Effects of Transcranial Direct Current Stimulation Combined with Physiotherapy on Gait Pattern, Balance, and Functionality in Stroke Patients. A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinic Question
2.2. Search Strategy and Database
2.3. Eligibility Criteria
2.4. Study Selection and Data Extraction
2.5. Methodological Quality Assessment and Risk of Bias
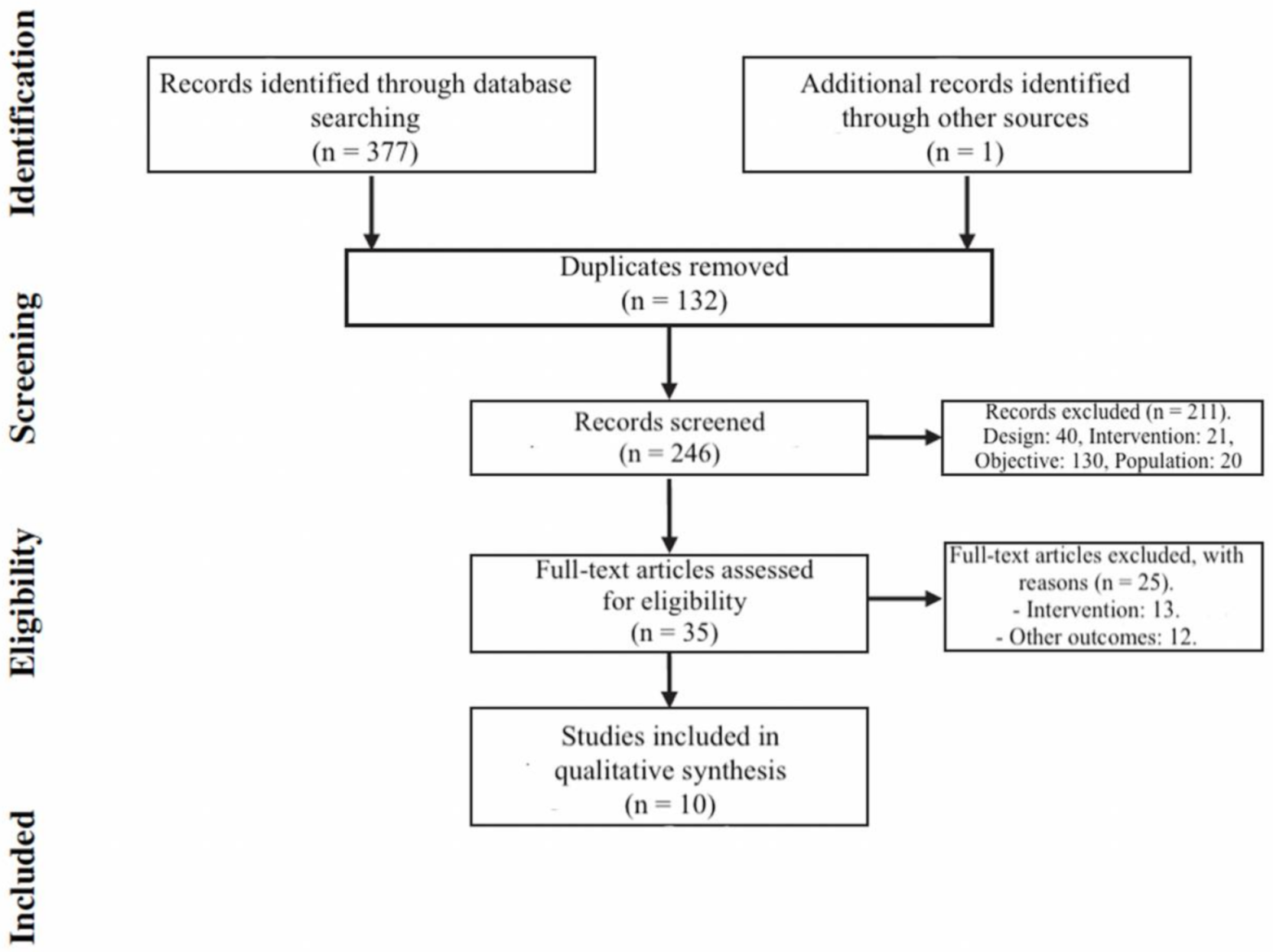
3. Results
3.1. Study Selection
3.2. Study Characteristics and Result Synthesis
3.3. Quality Assessment
3.4. Risk of Bias
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Danzl MM, Chelette KC, Lee K, Lykins D, Sawaki L. Brain stimulation paired with novel locomotor training with robotic gait orthosis in chronic stroke: a feasibility study. NeuroRehabilitation, 2013;33(1):67-76. Article excluded because it uses robotic therapy
- Geroin C, Picelli A, Munari D, Waldner A, Tomelleri C, Smania N. Combined transcranial direct current stimulation and robot-assisted gait training in patients with chronic stroke: a preliminary comparison. Clin Rehabi, 25(6):537–48. Article excluded because it uses robotic therapy
- Leon D, Cortes M, Elder J, Kumru H, Laxe S, Edwards DJ, et al. tDCS does not enhance the effects of robot-assisted gait training in patients with subacute stroke. Restor Neurol Neurosci, 2017;35(4):377–84. Article excluded because it uses robotic therapy
- Seo HG, Lee WH, Lee SH, Yi Y, Kim KD, Oh B-M. Robotic-assisted gait training combined with transcranial direct current stimulation in chronic stroke patients: A pilot double-blind, randomized controlled trial. Restor Neurol Neurosci, 2017;35(5):527–36. Article excluded because it uses robotic therapy
- Picelli A, Chemello E, Castellazzi P, Roncari L, Waldner A, Saltuari L, et al. Combined effects of transcranial direct current stimulation (tDCS) and transcutaneous spinal direct current stimulation (tsDCS) on robot-assisted gait training in patients with chronic stroke: A pilot, double blind, randomized controlled trial. Restor Neurol Neurosci, 2015;33(3):357–68. Article excluded because it uses other stimulations (spinal direct current stimulation)
- Picelli A, Brugnera A, Filippetti M, Mattiuz N, Chemello E, Modenese A, et al. Effects of two different protocols of cerebellar transcranial direct current stimulation combined with transcutaneous spinal direct current stimulation on robot-assisted gait training in patients with chronic supratentorial stroke: A single blind, randomized controlled trial. Restor Neurol Neurosci. 2019;37(2):97–107. Article excluded because it uses other stimulations (cerebellar transcranial direct current stimulation)
- Picelli A, Chemello E, Castellazzi P, Filippetti M, Brugnera A, Gandolfi M, et al. Combined effects of cerebellar transcranial direct current stimulation and transcutaneous spinal direct current stimulation on robot-assisted gait training in patients with chronic brain stroke: A pilot, single blind, randomized controlled trial. Restor Neurol Neurosci, 2018;36(2):161–71. Article excluded because it uses other stimulations (cerebellar transcranial direct current stimulation)
- Ang KK, Guan C, Phua KS, Wang C, Zhao L, Teo WP, et al. Facilitating effects of transcranial direct current stimulation on motor imagery brain-computer interface with robotic feedback for stroke rehabilitation. Arch Phys Med Rehabil, 2015;96(3):S79–87. Article excluded because it uses robotic therapy
- Cho HS, Cha HG. Effect of mirror therapy with tDCS on functional recovery of the upper extremity of stroke patients. Journal of physical therapy science, 2015;27(4), 1045-47. Article excluded because it assesses the upper limb
- Zheng X, Schlaug G. Structural white matter changes in descending motor tracts correlate with improvements in motor impairment after undergoing a treatment course of tDCS and physical therapy. Frontiers in human neuroscience, 2015;9, 229. Article excluded because it assesses the upper limb
- Allman C, Amadi U, Winkler AM, Wilkins L, Filippini N, et al. Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Science translational medicine, 2016;8(330),330re1-330re1. Article excluded because it assesses the upper limb
- Rocha S, Silva E, Foerster Á, Wiesiolek C, Chagas AP, et al. The impact of transcranial direct current stimulation (tDCS) combined with modified constraint-induced movement therapy (mCIMT) on upper limb function in chronic stroke: a double-blind randomized controlled trial. Disability and rehabilitation, 2016;38(7), 653-60. Article excluded because it assesses the modified constraint-induced movement therapy
- Cha HK, Ji SG, Kim MK, Chang JS. Effect of transcranial direct current stimulation of function in patients with stroke. Journal of physical therapy science, 2014;26(3), 363-65. Article excluded because it assesses the upper limb
- Tedesco Triccas L, Burridge JH, Hughes AM, Meadmore KL, Donovan-Hall M, Rothwell JC, et al. A qualitative study exploring views and experiences of people with stroke undergoing transcranial direct current stimulation and upper limb robot therapy. Top Stroke Rehabil, 2018;20,1–9. Article excluded because it uses a qualitative design
- Sattler V, Acket B, Raposo N, Albucher JF, Thalamas C, et al. Anodal tDCS combined with radial nerve stimulation promotes hand motor recovery in the acute phase after ischemic stroke. Neurorehabilitation and neural repair, 2015;29(8), 743-54. Article excluded because it uses other stimulations (radial nerve stimulation)
- Ilić NV, Dubljanin-Raspopović E, Nedeljković U, Tomanović-Vujadinović S, Milanović SD, et al. Effects of anodal tDCS and occupational therapy on fine motor skill deficits in patients with chronic stroke. Restorative neurology and neuroscience, 2016;34(6), 935-945. Article excluded because it assesses the upper limb
- Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabilitation and neural repair, 2011;25(9), 819-29. Article excluded because it assesses the modified constraint-induced movement therapy
- Flöel A. tDCS-enhanced motor and cognitive function in neurological diseases. Neuroimage, 2014;85, 934-47. Article excluded because it is a systematic review
- Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Archives of neurology, 2008;65(12), 1571-1576. Article excluded because it is a systematic review
- Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology, 2017;128(1),56-92. Article excluded because it is a systematic review
- Ambrosini E, Ferrante S, Ferrigno G, Molteni F, Pedrocchi A. Cycling induced by electrical stimulation improves muscle activation and symmetry during pedaling in hemiparetic patients. IEEE Trans neural Syst Rehabil Eng a Publ IEEE Eng Med Biol Soc. 2012;20(3):320–30. Article excluded because it uses other stimulations
- Yotnuengnit P, Bhidayasiri R, Donkhan R, Chaluaysrimuang J, Piravej K. Effects of transcranial direct current stimulation plus physical therapy on gait in patients with Parkinson disease: a randomized controlled trial. American journal of physical medicine & rehabilitation, 2018;97(1), 7-15. Article excluded because it included participants with Parkinson disease
- Grecco LA, Duarte NA, Zanon N, Galli M, Fregni F, Oliveira CS. Effect of a single session of transcranial direct-current stimulation on balance and spatiotemporal gait variables in children with cerebral palsy: a randomized sham-controlled study. Brazilian journal of physical therapy, 2014;18(5), 419-27. Article excluded because it included participants with cerebral palsy
- Kumru H, Murillo N, Benito-Penalva J, Tormos JM, Vidal J. Transcranial direct current stimulation is not effective in the motor strength and gait recovery following motor incomplete spinal cord injury during Lokomat® gait training. Neuroscience letters, 2016;620,143-47. Article excluded because it included participants with Spinal Cord Injury
- Kaski D, Dominguez RO, Allum JH, Islam AF, Bronstein AM. Combining physical training with transcranial direct current stimulation to improve gait in Parkinson’s disease: a pilot randomized controlled study. Clinical rehabilitation, 2014;28(11),1115-24. Article excluded because it included participants with Parkinson disease
References
- Woolley, S.M. Characteristics of Gait in Hemiplegia. Top. Stroke Rehabil. 2001, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Veerbeek, J.M.; Wegen, E.E.H.; Wel, B.C.; Kwakkel, G. Is Accurate Prediction of Gait in Nonambulatory Stroke Patients Possible Within 72 Hours Poststroke? Neurorehabil. Neural. Repair 2010, 25, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.C.; Rao, N.; Muthukrishnan, S.; Aruin, A.S. A textured insole improves gait symmetry in individuals with stroke. Disabil. Rehabil. 2017, 40, 2798–2802. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef]
- Prakash, C.; Kumar, R.; Mittal, N. Recent developments in human gait research: Parameters, approaches, applications, machine learning techniques, datasets and challenges. Artif. Intell. Rev. 2016, 49, 1–40. [Google Scholar] [CrossRef]
- Dobkin, B.H. Clinical practice. Rehabilitation after Stroke. N. Engl. J. Med. 2005, 352, 1677–1684. [Google Scholar] [CrossRef]
- Khanittanuphong, P.; Tipchatyotin, S. Correlation of the gait speed with the quality of life and the quality of life classified according to speed-based community ambulation in Thai stroke survivors. Neurorehabilition 2017, 41, 135–141. [Google Scholar] [CrossRef]
- Park, J.; Kim, T.H. The effects of balance and gait function on quality of life of stroke patients. Neurorehabilition 2019, 44, 37–41. [Google Scholar] [CrossRef]
- Grau-Pellicer, M.; Chamarro-Lusar, A.; Medina-Casanovas, J.; Serdà Ferrer, B.C. Walking speed as a predictor of community mobility and quality of life after stroke. Top. Stroke Rehabil. 2019, 26, 349–358. [Google Scholar] [CrossRef]
- Pollock, A.; Baer, G.; Campbell, P.; Choo, P.L.; Forster, A.; Morris, J.; Pomeroy, V.M.; Langhorne, P. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst. Rev. 2014, 4, CD001920. [Google Scholar] [CrossRef]
- Zeiler, S.R.; Krakauer, J.W. The interaction between training and plasticity in the poststroke brain. Curr. Opin. Neurol. 2013, 26, 609–616. [Google Scholar] [CrossRef]
- Schröder, J.; Truijen, S.; Van Criekinge, T.; Saeys, W. Feasibility and effectiveness of repetitive gait training early after stroke: A systematic review and meta-analysis. J. Rehabil. Med. 2019, 51, 78–88. [Google Scholar] [CrossRef]
- Hordacre, B.; Moezzi, B.; Ridding, M.C. Neuroplasticity and network connectivity of the motor cortex following stroke: A transcranial direct current stimulation study. Hum. Brain Map. 2018, 39, 3326–3339. [Google Scholar] [CrossRef]
- Murase, N.; Duque, J.; Mazzocchio, R.; Cohen, L.G. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. 2004, 55, 400–409. [Google Scholar] [CrossRef]
- Liew, S.L.; Santarnecchi, E.; Buch, E.R.; Cohen, L.G. Non-invasive brain stimulation in neurorehabilitation: Local and distant effects for motor recovery. Front. Hum. Neurosci. 2014, 8, 378. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Marquez, J.; Vliet, P.V.; Mcelduff, P.; Lagopoulos, J.; Parsons, M. Transcranial Direct Current Stimulation (tDCS): Does it Have Merit in Stroke Rehabilitation? A Systematic Review. Int. J. Stroke 2013, 10, 306–316. [Google Scholar] [CrossRef]
- Kang, N.; Summers, J.J.; Cauraugh, J.H. Transcranial direct current stimulation facilitates motor learning post-stroke: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2015, 87, 345–355. [Google Scholar] [CrossRef]
- Madhavan, S.; Shah, B. Enhancing Motor Skill Learning with Transcranial Direct Current Stimulation-A Concise Review with Applications to Stroke. Front. Psychiatry 2012, 3, 66. [Google Scholar] [CrossRef]
- Jeffery, D.T.; Norton, J.A.; Roy, F.D.; Gorassin, M.A. Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Exp. Brain Res. 2007, 82, 281–287. [Google Scholar] [CrossRef]
- Vaz, P.G.; Salazar, A.P.D.S.; Stein, C.; Marchese, R.R.; Lukrafka, J.L.; Plentz, R.D.M.; Pagnussat, A.S. Noninvasive brain stimulation combined with other therapies improves gait speed after stroke: A systematic review and meta-analysis. Top. Stroke Rehabil. 2019, 26, 201–213. [Google Scholar] [CrossRef]
- Fleming, M.K.; Pavlou, M.; Newham, D.J.; Sztriha, L.; Teo, J.T. Non-invasive brain stimulation for the lower limb after stroke: What do we know so far and what should we be doing next? Disabil. Rehabil. 2017, 39, 714–720. [Google Scholar] [CrossRef]
- Tien, H.H.; Liu, W.Y.; Chen, Y.L.; Wu, Y.C.; Lien, H.Y. Transcranial direct current stimulation for improving ambulation after stroke: A systematic review and meta-analysis. Int. J. Rehabil. Res. 2020, 43, 299. [Google Scholar] [CrossRef]
- Kang, N.; Da Lee, R.; Lee, J.H.; Hwang, M.H. Functional Balance and Postural Control Improvements in Patients With Stroke After Noninvasive Brain Stimulation: A Meta-analysis. Arch. Phys. Med. Rehabil. 2020, 101, 141–153. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- González de Dios, J.; Buñuel Álvarez, J.C.; Aparicio Rodrigo, M. Listas guía de comprobación de revisiones sistemáticas y metaanálisis: Declaración PRISMA. Evid. Pediatr. 2011, 7, 97. [Google Scholar]
- Andrade, S.M.; Ferreira, J.J.D.A.; Rufino, T.S.; Medeiros, G.; Brito, J.D.; da Silva, M.A.; Moreira, R.D.N. Effects of different montages of transcranial direct current stimulation on the risk of falls and lower limb function after stroke. Neurol. Res. 2017, 39, 1037–1043. [Google Scholar] [CrossRef]
- Chang, M.C.; Kim, D.Y.; Park, D.H. Enhancement of Cortical Excitability and Lower Limb Motor Function in Patients With Stroke by Transcranial Direct Current Stimulation. Brain Stimul. 2015, 8, 561–566. [Google Scholar] [CrossRef]
- Fusco, A.; Assenza, F.; Iosa, M.; Izzo, S.; Altavilla, R.; Paolucci, S.; Vernieri, F. The ineffective role of cathodal tDCS in enhancing the functional motor outcomes in early phase of stroke rehabilitation: An experimental trial. Biomed Res. Int. 2014, 2014, 547290. [Google Scholar] [CrossRef]
- Klomjai, W.; Aneksan, B.; Pheungphrarattanatrai, A.; Chantanachai, T.; Choowong, N.; Bunleukhet, S.; Auvichayapat, P.; Nilanon, Y.; Hiengkaew, V. Effect of single-session dual-tDCS before physical therapy on lower-limb performance in sub-acute stroke patients: A randomized sham-controlled crossover study. Ann. Phys. Rehabil. Med. 2018, 61, 286–291. [Google Scholar] [CrossRef]
- Ojardias, E.; Azé, O.D.; Luneau, D.; Mednieks, J.; Condemine, A.; Rimaud, D.; Chassagne, P.; Giraux, P. The Effects of Anodal Transcranial Direct Current Stimulation on the Walking Performance of Chronic Hemiplegic Patients. Neuromodulation 2019, 23, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Park, S.D.; Kim, J.Y.; Song, H.S. Effect of application of transcranial direct current stimulation during task-related training on gait ability of patients with stroke. J. Phys. Ther. Sci. 2015, 27, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Saeys, W.; Vereeck, L.; Lafosse, C.; Truijen, S.; Wuyts, F.L.; Van De Heyning, P. Transcranial direct current stimulation in the recovery of postural control after stroke: A pilot study. Disabil. Rehabil. 2015, 37, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.K.; Jee, S.J.; Kim, Y.W. Effect of transcranial direct current stimulation on postural stability and lower extremity strength in hemiplegic stroke patients. Ann. Rehabil. Med. 2013, 37, 759–765. [Google Scholar] [CrossRef]
- Tahtis, V.; Kaski, D.; Seemungal, B.M. The effect of single session bi-cephalic transcranial direct current stimulation on gait performance in sub-acute stroke: A pilot study. Restor. Neurol. Neurosci. 2014, 32, 527–532. [Google Scholar] [CrossRef]
- Utarapichat, S.; Kitisomprayoonkul, W. Effects of Transcranial Direct Current Stimulation on Motor Activity of Lower Limb Muscles in Chronic Stroke. J. Med. Assoc. Thai. 2018, 101, 131–136. [Google Scholar]
- Madhavan, S.; Weber, K.A., II; Stinear, J.W. Non-invasive brain stimulation enhances fine motor control of the hemiparetic ankle: Implications for rehabilitation. Exp. Brain Res. 2011, 209, 9–17. [Google Scholar] [CrossRef]
- Kaski, D.; Dominguez, R.O.; Allum, J.H.; Bronstein, A.M. Improving Gait and Balance in Patients With Leukoaraiosis Using Transcranial Direct Current Stimulation and Physical Training: An Exploratory Study. Neurorehabil. Neural. Repair 2013, 27, 864–871. [Google Scholar] [CrossRef]
- Danzl, M.M.; Chelette, K.C.; Lee, K.; Lykins, D.; Sawaki, L. Brain stimulation paired with novel locomotor training with robotic gait orthosis in chronic stroke: A feasibility study. NeuroRehabilitation 2013, 33, 67–76. [Google Scholar] [CrossRef]
- Geroin, C.; Picelli, A.; Munari, D.; Waldner, A.; Tomelleri, C.; Smania, N. Combined transcranial direct current stimulation and robot-assisted gait training in patients with chronic stroke: A preliminary comparison. Clin. Rehabil. 2011, 25, 537–548. [Google Scholar] [CrossRef]
- Seo, H.G.; Lee, W.H.; Lee, S.H.; Yi, Y.; Kim, K.D.; Oh, B.M. Robotic-assisted gait training combined with transcranial direct current stimulation in chronic stroke patients: A pilot double-blind, randomized controlled trial. Restor. Neurol. Neurosci. 2017, 35, 527–536. [Google Scholar] [CrossRef]
- Boggio, P.S.; Nunes, A.; Rigonatti, S.P.; Nitsche, M.A.; Pascual-Leone, A.; Fregni, F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor. Neurol. Neurosci. 2007, 25, 123–129. [Google Scholar]
- Khedr, E.M.; Shawky, O.A.; El-Hammady, D.H.; Rothwell, J.C.; Darwish, E.S.; Mostafa, O.M.; Tohamy, A.M. Effect of anodal versus cathodal transcranial direct current stimulation on stroke rehabilitation: A pilot randomized controlled trial. Neurorehabil. Neural. Repair 2013, 27, 592–601. [Google Scholar] [CrossRef]
- Leon, D.; Cortes, M.; Elder, J.; Kumru, H.; Laxe, S.; Edwards, D.J.; Tormos, J.M.; Bernabeu, M.; Pascualleone, A. tDCS does not enhance the effects of robot-assisted gait training in patients with subacute stroke. Restor. Neurol. Neurosci. 2017, 35, 377–384. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Dileone, M.; Capone, F.; Pellegrino, G.; Ranieri, F.; Musumeci, G.; Florio, L.; Di Pino, G.; Fregni, F. Immediate and late modulation of interhemipheric imbalance with bilateral transcranial direct current stimulation in acute stroke. Brain Stimul. 2014, 7, 841–848. [Google Scholar] [CrossRef]

| N° | Terms |
|---|---|
| 1 | Stroke OR cerebrovascular accident OR CVA 1. |
| 2 | Transcranial direct current stimulation OR tDCS 2. |
| 3 | Gait OR gait disorders, neurologic OR gait training OR neurological gait OR gait parameters OR balance OR stability OR postural balance OR postural stability OR kinematic OR kinetic OR gait analysis |
| 4 | Physiotherapy OR physical therapy OR rehabilitation OR exercise OR intervention |
| 5 | 1 AND 2 AND 3 AND 3 |
| Trial | N | Assessment Tool | Intervention | Protocol | Results |
|---|---|---|---|---|---|
| Andrade et al., 2011 | 60, Acute | RF, BBS, 6MWT | tDCS + PT.
| 10 sessions, 2 weeks. Stimulation time not indicated. Multimodal tDCS, 2 mA, 35 cm2 electrodes. Anode: over the ipsilateral hemisphere to the stroke. Cathode: over the contralateral hemisphere to the stroke. | Baseline vs. post-treatment and 1 and 3 months follow-up. RF improved compared to sham (p < 0.05) after treatment and 3 months follow-up. No differences between real groups. BBS and 6MWT, significantly improved in real than in sham groups. Group B: more improvements in BBS (p = 0.001–p = 0.007), and in 6MWT (no significant) than A–C, respectively. Group A–C showed similar performance in all test (p > 0.005) |
| Chang et al., 2015 | 24, Acute | FAC, FMA-LE, BBS, TSGP | tDCS + PT.
| 10 sessions, 2 weeks. Anodal tDCS, 10 min, 2 mA, A: 7.07 cm2, C: 28.2 cm2. Anode over the tibialis anterior area of the ipsilateral hemisphere to the stroke. | Baseline vs. post-treatment. All motor functions improved significantly. FMA-LE improved significantly (p = 0.023) in tDCS group. No significant differences between groups in FAC (p = 0.077), BBS (0.759), and gait analysis. |
| Fusco et al., 2014 | 11, acute, sub-acute, chronic | TUG, 6MWT, 10MWT, RMI, FAC. | tDCS followed by PT.
| 10 sessions, 2 weeks. Cathodal tDCS, 10 min, 1.5 mA, 35 cm2 electrodes. Cathode: over the contralateral hemisphere to the stroke. | T0 baseline, T1 post-treatment, T2 1 month follow-up, T3 75–100 days after treatment. 10 MWT, 6MWT y TUG improved significantly in both groups after all measurements without differences between groups. FAC (p = 0.931) RMI (p = 0.537) did not show significant changes between both groups |
| Klomjai et al., 2018 | 19, sub-acute | SKE, TUG. | tDCS followed by PT.
| 1 session, 1 sham session, 1-week interval. Dual tDCS, 2 mA, 20 min, 35 cm2 electrodes. Electrodes over the leg area of M1. Anode: over the ipsilateral hemisphere to the stroke. Cathode: over the contralateral hemisphere to the stroke. | Baseline vs. post-treatment. TUG increased significantly in both groups, but both groups did not differ No change in strength was found in either both groups |
| Ojardias et al., 2019 | 18, chronic | 6MWT, TSGP, CDP | tDCS compared to sham.
| 1session, 1 sham session, 11 days interval. Anodal tDCS, 2 mA, 20 min, 25 cm2 electrodes. Anode: over the ipsilateral hemisphere to the stroke. Leg area of M1. | T0 baseline, T1 9 days after first treatment, T2 9 days after second treatment. 6MWT significantly improved on real tDCS after 1 h after stimulation (p = 0.038). No significant differences were observed for the other evaluation. |
| Park et al., 2015 | 24, chronic | TSGP. | tDCS + TRT PT.
| 12 sessions, 3 days/week, 4 weeks Dual tDCS, 2 mA, 15 min. Electrodes over the leg area of M1. Anode: over the ipsilateral hemisphere to the stroke. Cathode: over the contralateral hemisphere to the stroke. | Baseline vs. post-treatment. All groups showed improvements in SPSP, SWPSP, and gait velocity. TT and TRT showed significant improvements (p < 0.05). TT group significantly improved compared with the TRT group |
| Saeys et al., 2015 | 31, acute, sub-acute | POMA. RMA. | tDCS + PT + TO.
| 16 sessions/4 weeks, 16 sham sessions/4 weeks. Dual tDCS, 1.5 mA, 15 min, 35 cm2 electrodes. Electrodes over the motor cortex (C3-C4 areas). Anode: over the ipsilateral hemisphere to the stroke. Cathode: over the contralateral hemisphere to the stroke. | T1 baseline, T2 mid of study, T3 post-treatment. Both groups improved significantly on all outcome measures (p < 0.001) without differences between both (p > 0.005). POMA significantly improved in real group at middle of the study (4 weeks) (p = 0.049). RMA showed an improvement on the leg and trunk sub-score (p = 0.045) in real group. |
| Sohn et al., 2013 | 11, acute | SB, SKE | tDCS + PT.
| 1 session, 1 sham session, 48 h interval. Anodal tDCS, 2 mA, 10 min, 35 cm2 electrodes. Anode: over the ipsilateral hemisphere to the stroke | Baseline vs. post-treatment. SB. Significant improvements with eyes opened and closed after real tDCS (p < 0.05). SKE. Significant improvements after real tDCS (p < 0.05) |
| Tahtis et al., 2014 | 14, sub-acute | TUG, POMA | tDCS compared to sham.
| 1 session. Dual tDCS, 2 mA, 15 min, 35 cm2 electrodes. Electrodes over the leg area of M1. Anode: over the ipsilateral hemisphere to the stroke. Cathode: over the contralateral hemisphere to the stroke. | Baseline vs. post-treatment. TUG improved significantly in real than sham group (p = 0.018). POMA showed no differences between groups (p = 0.897). |
| Utarapichat et al., 2018 | 10, chronic | RMS and MF of VMO and TA, TUG. | tDCS compared to sham.
| 1 session, 1 sham session, 48 h interval. Anodal tDCS, 2 mA, 10 min, A: 10.1 cm2, C: 25 cm2. Anode over the leg area of M1 (ipsilateral hemisphere to the stroke). | Baseline vs. post-treatment. There were not differences between tDCS and sham stimulation (p > 0.05) all measures. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-López, V.; Molina-Rueda, F.; Jiménez-Jiménez, S.; Alguacil-Diego, I.M.; Carratalá-Tejada, M. Effects of Transcranial Direct Current Stimulation Combined with Physiotherapy on Gait Pattern, Balance, and Functionality in Stroke Patients. A Systematic Review. Diagnostics 2021, 11, 656. https://doi.org/10.3390/diagnostics11040656
Navarro-López V, Molina-Rueda F, Jiménez-Jiménez S, Alguacil-Diego IM, Carratalá-Tejada M. Effects of Transcranial Direct Current Stimulation Combined with Physiotherapy on Gait Pattern, Balance, and Functionality in Stroke Patients. A Systematic Review. Diagnostics. 2021; 11(4):656. https://doi.org/10.3390/diagnostics11040656
Chicago/Turabian StyleNavarro-López, Víctor, Francisco Molina-Rueda, Samuel Jiménez-Jiménez, Isabel M Alguacil-Diego, and María Carratalá-Tejada. 2021. "Effects of Transcranial Direct Current Stimulation Combined with Physiotherapy on Gait Pattern, Balance, and Functionality in Stroke Patients. A Systematic Review" Diagnostics 11, no. 4: 656. https://doi.org/10.3390/diagnostics11040656
APA StyleNavarro-López, V., Molina-Rueda, F., Jiménez-Jiménez, S., Alguacil-Diego, I. M., & Carratalá-Tejada, M. (2021). Effects of Transcranial Direct Current Stimulation Combined with Physiotherapy on Gait Pattern, Balance, and Functionality in Stroke Patients. A Systematic Review. Diagnostics, 11(4), 656. https://doi.org/10.3390/diagnostics11040656







