Associations between Pre-Bariatric High-Sensitivity C-Reactive Protein and Post-Surgery Outcomes
Abstract
1. Introduction
2. Material and Methods
3. Laboratory Assessment
4. Anthropometric Indices
5. Definition
6. Two-Dimensional Shear Wave Elastography and Ultrasonography
7. Gastric Bypass Surgery
8. Post-Surgery Follow-up
9. Success Rate
10. Statistical Analyses
11. Results
11.1. Demographic Data
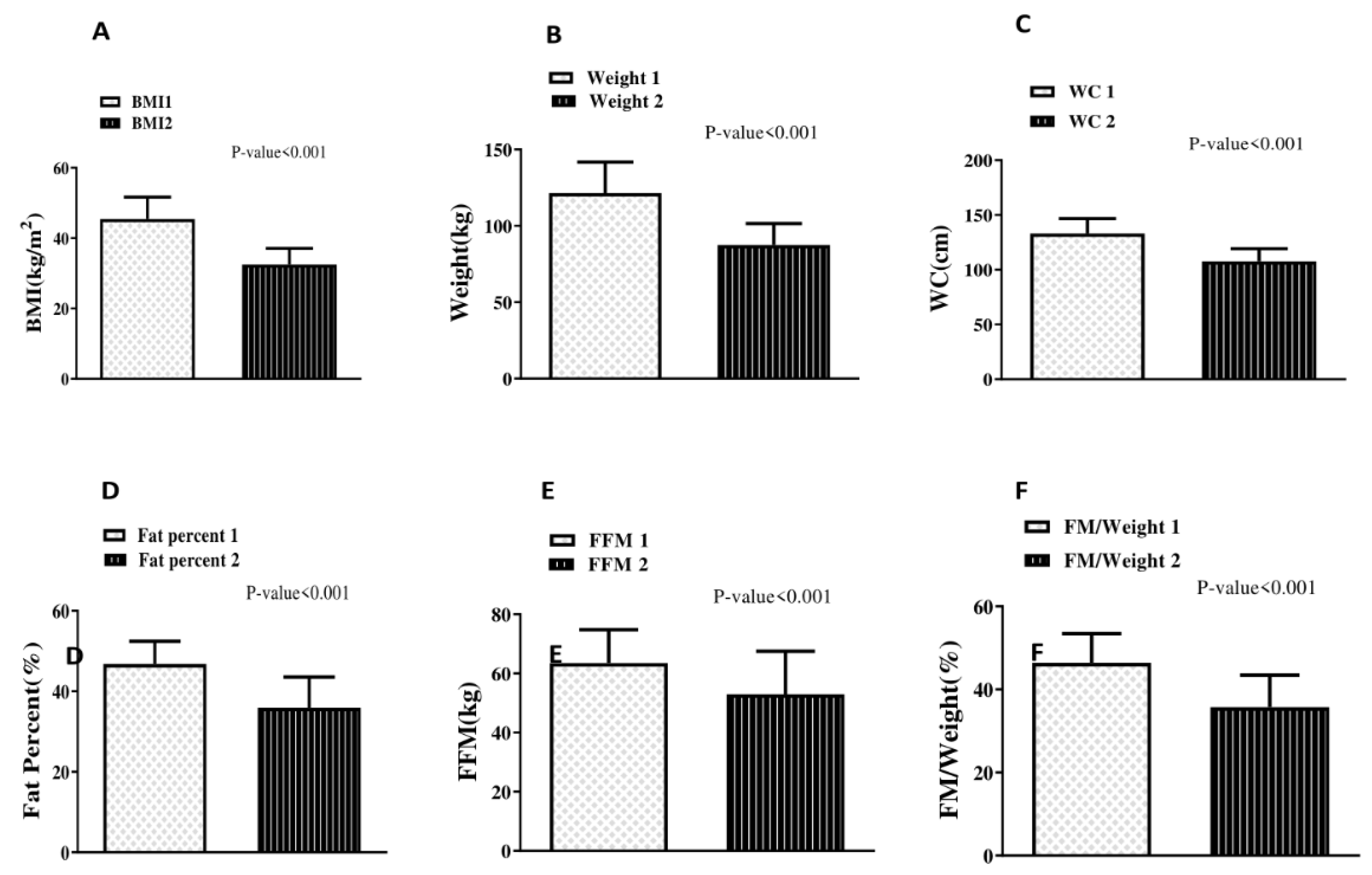
11.2. Anthropometric Indices before and after Surgery
11.3. Liver and Inflammation Status before and after Surgery
11.4. The Relationship between hs-CRP Levels and Liver Status after Bariatric Surgery
11.5. Association between Baseline hs-CRP and Post-Surgery Success Rate and Liver Status at Regression Analyses
12. Discussion
12.1. Hs-CRP Changes after Bariatric Surgery
12.2. Liver Status Changes after Bariatric Surgery
12.3. Hs-CRP and Weight Loss after Bariatric Surgery
12.4. Hs-CRP and Liver Status after Bariatric Surgery
13. Limitations
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wexler, D.; Hu, F.B.; Manson, J.E. Mediating effects of inflammatory biomarkers on insulin resistance associated with obesity. Obes. Res. 2005, 13, 1772–1783. [Google Scholar] [CrossRef]
- Wahba, I.M.; Mak, R.H. Obesity and obesity-initiated metabolic syndrome: Mechanistic links to chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 550–562. [Google Scholar] [CrossRef]
- Lee, Y.H.; Pratley, R.E. The evolving role of inflammation in obesity and the metabolic syndrome. Curr. Diab. Rep. 2005, 5, 70–75. [Google Scholar] [CrossRef]
- Wisse, B.E. The inflammatory syndrome: The role of adipose tissue cytokines in metabolic disorders linked to obesity. J. Am. Soc. Nephrol. 2004, 15, 2792–2800. [Google Scholar] [CrossRef] [PubMed]
- Maachi, M.; Pieroni, L.; Bruckert, E.; Jardel, C.; Fellahi, S.; Hainque, B.; Capeau, J.; Bastard, J.P. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Lau, D.C.; Dhillon, B.; Yan, H.; Szmitko, P.E.; Verma, S. Adipokines: Molecular links between obesity and atheroslcerosis. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2031–H2041. [Google Scholar] [CrossRef]
- Hawkins, M.A. Markers of increased cardiovascular risk: Are we measuring the most appropriate parameters? Obes. Res. 2004, 12 (Suppl. 2), 107s–114s. [Google Scholar] [CrossRef]
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The evidence report. Obes. Res. 1998, 6 (Suppl. 2), 51s–209s.
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L. Obesity and the risk of myocardial infarction in 27000 participants from 52 countries: A case–control study. Lancet 2005, 366, 1640–1649. [Google Scholar] [CrossRef]
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Illan-Gomez, F.; Gonzalvez-Ortega, M.; Orea-Soler, I.; Alcaraz-Tafalla, M.S.; Aragón-Alonso, A.; Pascual-Díaz, M.; Pérez-Paredes, M.; Lozano-Almela, M.L. Obesity and inflammation: Change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes. Surg. 2012, 22, 950–955. [Google Scholar] [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric surgery: A systematic review and meta-analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef]
- Askarpour, M.; Khani, D.; Sheikhi, A.; Ghaedi, E.; Alizadeh, S. Effect of Bariatric Surgery on Serum Inflammatory Factors of Obese Patients: A Systematic Review and Meta-Analysis. Obes. Surg. 2019, 29, 2631–2647. [Google Scholar] [CrossRef] [PubMed]
- Lassailly, G.; Caiazzo, R.; Buob, D.; Pigeyre, M.; Verkindt, H.; Labreuche, J.; Raverdy, V.; Leteurtre, E.; Dharancy, S.; Louvet, A. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology 2015, 149, 379–388. [Google Scholar] [CrossRef]
- Schneck, A.S.; Anty, R.; Patouraux, S.; Bonnafous, S.; Rousseau, D.; Lebeaupin, C.; Bailly-Maitre, B.; Sans, A.; Tran, A.; Gugenheim, J. Roux-en-Y gastric bypass results in long-term remission of hepatocyte apoptosis and hepatic histological features of non-alcoholic steatohepatitis. Front. Physiol. 2016, 7, 344. [Google Scholar] [CrossRef]
- Seki, Y.; Kakizaki, S.; Horiguchi, N.; Hashizume, H.; Tojima, H.; Yamazaki, Y.; Sato, K.; Kusano, M.; Yamada, M.; Kasama, K. Prevalence of nonalcoholic steatohepatitis in Japanese patients with morbid obesity undergoing bariatric surgery. J. Gastroenterol. 2016, 51, 281–289. [Google Scholar] [CrossRef]
- Bona, D.; Micheletto, G.; Bonitta, G.; Panizzo, V.; Cavalli, M.; Rausa, E.; Cirri, S.; Aiolfi, A. Does C-reactive Protein Have a Predictive Role in the Early Diagnosis of Postoperative Complications After Bariatric Surgery? Systematic Review and Bayesian Meta-analysis. Obes. Surg. 2019, 29, 3448–3456. [Google Scholar]
- Agrawal, V.; Krause, K.R.; Chengelis, D.L.; Zalesin, K.C.; Rocher, L.L.; McCullough, P.A. Relation between degree of weight loss after bariatric surgery and reduction in albuminuria and C-reactive protein. Surg. Obes. Relat. Dis. 2009, 5, 20–26. [Google Scholar] [CrossRef]
- Hakeam, H.A.; O’Regan, P.J.; Salem, A.M.; Bamehriz, F.Y.; Jomaa, L.F. Inhibition of C-Reactive Protein in Morbidly Obese Patients After Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2009, 19, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Jouan, Y.; Blasco, H.; Bongrani, A.; Couet, C.; Dupont, J.; Maillot, F. Preoperative Chemerin Level Is Predictive of Inflammatory Status 1 Year After Bariatric Surgery. Obes. Surg. 2020, 30, 3852–3861. [Google Scholar]
- Mallipedhi, A.; Prior, S.; Barry, J.; Caplin, S.; Baxter, J.N.; Stephens, J.W. Changes in inflammatory markers after sleeve gastrectomy in patients with impaired glucose homeostasis and type 2 diabetes. Surg. Obes. Relat. Dis. 2014, 10, 1123–1128. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.J.; Choi, C.Y.; Cho, N.J.; Gil, H.W.; Lee, E.Y. Bariatric Surgery can Reduce Albuminuria in Patients with Severe Obesity and Normal Kidney Function by Reducing Systemic Inflammation. Obes. Surg. 2018, 28, 831–837. [Google Scholar]
- Juiz-Valiña, P.; Pena-Bello, L.; Cordido, M.; Outeiriño-Blanco, E.; Pértega, S.; Varela-Rodriguez, B.; Garcia-Brao, M.J.; Mena, E.; Sangiao-Alvarellos, S.; Cordido, F. Altered GH-IGF-1 Axis in Severe Obese Subjects is Reversed after Bariatric Surgery-Induced Weight Loss and Related with Low-Grade Chronic Inflammation. J. Clin. Med. 2020, 9, 2614. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Ma, B.; Gao, J.Y.; Yin, J.; Qu, S. Insulin-Like Growth Factor 1 Related to Chronic Low-Grade Inflammation in Patients with Obesity and Early Change of its Levels After Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2020, 30, 3326–3332. [Google Scholar]
- Vilarrasa, N.; Vendrell, J.; Sánchez-Santos, R.; Broch, M.; Megia, A.; Masdevall, C.; Gomez, N.; Soler, J.; Pujol, J.; Bettonica, C. Effect of weight loss induced by gastric bypass on proinflammatory interleukin-18, soluble tumour necrosis factor-alpha receptors, C-reactive protein and adiponectin in morbidly obese patients. Clin. Endocrinol. 2007, 67, 679–686. [Google Scholar] [CrossRef]
- Randell, E.W.; Twells, L.K.; Gregory, D.M.; Lester, K.K.; Daneshtalab, N.; Dillon, C.; Pace, D.; Smith, C.; Boone, D. Pre-operative and post-operative changes in CRP and other biomarkers sensitive to inflammatory status in patients with severe obesity undergoing laparoscopic sleeve gastrectomy. Clin. Biochem. 2018, 52, 13–19. [Google Scholar]
- Min, T.; Prior, S.L.; Dunseath, G.; Churm, R.; Barry, J.D.; Stephens, J.W. Temporal Effects of Bariatric Surgery on Adipokines, Inflammation and Oxidative Stress in Subjects with Impaired Glucose Homeostasis at 4 Years of Follow-up. Obes. Surg. 2020, 30, 1712–1718. [Google Scholar]
- Uehara, D.; Seki, Y.; Kakizaki, S.; Horiguchi, N.; Tojima, H.; Yamazaki, Y.; Sato, K.; Yamada, M.; Uraoka, T.; Kasama, K. Long-term Results of Bariatric Surgery for Non-alcoholic Fatty Liver Disease/Non-alcoholic Steatohepatitis Treatment in Morbidly Obese Japanese Patients. Obes. Surg. 2019, 29, 1195–1201. [Google Scholar] [CrossRef]
- Pardina, E.; Ferrer, R.; Baena-Fustegueras, J.A.; Rivero, J.; Lecube, A.; Fort, J.M.; Rivero, J.; Lecube, A.; Fort, J.M.; Vargas, V.; et al. Only C-reactive protein, but not TNF-α or IL6, reflects the improvement in inflammation after bariatric surgery. Obes. Surg. 2012, 22, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, S.; Jamadar, P.; Stier, C.; Bottino, V.; Weiner, R.A.; Runkel, N. The role of C-reactive protein after surgery for obesity and metabolic disorders. Surg. Obes. Relat. Dis. 2020, 16, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; McKechnie, T.; Doumouras, A.G.; Handler, C.; Eskicioglu, C.; Gmora, S.; Anvari, M.; Hong, D. Diagnostic Value of C-Reactive Protein Levels in Postoperative Infectious Complications After Bariatric Surgery: A Systematic Review and Meta-Analysis. Obes. Surg. 2019, 29, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; McMeekin, S.; Wilson, R.J.; Miller, G.V.; Langlands, F.E.; Wong, W.; Peter, M.; Giles, M.S. Predictive value of C-Reactive protein for complications post-laparoscopic roux-en-Y gastric bypass. Obes. Surg. 2017, 27, 709.e15. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.D.; Nicklas, B.J.; Fernandez, A. Serial changes in inflammatory biomarkers after Roux-en-Y gastric bypass surgery. Surg. Obes. Relat. Dis. 2011, 7, 618–624. [Google Scholar] [CrossRef]
- Compher, C.; Badellino, K.O. Obesity and inflammation: Lessons from bariatric surgery. J. Parenter. Enter. Nutr. 2008, 32, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarian, H.; Bhutta, H.Y.; Heshmati, K.; Kunju, S.U.; Sheu, E.G.; Tavakkoli, A. Pre-operative Predictors of Weight Loss and Weight Regain Following Roux-en-Y Gastric Bypass Surgery: A Prospective Human Study. Obes. Surg. 2020. [Google Scholar] [CrossRef]
- Carbone, F.; Nulli Migliola, E.; Bonaventura, A.; Vecchié, A.; De Vuono, S.; Ricci, M.A.; Vaudo, G.; Boni, M.; Dallegri, F.; Montecucco, F. High serum levels of C-reactive protein (CRP) predict beneficial decrease of visceral fat in obese females after sleeve gastrectomy. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 494–500. [Google Scholar] [CrossRef]
- Bonaventura, A.; Liberale, L.; Carbone, F.; Scopinaro, N.; Camerini, G.; Papadia, F.S.; Cordera, R.; Dallegri, F.; Adami, G.F.; Montecucco, F. High baseline C-reactive protein levels predict partial type 2 diabetes mellitus remission after biliopancreatic diversion. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 423–429. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Deitel, M.; Gawdat, K.; Melissas, J. Reporting weight loss. Obes. Surg. 2007, 17, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Galassetti, P. Inflammation and oxidative stress in obesity, metabolic syndrome, and diabetes. Exp. Diabetes Res. 2012, 2012, 943706. [Google Scholar] [CrossRef] [PubMed]
- Yeniova, A.O.; Küçükazman, M.; Ata, N.; Dal, K.; Kefeli, A.; Başyiğit, S.; Dal, K.; Kefeli, A.; Başyiğit, S.; Aktaş, B.; et al. High-sensitivity C-reactive protein is a strong predictor of non-alcoholic fatty liver disease. Hepato-gastroenterology 2014, 61, 422–425. [Google Scholar]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.L.; Rifai, N.; Tracy, R.P.; Roberts, W.L.; Alexander, R.W.; Biasucci, L.M.; Catravas, J.D.; Cole, T.G.; Cooper, G.R.; Khan, B.V. CDC/AHA workshop on markers of inflammation and cardiovascular disease: Application to clinical and public health practice—Report from the laboratory science discussion group. Circulation 2004, 110, e545–e549. [Google Scholar] [CrossRef] [PubMed]
- Bisoendial, R.J.; Kastelein, J.J.; Stroes, E.S. C-reactive protein and atherogenesis: From fatty streak to clinical event. Atherosclerosis 2007, 195, e10-8. [Google Scholar] [CrossRef]
- Mendall, M.A.; Strchan, D.P.; Butland, B.K.; Ballam, L.; Morris, J.; Sweetnam, P.M.; Elwood, P.C. C-reactive protein: Relation to total mortality, cardiovascular mortality and cardiovascular mortality and cardiovascular risk factors in men. Eur. Heart J. 2000, 21, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandris, C.; Lauro, R.; Presta, I.; Sesti, G. C-reactive protein induces phosphorylation of insulin receptor substrate-1 on Ser307 and Ser 612 in L6 myocytes, thereby impairing the insulin signalling pathway that promotes glucose transport. Diabetologia 2007, 50, 840–849. [Google Scholar] [CrossRef]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta 2019, 49, 35–44. [Google Scholar] [CrossRef]
- Kumar, R.; Porwal, Y.C.; Dev, N.; Kumar, P.; Chakravarthy, S.; Kumawat, A. Association of high-sensitivity C-reactive protein (hs-CRP) with non-alcoholic fatty liver disease (NAFLD) in Asian Indians: A cross-sectional study. J. Fam. Med. Prim. Care 2020, 9, 390–394. [Google Scholar]
- Zagorski, S.M.; Papa, N.N.; Chung, M.H. The effect of weight loss after gastric bypass on C-reactive protein levels. Surg. Obes. Relat. Dis. 2005, 1, 81–85. [Google Scholar] [CrossRef]
- Cancello, R.; Clement, K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1141–1147. [Google Scholar] [CrossRef]
- Popović-Dragonjić, L.; Jovanović, M.; Vrbić, M.; Konstantinović, L.J.; Dragonjić, K.V. High sensitivity C-reactive protein as prediction factor of disease progression in patients with chronic hepatitis C and mild liver steatosis. Acta Med. Median. 2010, 49, 14–18. [Google Scholar]
- Bower, G.; Toma, T.; Harling, L.; Jiao, L.R.; Efthimiou, E.; Darzi, A.; Athanasiou, T.; Ashrafian, H. Bariatric Surgery and Non-Alcoholic Fatty Liver Disease: A Systematic Review of Liver Biochemistry and Histology. Obes. Surg. 2015, 25, 2280–2289. [Google Scholar] [CrossRef] [PubMed]
- Nickel, F.; Tapking, C.; Benner, L.; Sollors, J.; Billeter, A.T.; Kenngott, H.G.; Bokhary, L.; Schmid, M.; von Frankenberg, M.; Fischer, L. Bariatric Surgery as an Efficient Treatment for Non-Alcoholic Fatty Liver Disease in a Prospective Study with 1-Year Follow-up. Obes. Surg. 2018, 28, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Mittendorfer, B.; Eagon, J.C.; Patterson, B.; Grant, L.; Feirt, N.; Seki, E.; Brenner, D.; Korenblat, K.; McCrea, J. Gastric Bypass Surgery Improves Metabolic and Hepatic Abnormalities Associated With Nonalcoholic Fatty Liver Disease. Gastroenterology 2006, 130, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lazenby, A.J.; Clements, R.H.; Sollors, J.; Billeter, A.T.; Kenngott, H.G.; Bokhary, L.; Schmid, M.; von Frankenberg, M.; Fischer, L. Resolution of Nonalcoholic Steatohepatits after Gastric Bypass Surgery. Obes. Surg. 2007, 17, 486–492. [Google Scholar] [CrossRef]
- Caiazzo, R.; Lassailly, G.; Leteurtre, E.; Baud, G.; Verkindt, H.; Raverdy, V.; Buob, D.; Pigeyre, M.; Mathurin, P.; Pattou, F. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease. Ann. Surg. 2014, 260, 893–899. [Google Scholar] [CrossRef]
- KFakhry, T.; Mhaskar, R.; Schwitalla, T.; Muradova, E.; Gonzalvo, J.P.; Murr, M.M. Bariatric surgery improves nonalcoholic fatty liver disease: A contemporary systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2019, 15, 502–511. [Google Scholar] [CrossRef]
- Hindle, A.K.; Edwards, C.; McCaffrey, T.; Fu, S.W.; Brody, F. Reactivation of adiponectin expression in obese patients after bariatric surgery. Surg. Endosc. 2010, 24, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, M.; Verhelst, X.; Geerts, A.; Lapauw, B.; Calders, P. Association of recently described adipokines with liver histology in biopsy-proven non-alcoholic fatty liver disease: A systematic review. Obes. Rev. 2016, 17, 68–80. [Google Scholar] [CrossRef]
- Villard, M.A.; Helm, M.C.; Kindel, T.L.; Goldblatt, M.I.; Gould, J.C.; Higgins, R.M. C-Reactive protein as a predictor of post-operative complications in bariatric surgery patients. Surg. Endosc. 2019, 33, 2479–2484. [Google Scholar] [CrossRef] [PubMed]
- Kröll, D.; Nakhostin, D.; Stirnimann, G.; Erdem, S.; Haltmeier, T.; Nett, P.C.; Borbély, Y.M. C-Reactive Protein on Postoperative Day 1: A Predictor of Early Intra-abdominal Infections After Bariatric Surgery. Obes. Surg. 2018, 28, 2760–2766. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, M.; Małczak, P.; Wierdak, M.; Walędziak, M.; Hady, H.R.; Diemieszczyk, I.; Proczko-Stepaniak, M.; Szymański, M.; Dowgiałło-Wnukiewicz, N.; Szeliga, J. Utility of Inflammatory Markers in Detection of Perioperative Morbidity After Laparoscopic Sleeve Gastrectomy, Laparoscopic Roux-en-Y Gastric Bypass, and One-Anastomosis Gastric Bypass-Multicenter Study. Obes. Surg. 2020, 30, 2971–2979. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Hildebrandt, C.; Bruckner, T.; Kenngott, H.; Linke, G.R.; Gehrig, T.; Büchler, M.W.; Müller-Stich, B.P. Excessive Weight Loss after Sleeve Gastrectomy: A Systematic Review. Obes. Surg. 2012, 22, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.; Anty, R.; Tordjman, J.; Verrijken, A.; Gual, P.; Tran, A.; Iannelli, A.; Gugenheim, J.; Bedossa, P.; Francque, S. C-reactive protein levels in relation to various features of non-alcoholic fatty liver disease among obese patients. J. Hepatol. 2011, 55, 660–665. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, B.I.; Yun, J.W.; Kim, J.W.; Park, D.I.; Cho, Y.K.; Sung, I.K.; Park, C.Y.; Sohn, C.I.; Jeon, W.K. Insulin resistance and C-reactive protein as independent risk factors for non-alcoholic fatty liver disease in non-obese Asian men. J. Gastroenterol. Hepatol. 2004, 19, 694698. [Google Scholar] [CrossRef]
- Yoneda, M.; Mawatari, H.; Fujita, K.; Iida, H.; Yonemitsu, K.; Kato, S.; Takahashi, H.; Kirikoshi, H.; Inamori, M.; Nozaki, Y. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J. Gastroenterol. 2007, 42, 573–582. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, K.; Ryu, S.; Chang, Y.; Kim, H.-R. High-normal levels of hs-CRP predict the development of non-alcoholic fatty liver in healthy men. PLoS ONE 2017, 12, e0172666. [Google Scholar] [CrossRef]
- Shaharyar, S.; Roberson, L.L.; Jamal, O.; Younus, A.; Blaha, M.J.; Ali, S.S.; Younus, A.; Blaha, M.; Ali, S.; Zide, K.; et al. Obesity and metabolic phenotypes (metabolically healthy and unhealthy variants) are significantly associated with prevalence of elevated C-reactive protein and hepatic steatosis in a large healthy Brazilian population. J. Obes. 2015, 2015, 178526. [Google Scholar] [CrossRef]
- Hindle, A.; de la Piedad Garcia, X.; Brennan, L. Early post-operative psychosocial and weight predictors of later outcome in bariatric surgery: A systematic literature review. Obes. Rev. 2017, 18, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Hashimoto, S.; Kawabe, N. Measurement of liver stiffness as a non-invasive method for diagnosis of non-alcoholic fatty liver disease. Hepatol. Res. 2015, 45, 142–151. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total |
|---|---|
| Male (%) | 18 (20) |
| Age (years) | 38.5 ± 11.1 |
| BMI (kg/m2) | 45.46 ± 6.26 |
| Weight (kg) | 121.34 ± 20.32 |
| Waist Circumference (cm) | 133.04 ± 13.6 |
| Height (m) | 1.62 ± 8.87 |
| Type 2 diabetes mellitus (%) | 25 (27.8) |
| Arterial hypertension (%) | 23 (25.6) |
| Metabolic syndrome (%) | 46 (51.1) |
| Variable | Before | After | p-Value |
|---|---|---|---|
| Liver stiffness measurement, kPa | 6.10 ± 1.25 | 5.42 ± 1.52 | 0.002 |
| AST (IU/L) | 21 (17.00; 29.00) | 19 (16.00; 25.00) | 0.027 |
| ALT (IU/L) | 25 (17.00; 38.50) | 16.5 (12.00; 25.00) | <0.001 |
| GGT (IU/L) | 27 (20.00; 34.50) | 14 (11.00; 19.00) | <0.001 |
| ALP (IU/L) | 196.25 ± 53.79 | 222.50 ± 65.61 | <0.001 |
| Platelets (number/μL) | 303.43 ± 71.20 | 280.47 ± 66.12 | <0.001 |
| FIB 4 | 0.53 (0.37; 0.73) | 0.66 (0.43; 0.94) | <0.001 |
| NFS | −1.35 (−2.44; −0.39) | −2.40 (−3.16; −1.41) | <0.001 |
| Steatosis (ultrasonography) (%) | <0.001 | ||
| Grade 0 | 5 (5.5) | 16 (18) | |
| Grade 1 | 19 (21.1) | 47 (52.8) | |
| Grade 2 | 53 (58.8) | 24 (27) | |
| Grade 3 | 13 (14.4) | 2 (2.2) | |
| Hs-CRP (mg/L) | 5.05 (2.40; 13.60) | 1.60 (0.80; 4.02) | <0.001 |
| hs-CRP (mg/L) | ||
|---|---|---|
| CC | Rho | p-Value |
| FIB-4 | 0.057 | 0.657 |
| NFS | 0.002 | 0.985 |
| Fibrosis (elastography) | 0.045 | 0.704 |
| Steatosis (ultrasonography) | 0.164 | 0.154 |
| Parameters | p | OR | 95% CI for OR | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Crude Model | FIB4 | 0.914 | −0.001 | −0.013 | 0.012 |
| NFS | 0.967 | 0.001 | −0.041 | 0.043 | |
| Success rate | 0.829 | 0.994 | 0.940 | 1.050 | |
| Fibrosis (Elastography) | 0.688 | 1.014 | 0.947 | 1.085 | |
| Steatosis (Sonography) | 0.077 | 1.05 | 0.99 | 1.11 | |
| Adjusted Model | FIB4 * | 0.866 | −0.018 | −0.237 | 0.200 |
| NFS ** | 0.442 | 0.254 | −0.403 | 0.912 | |
| Success rate * | 0.479 | 1.466 | 0.508 | 4.226 | |
| Fibrosis * (Elastography) | 0.389 | −0.382 | −1.264 | 0.499 | |
| Steatosis * (Sonography) | 0.674 | 0.206 | 0.753 | 1.165 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamialahmadi, T.; Nematy, M.; Bo, S.; Ponzo, V.; Jangjoo, A.; Goshayeshi, L.; Tasbandi, A.; Nikiforov, N.G.; Sahebkar, A. Associations between Pre-Bariatric High-Sensitivity C-Reactive Protein and Post-Surgery Outcomes. Diagnostics 2021, 11, 721. https://doi.org/10.3390/diagnostics11040721
Jamialahmadi T, Nematy M, Bo S, Ponzo V, Jangjoo A, Goshayeshi L, Tasbandi A, Nikiforov NG, Sahebkar A. Associations between Pre-Bariatric High-Sensitivity C-Reactive Protein and Post-Surgery Outcomes. Diagnostics. 2021; 11(4):721. https://doi.org/10.3390/diagnostics11040721
Chicago/Turabian StyleJamialahmadi, Tannaz, Mohsen Nematy, Simona Bo, Valentina Ponzo, Ali Jangjoo, Ladan Goshayeshi, Aida Tasbandi, Nikita G. Nikiforov, and Amirhossein Sahebkar. 2021. "Associations between Pre-Bariatric High-Sensitivity C-Reactive Protein and Post-Surgery Outcomes" Diagnostics 11, no. 4: 721. https://doi.org/10.3390/diagnostics11040721
APA StyleJamialahmadi, T., Nematy, M., Bo, S., Ponzo, V., Jangjoo, A., Goshayeshi, L., Tasbandi, A., Nikiforov, N. G., & Sahebkar, A. (2021). Associations between Pre-Bariatric High-Sensitivity C-Reactive Protein and Post-Surgery Outcomes. Diagnostics, 11(4), 721. https://doi.org/10.3390/diagnostics11040721








