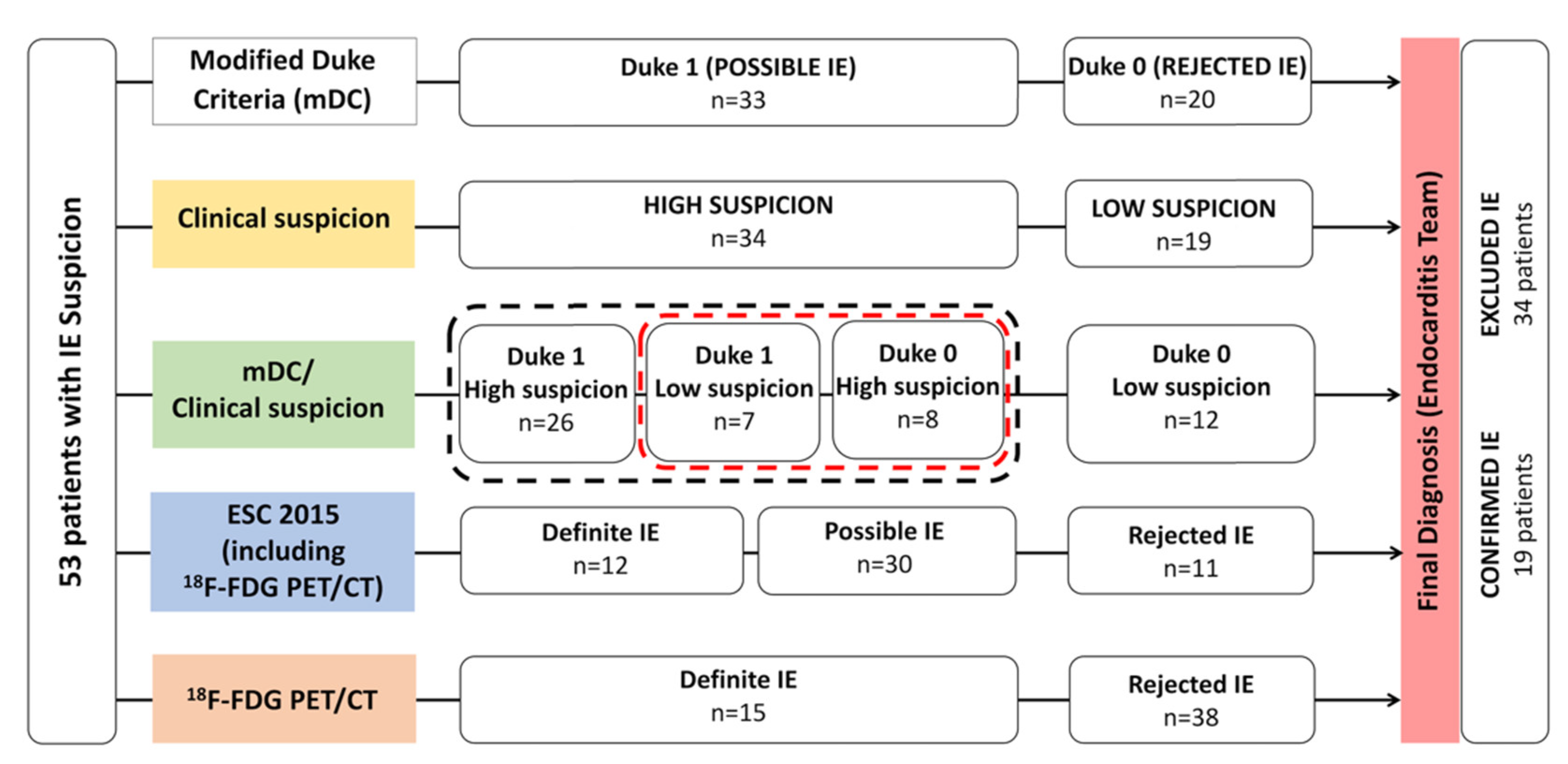
3. Results
3.1. Overall Population
3.2. Modified Duke Criteria (mDC), Clinical Suspicion Degree, and Combined mDC/Clinical Suspicion
3.3. 18F-FDG PET/CT
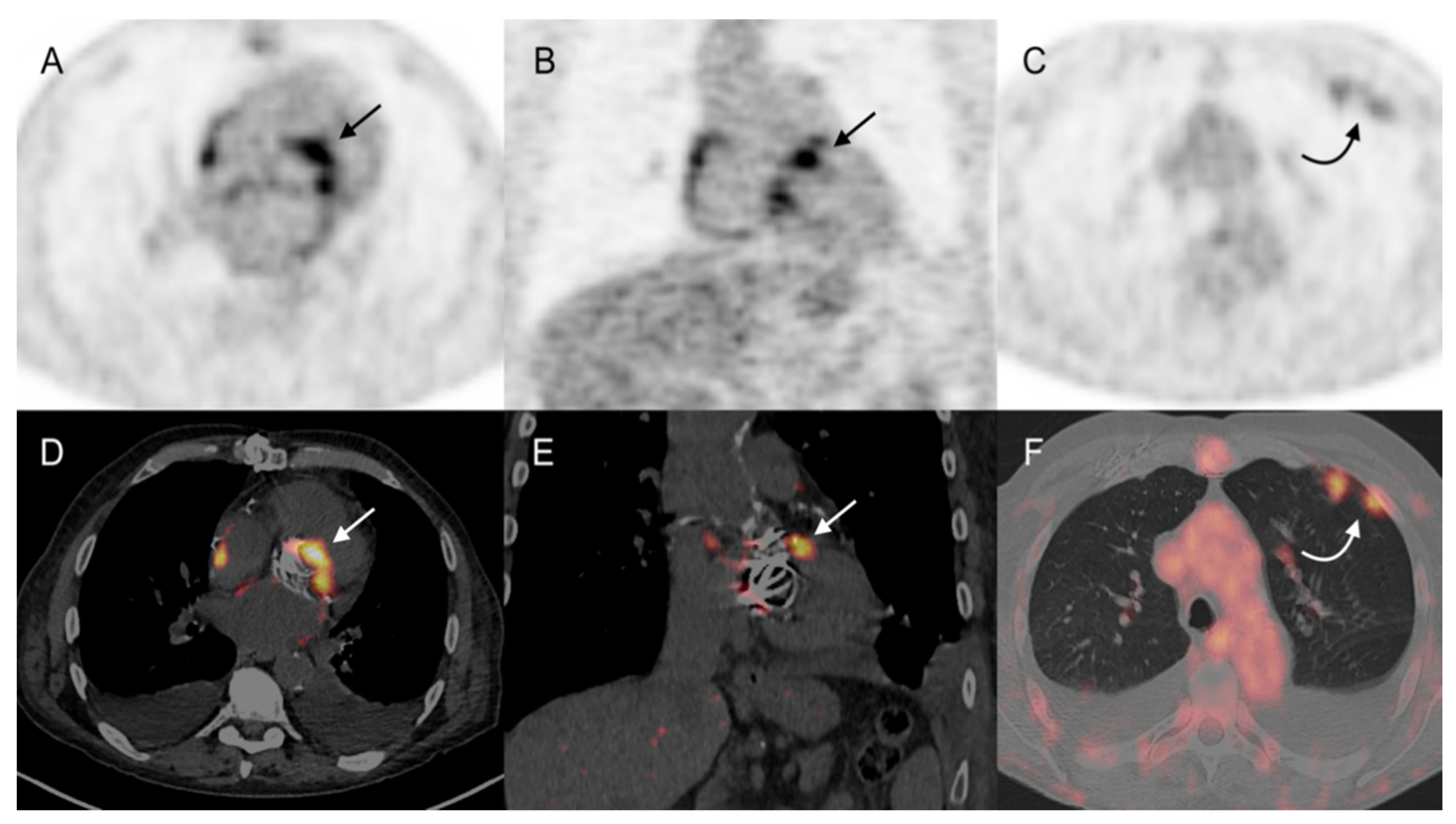
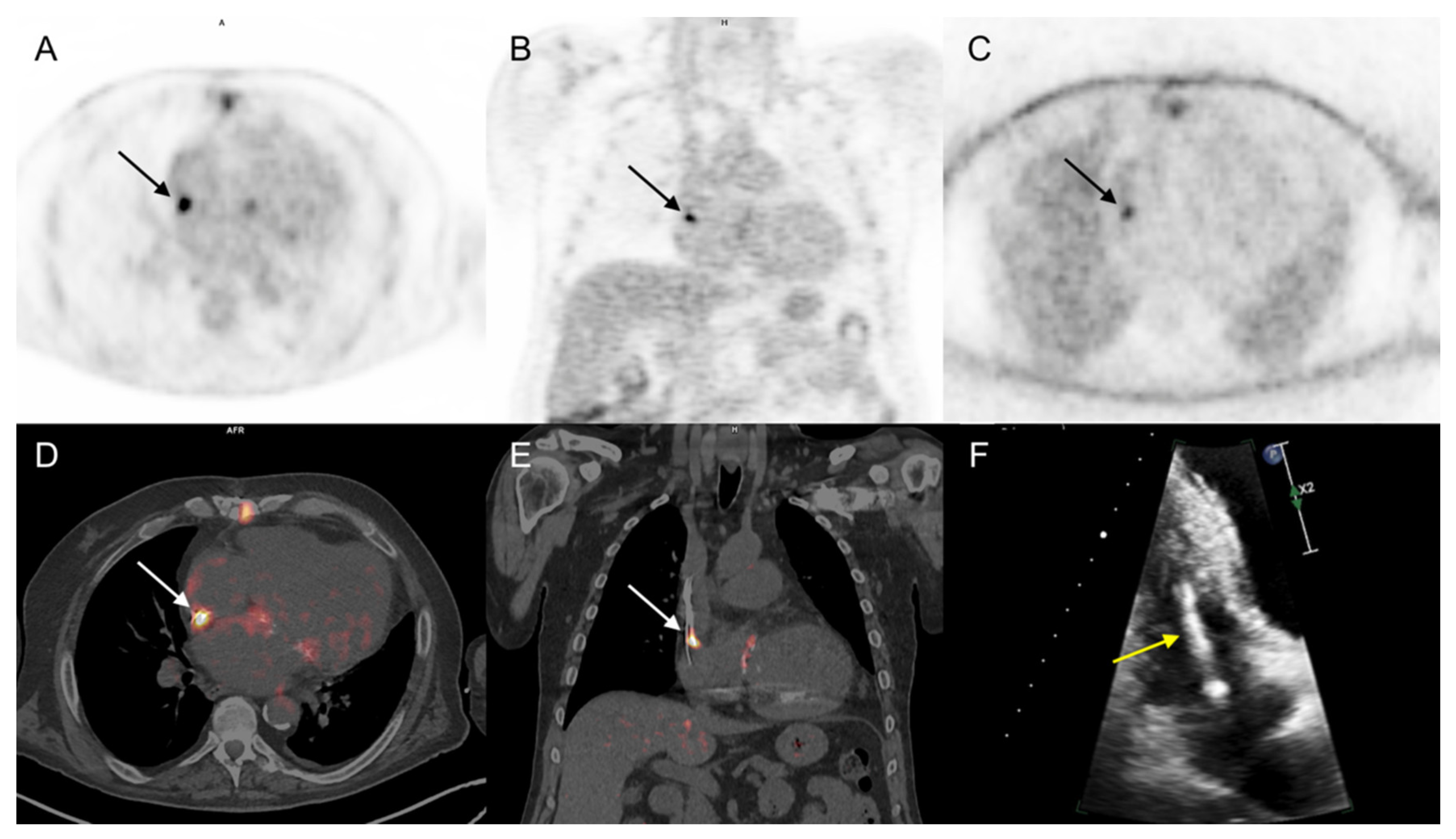
3.3.1. Cardiac Area Investigation
3.3.2. Extra-Cardiac Infection Assessment
3.3.3. Impact on Patient Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thuny, F.; Grisoli, D.; Collart, F.; Habib, G.; Raoult, D. Management of infective endocarditis: Challenges and perspectives. Lancet 2012, 379, 965–975. [Google Scholar] [CrossRef]
- Slipczuk, L.; Codolosa, J.N.; Davila, C.D.; Romero-Corral, A.; Yun, J.; Pressman, G.S.; Figueredo, V.M. Infective Endocarditis Epidemiology Over Five Decades: A Systematic Review. PLoS ONE 2013, 8, e82665. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, J.V.G.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis. The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Vieira, M.L.C.; Grinberg, M.; Pomerantzeff, P.M.A.; Andrade, J.L.; Mansur, A.J. Repeated echocardiographic examinations of patients with suspected infective endocarditis. Heart 2004, 90, 1020–1024. [Google Scholar] [CrossRef]
- Bruun, N.E.; Habib, G.; Thuny, F.; Sogaard, P. Cardiac imaging in infectious endocarditis. Eur. Heart J. 2014, 35, 624–632. [Google Scholar] [CrossRef]
- Rouzet, F.; Chequer, R.; Benali, K.; Lepage, L.; Ghodbane, W.; Duval, X.; Iung, B.; Vahanian, A.; Le Guludec, D.; Hyafil, F. Respective Performance of 18F-FDG PET and Radiolabeled Leukocyte Scintigraphy for the Diagnosis of Prosthetic Valve Endocarditis. J. Nucl. Med. 2014, 55, 1980–1985. [Google Scholar] [CrossRef]
- Sánchez-Enrique, C.; Olmos, C.; Jiménez-Ballvé, A.; Fernández-Pérez, C.; Ferrera, C.; Pérez-Castejón, M.J.; Candil, A.O.; Delgado-Bolton, R.; Carnero, M.; Maroto, L.; et al. Usefulness of 18F Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Infective Endocarditis in Daily Practice. JACC Cardiovasc. Imaging 2018, 11, 1920–1922. [Google Scholar] [CrossRef]
- Chen, W.; Sajadi, M.M.; Dilsizian, V. Merits of FDG PET/CT and Functional Molecular Imaging Over Anatomic Imag-ing With Echocardiography and CT Angiography for the Diagnosis of Cardiac Device Infections. JACC Cardiovasc. Imaging 2018, 11, 1679–1691. [Google Scholar] [CrossRef]
- Duval, X.; Le Moing, V.; Tubiana, S.; Esposito-Farèse, M.; Ilic-Habensus, E.; Leclercq, F.; Bourdon, A.; Goehringer, F.; Selton-Suty, C.; Chevalier, E.; et al. Impact of Systematic Whole-body 18F-Fluorodeoxyglucose PET/CT on the Management of Patients Suspected of Infective Endocarditis: The Prospective Multicenter TEPvENDO Study. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Tessonier, L.; Mancini, J.; Mainardi, J.-L.; Fernandez-Gerlinger, M.-P.; Lussato, D.; Attias, D.; Cammilleri, S.; Weinmann, P.; Hagege, A.; et al. Comparison Between ESC and Duke Criteria for the Diagnosis of Prosthetic Valve Infective Endocarditis. JACC Cardiovasc. Imaging 2020, 13, 2605–2615. [Google Scholar] [CrossRef]
- Mathieu, C.; Mikaïl, N.; Benali, K.; Iung, B.; Duval, X.; Nataf, P.; Jondeau, G.; Hyafil, F.; Le Guludec, D.; Rouzet, F. Characterization of 18F-Fluorodeoxyglucose Uptake Pattern in Noninfected Prosthetic Heart Valves. Circ. Cardiovasc. Imaging. 2017, 10, e005585. [Google Scholar] [PubMed]
- Scholtens, A.M.; Swart, L.E.; Verberne, H.J.; Tanis, W.; Lam, M.G.; Budde, R.P. Confounders in FDG-PET/CT Imaging of Suspected Prosthetic Valve Endocarditis. JACC Cardiovasc. Imaging 2016, 9, 1462–1465. [Google Scholar] [CrossRef]
- Calais, J.; Touati, A.; Grall, N.; Laouénan, C.; Benali, K.; Mahida, B.; Vigne, J.; Hyafil, F.; Iung, B.; Duval, X.; et al. Diagnostic Impact of 18 F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography and White Blood Cell SPECT/Computed Tomography in Patients With Suspected Cardiac Implantable Electronic Device Chronic Infection. Circ. Cardiovasc. Imaging 2019, 12, e007188. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, M.N.; Roque, A.; Fernández-Hidalgo, N.; Cuéllar-Calabria, H.; Ferreira-González, I.; Gonzàlez-Alujas, M.T.; Oristrell, G.; Gracia-Sánchez, L.; González, J.J.; Rodríguez-Palomares, J.; et al. Improving the Diagnosis of Infective Endocarditis in Prosthetic Valves and Intracardiac Devices With 18F-Fluordeoxyglucose Positron Emission Tomography/Computed Tomography Angiography: Initial Results at an Infective Endocarditis Referral Center. Circulation 2015, 132, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Erba, P.A.; Lancellotti, P.; Vilacosta, I.; Gaemperli, O.; Rouzet, F.; Hacker, M.; Signore, A.; Slart, R.H.J.A.; Habib, G. Recommendations on nuclear and multimodality imaging in IE and CIED infections. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1795–1815. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Glaudemans, A.W.J.M.; Touw, D.J.; van Melle, J.P.; Willems, T.P.; Maass, A.H.; Natour, E.; Prakken, N.H.J.; Borra, R.J.H.; van Geel, P.P.; et al. Diagnostic value of imaging in infective endocarditis: A systematic review. Lancet Infect. Dis. 2017, 17, e1–e14. [Google Scholar] [CrossRef]
- Swart, L.E.; Gomes, A.; Scholtens, A.M.; Sinha, B.; Tanis, W.; Lam, M.G.; van der Vlugt, M.J.; Streukens, S.A.F.; Aarntzen, E.H.; Bucerius, J.; et al. Improving the Diagnostic Performance of 18 F-Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography in Prosthetic Heart Valve Endocarditis. Circulation 2018, 138, 1412–1427. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, A.; Van Aarnhem, E.; Budde, R. Effect of antibiotics on FDG-PET/CT imaging of prosthetic heart valve endocarditis. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1223. [Google Scholar] [CrossRef] [PubMed]
- Sag, S.J.M.; Menhart, K.; Grosse, J.; Hitzenbichler, F.; Hanses, F.; Mohr, A.; Salzberger, B.; Zerdzitzki, M.; Hilker, M.; Rupprecht, L.; et al. Diagnostic value of FDG PET/CT imaging in patients with surgically managed infective endocarditis: Results of a retrospective analysis at a tertiary center. J. Nucl. Cardiol. 2020, 22, 1–14. [Google Scholar] [CrossRef]
- Abikhzer, G.; Martineau, P.; Grégoire, J.; Finnerty, V.; Harel, F.; Pelletier-Galarneau, M. [18F]FDG-PET CT for the evaluation of native valve endocarditis. J. Nucl. Cardiol. 2020, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- El-Dalati, S.; Murthy, V.L.; Owczarczyk, A.B.; Fagan, C.; Riddell, J., 4th; Cinti, S.; Weinberg, R.L. Correlating cardiac F-18 FDG PET/CT results with intra-operative findings in infectious endocarditis. J. Nucl Cardiol. 2021, 28, 289–294. [Google Scholar] [CrossRef]
- Chen, W.; Dilsizian, V. FDG PET/CT for the diagnosis and management of infective endocarditis: Expert consensus vs evidence-based practice. J. Nucl. Cardiol. 2018, 26, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Lakkas, L.; Serim, B.D.; Fotopoulos, A.; Iakovou, I.; Doumas, A.; Korkmaz, U.; Michalis, L.K.; Sioka, C. Infection of cardiac prosthetic valves and implantable electronic devices: Early diagnosis and treatment. Acta Cardiol. 2020, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Camargo, R.A.; Sommer Bitencourt, M.; Meneghetti, J.C.; Soares, J., Jr.; Gonçalves, L.F.T.; Buchpiguel, C.A.; Paixão, M.R.; Felicio, M.F.; de Matos Soeiro, A.; Strabelli, T.M.V.; et al. The Role of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in the Diagnosis of Left-sided Endocarditis: Native vs Prosthetic Valves Endocarditis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 70, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Wahadat, A.R.; Tanis, W.; Swart, L.E.; Scholtens, A.; Krestin, G.P.; Van Mieghem, N.M.D.A.; Schurink, C.A.M.; Van Der Spoel, T.I.G.; Brink, F.S.V.D.; Vossenberg, T.; et al. Added value of 18F-FDG-PET/CT and cardiac CTA in suspected transcatheter aortic valve endocarditis. J. Nucl. Cardiol. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Boursier, C.; Duval, X.; Bourdon, A.; Imbert, L.; Mahida, B.; Chevalier, E.; Claudin, M.; Hoen, B.; Goehringer, F.; Selton-Suty, C.; et al. ECG-Gated Cardiac FDG PET Acquisitions Significantly Improve Detectability of Infective Endocarditis. JACC Cardiovasc. Imaging 2020, 13, 2691–2693. [Google Scholar] [CrossRef]
- Kamani, C.H.; Allenbach, G.; Jreige, M.; Pavon, A.G.; Meyer, M.; Testart, N.; Firsova, M.; Vieira, V.F.; Boughdad, S.; LaLonde, M.N.; et al. Diagnostic Performance of 18F-FDG PET/CT in Native Valve Endocarditis: Systematic Review and Bivariate Meta-Analysis. Diagnostics 2020, 10, 754. [Google Scholar] [CrossRef]
- Wahadat, A.R.; Tanis, W.; Scholtens, A.M.; Bekker, M.; Graven, L.H.; Swart, L.E.; den Harder, A.M.; Lam, M.G.; De Heer, L.M.; Roos-Hesselink, J.W.; et al. Normal imaging findings after aortic valve implantation on 18F-Fluorodeoxyglucose positron emission tomography with computed tomography. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2020. [Google Scholar] [CrossRef]
- Caiati, C.; Pollice, P.; Lepera, M.E.; Favale, S. Pacemaker Lead Endocarditis Investigated with Intracardiac Echocardiography: Factors Modulating the Size of Vegetations and Larger Vegetation Embolic Risk during Lead Extraction. Antibiotics 2019, 8, 228. [Google Scholar] [CrossRef]
- Narducci, M.L.; Pelargonio, G.; Russo, E.; Marinaccio, L.; Di Monaco, A.; Perna, F.; Bencardino, G.; Casella, M.; Di Biase, L.; Santangeli, P.; et al. Usefulness of Intracardiac Echocardiography for the Diagnosis of Cardiovascular Implantable Electronic Device–Related Endocarditis. J. Am. Coll. Cardiol. 2013, 61, 1398–1405. [Google Scholar] [CrossRef]
- Roque, A.; Pizzi, M.N.; Fernández-Hidalgo, N.; Permanyer, E.; Cuellar-Calabria, H.; Romero-Farina, G.; Ríos, R.; Almirante, B.; Castell-Conesa, J.; Escobar, M.; et al. Morpho-metabolic post-surgical patterns of non-infected prosthetic heart valves by [18F]FDG PET/CTA: “normality” is a possible diagnosis. Eur. Heart J. Cardiovasc. Imaging 2019, 21, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Holle, S.L.K.; Andersen, M.H.; Klein, C.F.; Bruun, N.E.; Tønder, N.; Haarmark, C.; Loft, A.; Høilund-Carlsen, P.F.; Bundgaard, H.; Iversen, K.K. Clinical usefulness of FDG-PET/CT for identification of abnormal extra-cardiac foci in patients with infective endocarditis. Int. J. Cardiovasc. Imaging 2020, 36, 939–946. [Google Scholar] [CrossRef]
- Orvin, K.; Goldberg, E.; Bernstine, H.; Groshar, D.; Sagie, A.; Kornowski, R.; Bishara, J. The role of FDG-PET/CT imaging in early detection of extra-cardiac complications of infective endocarditis. Clin. Microbiol. Infect. 2015, 21, 69–76. [Google Scholar] [CrossRef]
- Federici, L.; Blondet, C.; Imperiale, A.; Sibilia, J.; Pasquali, J.-L.; Pflumio, F.; Goichot, B.; Blaison, G.; Weber, J.-C.; Christmann, D.; et al. Value of 18F-FDG-PET/CT in patients with fever of unknown origin and unexplained prolonged inflammatory syndrome: A single centre analysis experience. Int. J. Clin. Pract. 2010, 64, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Van Geel, P.P.; Santing, M.; Prakken, N.H.J.; Ruis, M.L.; Van Assen, S.; Slart, R.H.J.A.; Sinha, B.; Glaudemans, A.W.J.M. Imaging infective endocarditis: Adherence to a diagnostic flowchart and direct comparison of imaging techniques. J. Nucl. Cardiol. 2020, 27, 592–608. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, L.; Wang, H.; Cai, H.; Xiang, Y.; Li, L. Effective radiation dose of 18f-fdg pet/ct: How much does diagnostic ct contribute? Radiat. Prot. Dosim. 2019, 187, 183–190. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Lee, W.H.; Li, Y.F.; Hong, W.X.; Hu, S.; Chan, C.; Liang, G.; Nguyen, I.; Ong, S.-G.; Churko, J.; et al. Assessment of the Radiation Effects of Cardiac CT Angiography Using Protein and Genetic Biomarkers. JACC Cardiovasc. Imaging 2015, 8, 873–884. [Google Scholar] [CrossRef]
| Characteristics | Values |
|---|---|
| Age (years), mean ± SD | 65 ± 19 |
| Sex, n (%) | |
| Female Male | 20 (38) 33 (62) |
| CRP (mg/L), mean (range) | 81.9 (4.0–280 ; N<4) |
| White blood cell (G/L), mean (range) | 10.0 (2.8–20.0 ; 4.1<N<10.5) |
| Material, n (%) Prosthetic valve * Biological Aortic Mitral Pulmonary Mechanic Aortic Mitral CEID LVAD Others ** | 43 (81) 22 (42) 15 (28) 11 (21) 5 (10) 2 (4) 9 (17) 4 (8) 6 (11) 24 (45) 4 (8) 4 (8) |
| Causative pathogen, n (%) | |
| Positive blood culture for IE Coagulase Negative Staphylococcus Staphylococcus aureus Streptococcus spp. Enterococcus spp. | 12 (23) 3 (25) 4 (33) 2 (17) 3 (25) |
| Ongoing antibiotic treatment, n (%) | 40 (75) |
| Modified Duke Criteria, n (%) | |
| Duke 0 (Rejected IE) Duke 1 (Possible IE) | 20 (38) 33 (62) |
| Clinical suspicion, n (%) | |
| Low High | 19 (36) 34 (64) |
| IE diagnostic probability, n (%) | |
| Duke 0/Low suspicion Duke 1/High suspicion Duke 1/Low suspicion Duke 0/High suspicion | 12 (23) 26 (49) 7 (13) 8 (15) |
| Extra-cardiac FDG PET/CT infected site, n (%) | 26 (49) |
| Se | Sp | PPV | NPV | Ac | |
|---|---|---|---|---|---|
| Modified Duke Criteria (mDC) | 84% (16/19) | 50% (17/34) | 48% (16/33) | 85% (17/20) | 62% (33/53) |
| Degree of Clinical Suspicion | 95% (18/19) | 53% (18/34) | 53% (18/34) | 95% (18/19) | 68% (36/53) |
| mDC+Clinical Suspicion | 95% (18/19) | 32% (11/34) | 44% (18/41) | 92% (11/12) | 55% (29/53) |
| 18F-FDG PET/CT | 79% (15/19) | 100% (34/34) | 100% (15/15) | 89% (34/38) | 92% (49/53) |
| 18F-FDG PET/CT * | 83% (15/18) | 100% (25/25) | 100% (15/15) | 89% (25/28) | 93% (40/43) |
| Duke Modified Criteria (mDC) | Clinical Suspicion | mDC+Clinical SUSPICION Degree | 18F-FDG PET/CT | |
|---|---|---|---|---|
| Modified Duke Criteria (mDC) | - | ns | ns | p = 0.003 |
| Clinical Suspicion degree | ns | - | ns | p = 0.001 |
| mDC+Clinical Suspicion degree | ns | ns | - | p = 0.001 |
| 18F-FDG PET/CT | p = 0.003 | p = 0.001 | p = 0.001 | - |
| n°, Age, Sex | mDC/Clinical Suspicion | Final Diagnosis | FDG PET | TTE | TEE | mDC Major Microbiological Evidence | mDC Minor Findings |
|---|---|---|---|---|---|---|---|
| 1, 68, F | Rej/High | no IE | - | - | - | - | Predisposition, microbiologic evidence (Pseudomonas aeruginosa) |
| 2, 43, M | Rej/High | no IE | - | - | - | - | vascular, phenomena |
| 3, 78, M | Rej/High | no IE | - | - | - | - | predisposition, fever |
| 4, 48, F | Rej/High | IE | PM lead | - | - | - | Predisposition, fever |
| 5, 50, M | Rej/High | IE | LVAD | - | - | - | Fever, microbiologic evidence (Staphylococcus aureus) |
| 6, 72, M | Rej/High | no IE | - | - | - | - | fever |
| 7, 76, F | Rej/High | no IE | - | - | - | Staphylococcus epidermidis | - |
| 8, 56, M | Rej/High | no IE | - | - | - | Coxiella burnetii IgG antiphase 1 > 1/800 | - |
| 9, 79, F | Poss/Low | no IE | - | - | - | Staphylococcus aureus | fever |
| 10, 82, M | Poss/Low | no IE | - | - | - | Staphylococcus epidermidis | fever |
| 11, 53, M | Poss/Low | no IE | - | Ao veg | Ao veg | - | predisposition |
| 12, 31, F | Poss/Low | no IE | - | - | - | - | Predisposition, fever, microbiologic evidence (Pantoea ananatis) |
| 13, 75, F | Poss/Low | no IE | - | Mit veg | Mit veg | - | fever |
| 14, 81, M | Poss/Low | no IE | - | - | Mit abs | - | fever |
| 15, 67, M | Poss/Low | no IE | - | - | - | Staphylococcus epidermidis | Predisposition, fever |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pretet, V.; Blondet, C.; Ruch, Y.; Martinez, M.; El Ghannudi, S.; Morel, O.; Hansmann, Y.; Schindler, T.H.; Imperiale, A. Advantages of 18F-FDG PET/CT Imaging over Modified Duke Criteria and Clinical Presumption in Patients with Challenging Suspicion of Infective Endocarditis. Diagnostics 2021, 11, 720. https://doi.org/10.3390/diagnostics11040720
Pretet V, Blondet C, Ruch Y, Martinez M, El Ghannudi S, Morel O, Hansmann Y, Schindler TH, Imperiale A. Advantages of 18F-FDG PET/CT Imaging over Modified Duke Criteria and Clinical Presumption in Patients with Challenging Suspicion of Infective Endocarditis. Diagnostics. 2021; 11(4):720. https://doi.org/10.3390/diagnostics11040720
Chicago/Turabian StylePretet, Valentin, Cyrille Blondet, Yvon Ruch, Matias Martinez, Soraya El Ghannudi, Olivier Morel, Yves Hansmann, Thomas H. Schindler, and Alessio Imperiale. 2021. "Advantages of 18F-FDG PET/CT Imaging over Modified Duke Criteria and Clinical Presumption in Patients with Challenging Suspicion of Infective Endocarditis" Diagnostics 11, no. 4: 720. https://doi.org/10.3390/diagnostics11040720
APA StylePretet, V., Blondet, C., Ruch, Y., Martinez, M., El Ghannudi, S., Morel, O., Hansmann, Y., Schindler, T. H., & Imperiale, A. (2021). Advantages of 18F-FDG PET/CT Imaging over Modified Duke Criteria and Clinical Presumption in Patients with Challenging Suspicion of Infective Endocarditis. Diagnostics, 11(4), 720. https://doi.org/10.3390/diagnostics11040720






