4D Doppler Ultrasound in High Grade Serous Ovarian Cancer Vascularity Evaluation—Preliminary Study
Abstract
1. Introduction
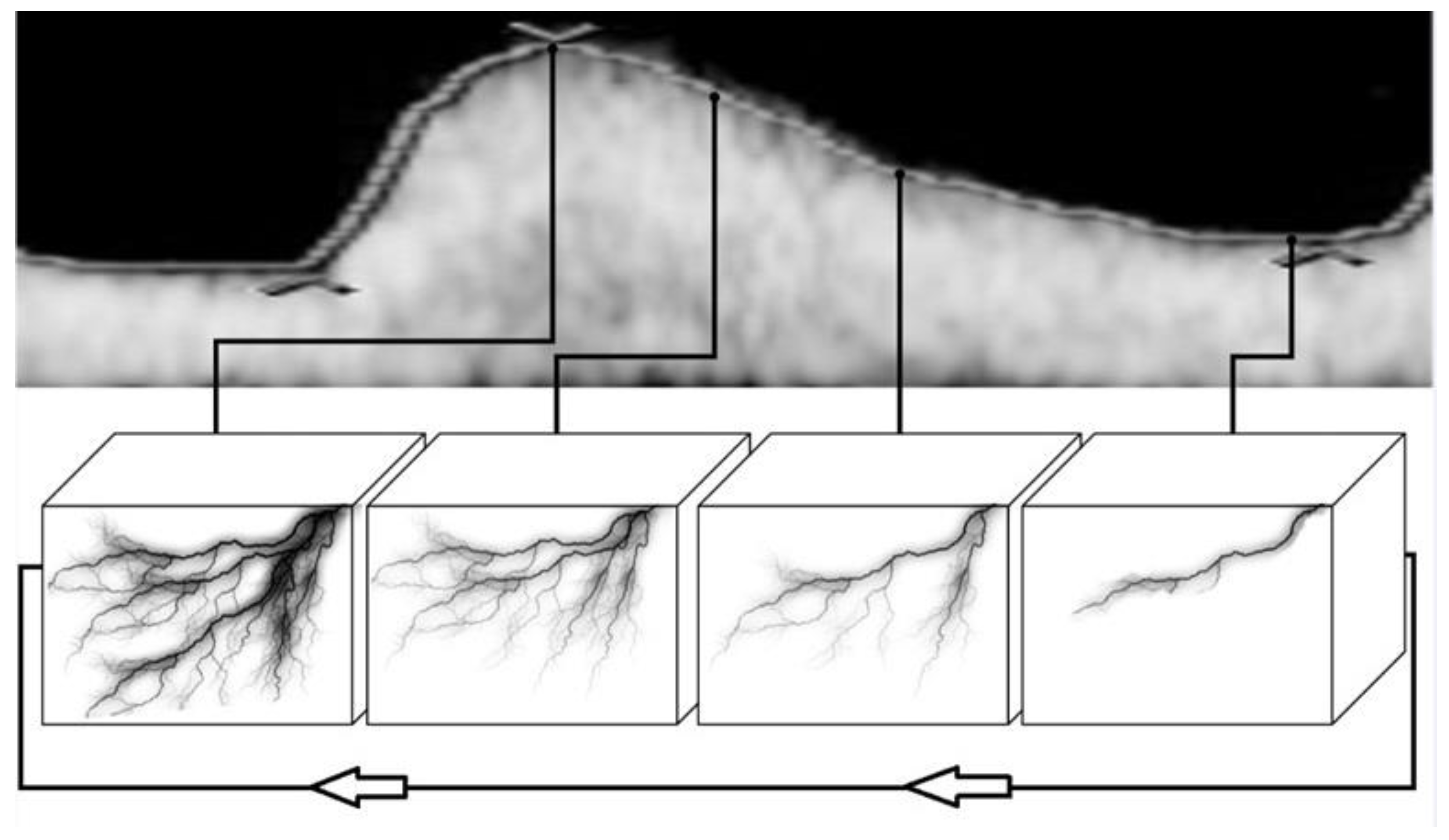
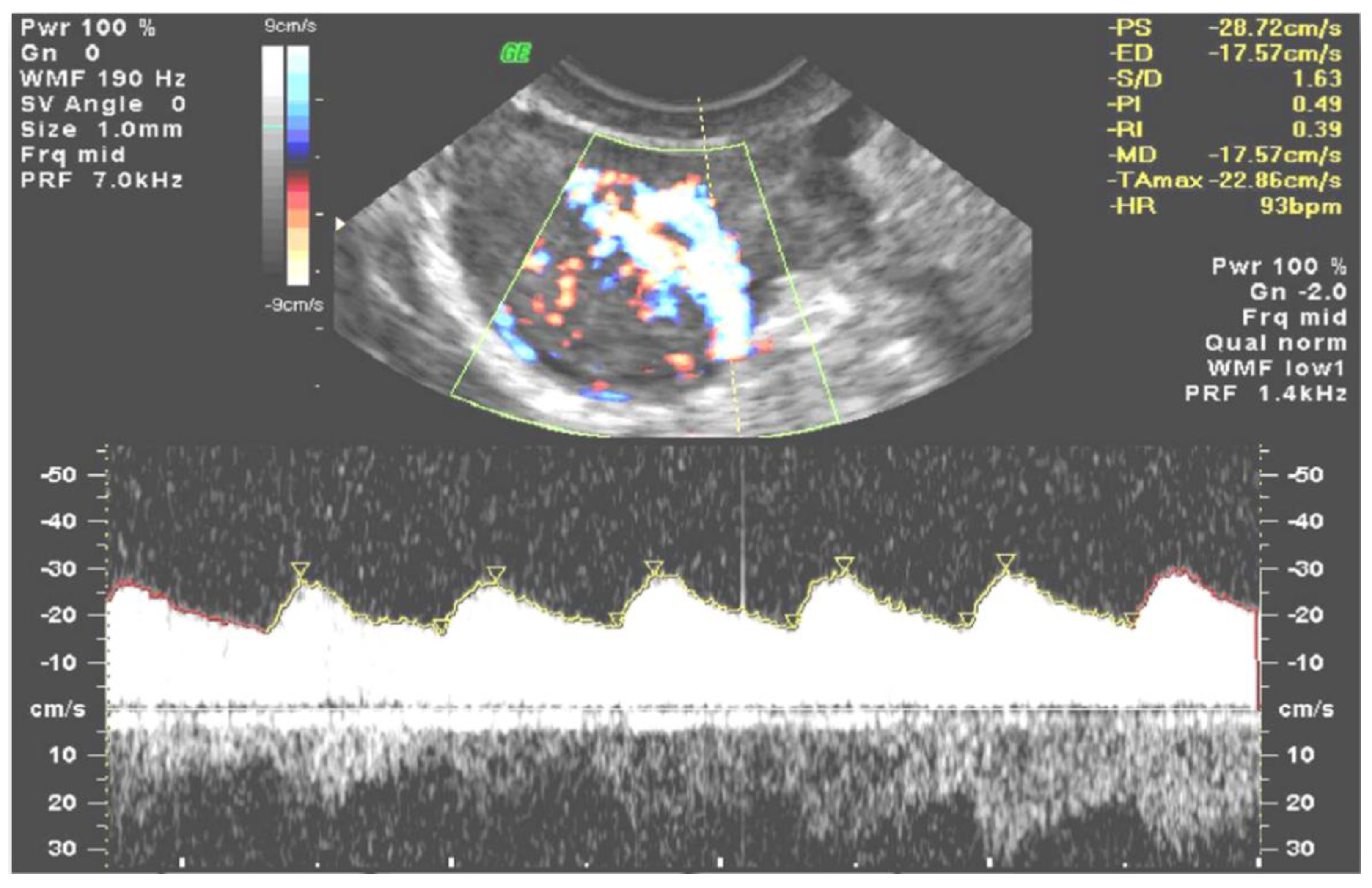
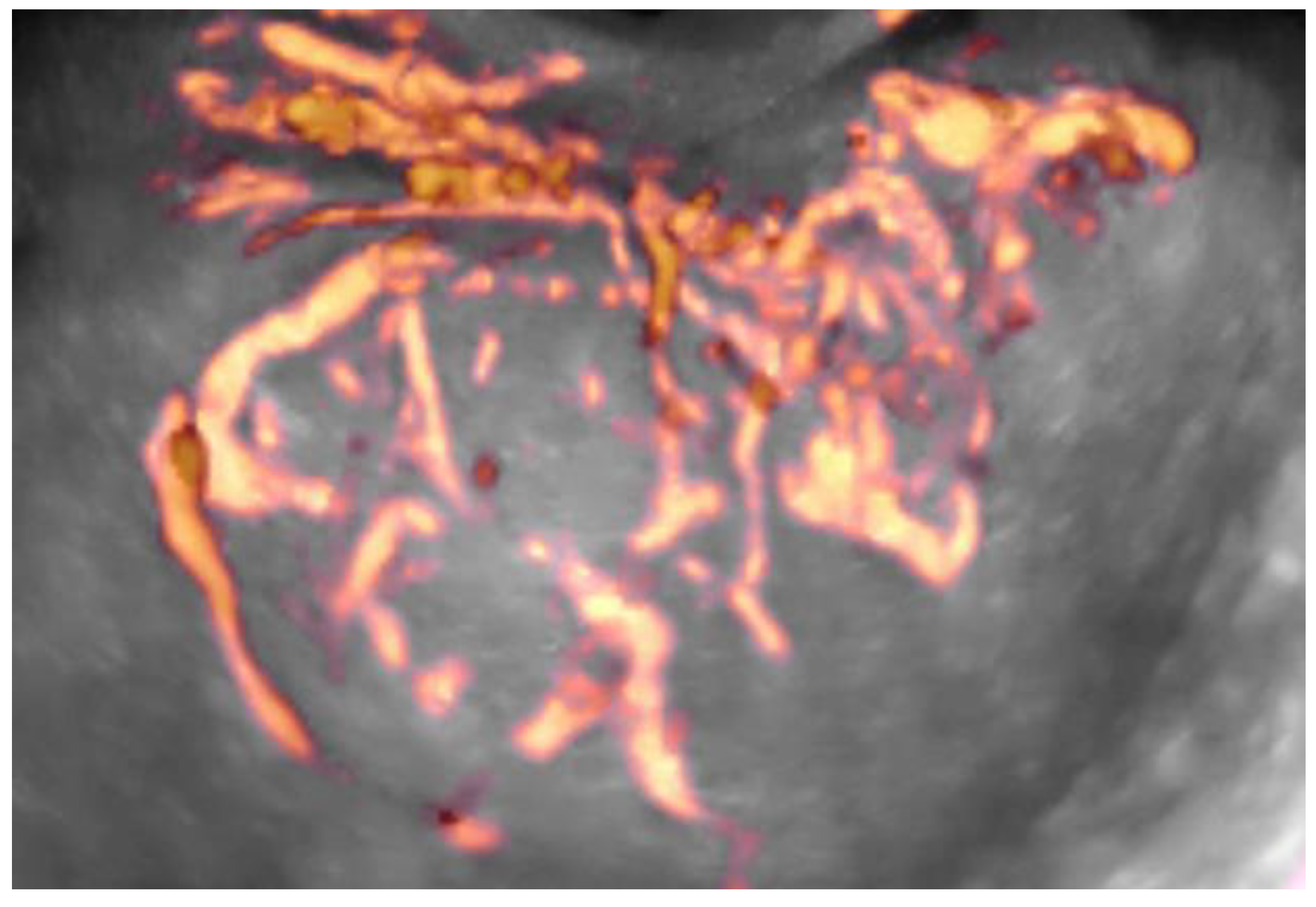
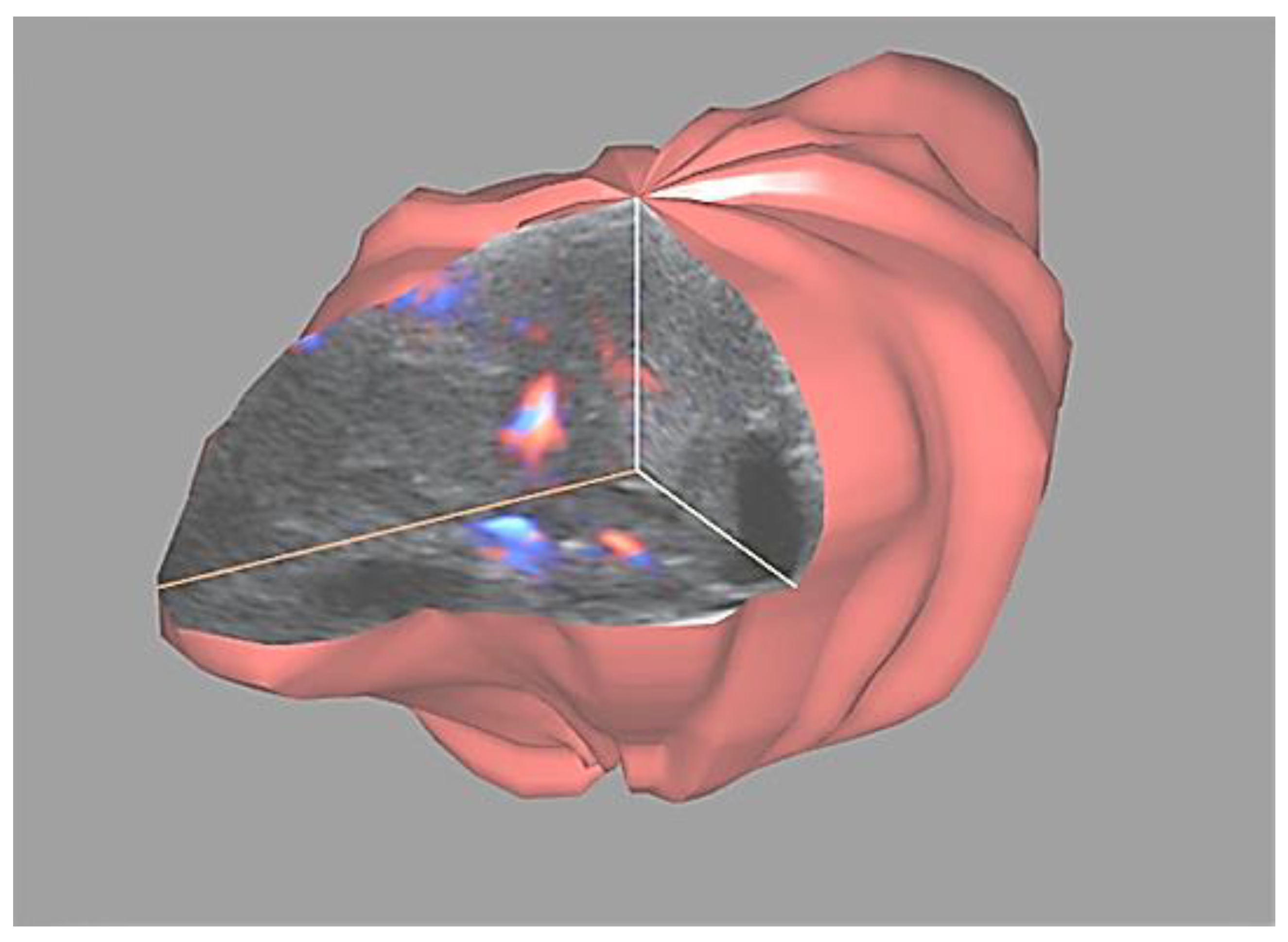
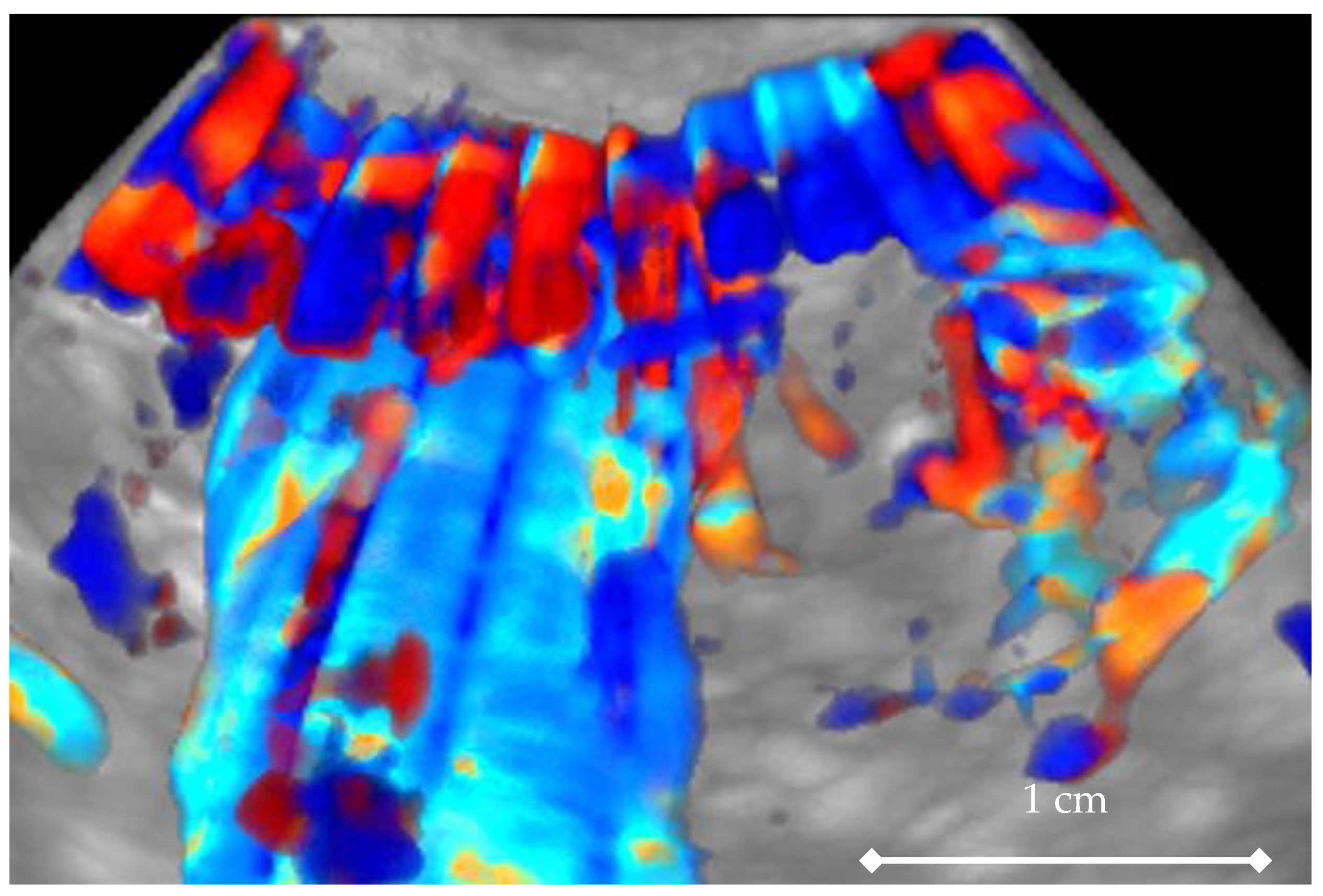
2. Materials and Methods
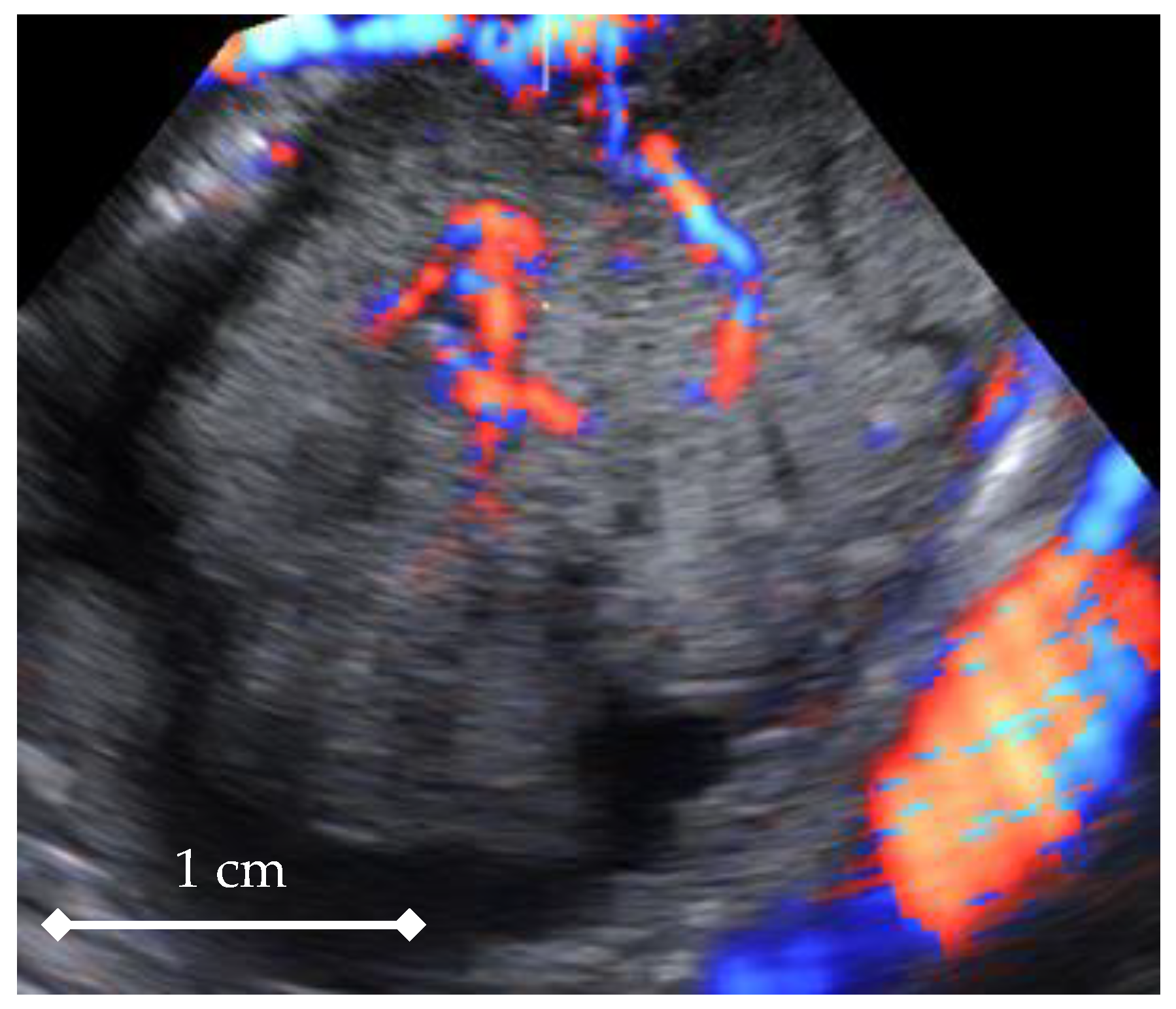
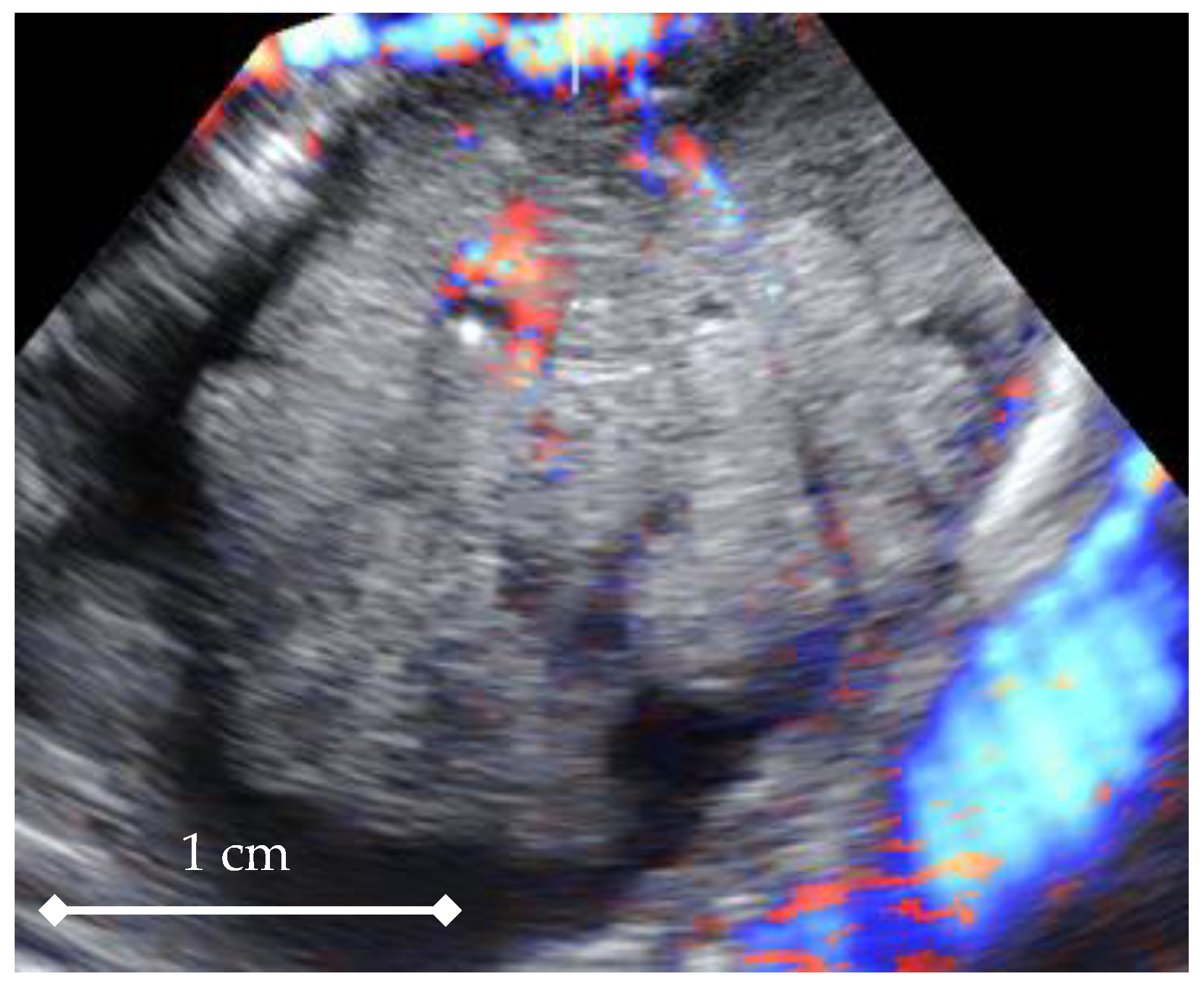
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alcázar, J.L.; Guerriero, S.; Laparte, C.; Ajossa, S.; Jurado, M. Contribution of power Doppler blood flow mapping to gray-scale ultrasound for predicting malignancy of adnexal masses in symptomatic and asymptomatic women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 99–105. [Google Scholar] [CrossRef]
- Alcázar, J.L.; Prka, M. Evaluation of two different methods for vascular sampling by three-dimensional power Doppler angi-ography in solid and cystic-solid adnexal masses. Ultrasound Obstet. Gynecol. 2009, 33, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, S.; Saba, L.; Alcazar, J.L.; Pascual, M.A.; Ajossa, S.; Perniciano, M.; Piras, A.; Sedda, F.; Peddes, C.; Fabbri, P.; et al. Past, present and future ultrasonographic techniques for analyzing ovarian masses. Women’s Health 2015, 11, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Sladkevicius, P.; Jokubkiene, L.; Timmerman, D.; Fischerova, D.; Van Holsbeke, C.; Franchi, D.; Savelli, L.; Epstein, E.; Fruscio, R.; Kaijser, J.; et al. Vessel morphology depicted by three-dimensional power Doppler ultrasound as second-stage test in adnexal tumors that are difficult to classify: Prospective diagnostic accuracy study. Ultrasound Obstet. Gynecol. 2021, 57, 324–334. [Google Scholar] [CrossRef]
- Silvestre, L.; Martins, W.P.; Candido-Dos-Reis, F.J. Limitations of three-dimensional power Doppler angiography in preoperative evaluation of ovarian tumors. J. Ovarian Res. 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Utrilla-Layna, J.; Alcázar, J.L.; Aubá, M.; Laparte, C.; Olartecoechea, B.; Errasti, T.; Juez, L.; Mínguez, J.Á.; Guerriero, S.; Jurado, M. Performance of three-dimensional power Doppler angiography as third-step assessment in differential diagnosis of adnex-al masses. Ultrasound Obstet. Gynecol. 2015, 45, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, J.L.; Jurado, M. Three-dimensional ultrasound for assessing women with gynecological cancer: A systematic review. Gynecol. Oncol. 2011, 120, 340–346. [Google Scholar] [CrossRef]
- Kudla, M.J.; Timor-Tritsch, I.E.; Hope, J.M.; Monteagudo, A.; Popiolek, D.; Monda, S.; Lee, C.J.; Arslan, A.A. Spherical tissue sampling in 3-dimensional power Doppler angiography: A new approach for evaluation of ovarian tumors. J. Ultrasound Med. 2008, 27, 425–433. [Google Scholar] [CrossRef]
- Meys, E.M.; Kaijser, J.; Kruitwagen, R.F.; Slangen, B.F.; Van Calster, B.; Aertgeerts, B.; Verbakel, J.Y.; Timmerman, D.; Van Gorp, T. Sub-jective assessment versus ultrasound models to diagnose ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer 2016, 58, 17–29. [Google Scholar] [CrossRef]
- Sladkevicius, P.; Valentin, L. Interobserver agreement in describing the ultrasound appearance of adnexal masses and in calcu-lating the risk of malignancy using logistic regression models. Clin. Cancer Res. 2015, 21, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Kudla, M.J.; Alcázar, J.L. Spatiotemporal image correlation using high-definition flow: A new method for assessing ovarian vascularization. J. Ultrasound Med. 2010, 29, 1469–1474. [Google Scholar] [CrossRef]
- Martins, W.P.; Welsh, A.W.; Lima, J.C.; Nastri, C.O.; Raine-Fenning, N.J. The “volumetric” pulsatility index as evaluated by spatio-temporal imaging correlation (STIC): A preliminary description of a novel technique, its application to the endometrium and an evaluation of its reproducibility. Ultrasound Med. Biol. 2011, 37, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Testa, A.C.; Bourne, T.; Ameye, L.; Jurkovic, D.; Van Holsbeke, C.; Paladini, D.; Van Calster, B.; Vergote, I.; Van Huffel, S.; et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet. Gynecol. 2008, 31, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Kudla, M.J.; Kandzia, T.; Alcázar, J.L. Comparison of two methods for calculating the mean vascularization index of ovarian stroma on the basis of spatio-temporal image correlation high-definition flow technology. Ultrasound Med. Biol. 2013, 39, 2202–2204. [Google Scholar] [CrossRef]
- Polanski, L.T.; Baumgarten, M.N.; Brosens, J.J.; Quenby, S.M.; Campbell, B.K.; Martins, W.P.; Raine-Fenning, N.J. 4-D assessment of en-dometrial vascularity using spatiotemporal image correlation: A study comparing spherical sampling and whole-tissue analysis. Ultrasound Med. Biol. 2015, 41, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xuan, Z.; Wang, Y. Diagnostic value of ultrasound score, color Doppler ultrasound RI and spiral CT for ovarian tumors. Oncol. Lett. 2019, 17, 5499–5504. [Google Scholar] [CrossRef]
- Alcázar, J.L. Tumor angiogenesis assessed by three-dimensional power Doppler ultrasound in early, advanced and metastatic ovarian cancer: A preliminary study. Ultrasound Obstet. Gynecol. 2006, 28, 325–329. [Google Scholar] [CrossRef]
- Timmerman, D.; Van Calster, B.; Testa, A.; Savelli, L.; Fischerova, D.; Froyman, W.; Wynants, L.; Van Holsbeke, C.; Epstein, E.; Franchi, D.; et al. Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis group. Am. J. Obstet. Gynecol. 2016, 214, 424–437. [Google Scholar] [CrossRef]
- Jokubkiene, L.; Sladkevicius, P.; Valentin, L. Does three-dimensional power Doppler ultrasound help in discrimination between benign and malignant ovarian masses? Ultrasound Obstet. Gynecol. 2007, 29, 215–225. [Google Scholar] [CrossRef]
- Alcázar, J.L.; Kudla, M.J. Ovarian stromal vessels assessed by spatiotemporal image correlation-high definition flow in women with polycystic ovary syndrome: A case-control study. Ultrasound Obstet. Gynecol. 2012, 40, 470–475. [Google Scholar] [CrossRef]
- Miyague, A.H.; Pavan, T.Z.; Grillo, F.W.; Teixeira, D.M.; Nastri, C.O.; Martins, W.P. Influence of attenuation on three-dimensional power Doppler indices and STIC volumetric pulsatility index: A flow phantom experiment. Ultrasound Obstet. Gynecol. 2014, 43, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Miyague, A.H.; Pavan, T.Z.; Soares, C.A.; De Catte, L.; Nastri, C.O.; Welsh, A.W.; Martins, W.P. Importance of pulse repetition fre-quency adjustment for 3- and 4-dimensional power doppler quantification. J. Ultrasound Med. 2015, 34, 2245–2251. [Google Scholar] [CrossRef]
- Miyague, A.; Rainne-Fenning, N.; Polanski, L.; Martinez, L.; Junior, E.; Pavan, T.; Martins, W. Assessing the repeatability of 3D doppler indices obtained by static 3D and stic power doppler: A combined in vitro/in vivo flow phantom study. Ultrasound Med. Biol. 2013, 39, S31. [Google Scholar] [CrossRef]
- Soares, C.A.; Pavan, T.Z.; Miyague, A.H.; Kudla, M.; Martins, W.P. Influence of pulse repetition frequency on 3-D power doppler quantification. Ultrasound Med. Biol. 2016, 42, 2887–2892. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.R.; Martins, W.P.; Soares, C.A.M.; Miyague, A.H.; Kudla, M.J.; Pavan, T.Z. Understanding the influence of flow velocity, wall motion filter, pulse repetition frequency, and aliasing on power doppler image quantification. J. Ultrasound Med. 2018, 37, 255–261. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.M.; Kim, Y.J.; Lee, J.Y.; Han, J.K.; Choi, B.I. High-definition flow Doppler ultrasonographic technique to assess hepatic vasculature compared with color or power Doppler ultrasonography: Preliminary experience. J. Ultrasound Med. 2008, 27, 1491–1501. [Google Scholar] [CrossRef]
- Kudla, M.J.; Alcazar, J.L. Does sphere volume affect the performance of three-dimensional power Doppler virtual vascular sampling for predicting malignancy in vascularized solid or cystic-solid adnexal masses? Ultrasound Obstet. Gynecol. 2010, 35, 602–608. [Google Scholar] [CrossRef]
- Martins, W.P.; Raine-Fenning, N.J.; Ferriani, R.A.; Nastri, C.O. Quantitative three-dimensional power Doppler angiography: A flow-free phantom experiment to evaluate the relationship between color gain, depth and signal artifact. Ultrasound Obstet. Gynecol. 2010, 35, 361–368. [Google Scholar] [CrossRef]
- Hildreth, K.L.; Ozemek, C.; Kohrt, W.M.; Blatchford, P.J.; Moreau, K.L. Vascular dysfunction across the stages of the menopausal transition is associated with menopausal symptoms and quality of life. Menopause 2018, 25, 1011–1019. [Google Scholar] [CrossRef]
- Moreau, K.L.; Hildreth, K.L.; Meditz, A.L.; Deane, K.D.; Kohrt, W.M. Endothelial function is impaired across the stages of the menopause transition in healthy women. J. Clin. Endocrinol. Metab. 2012, 97, 4692–4700. [Google Scholar] [CrossRef]

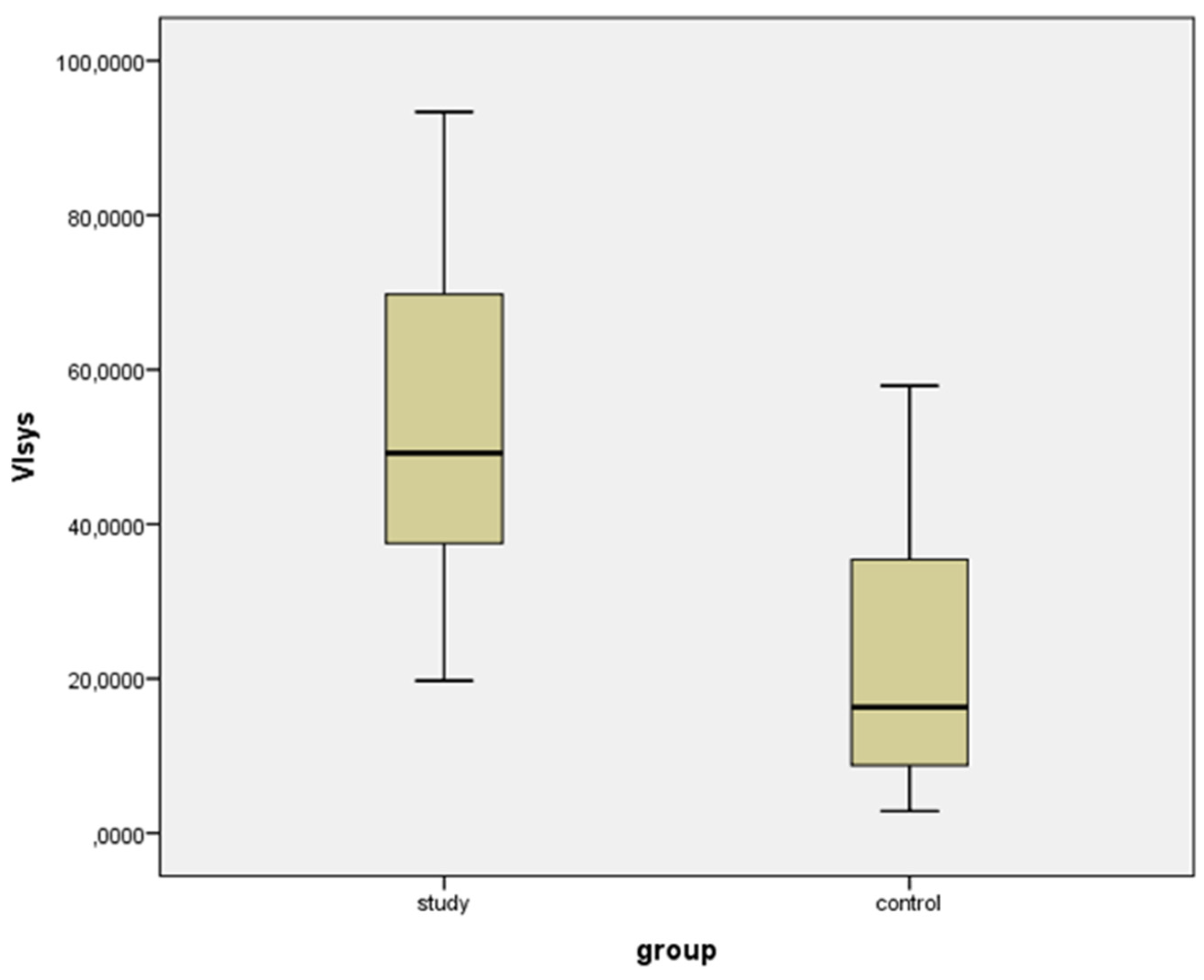
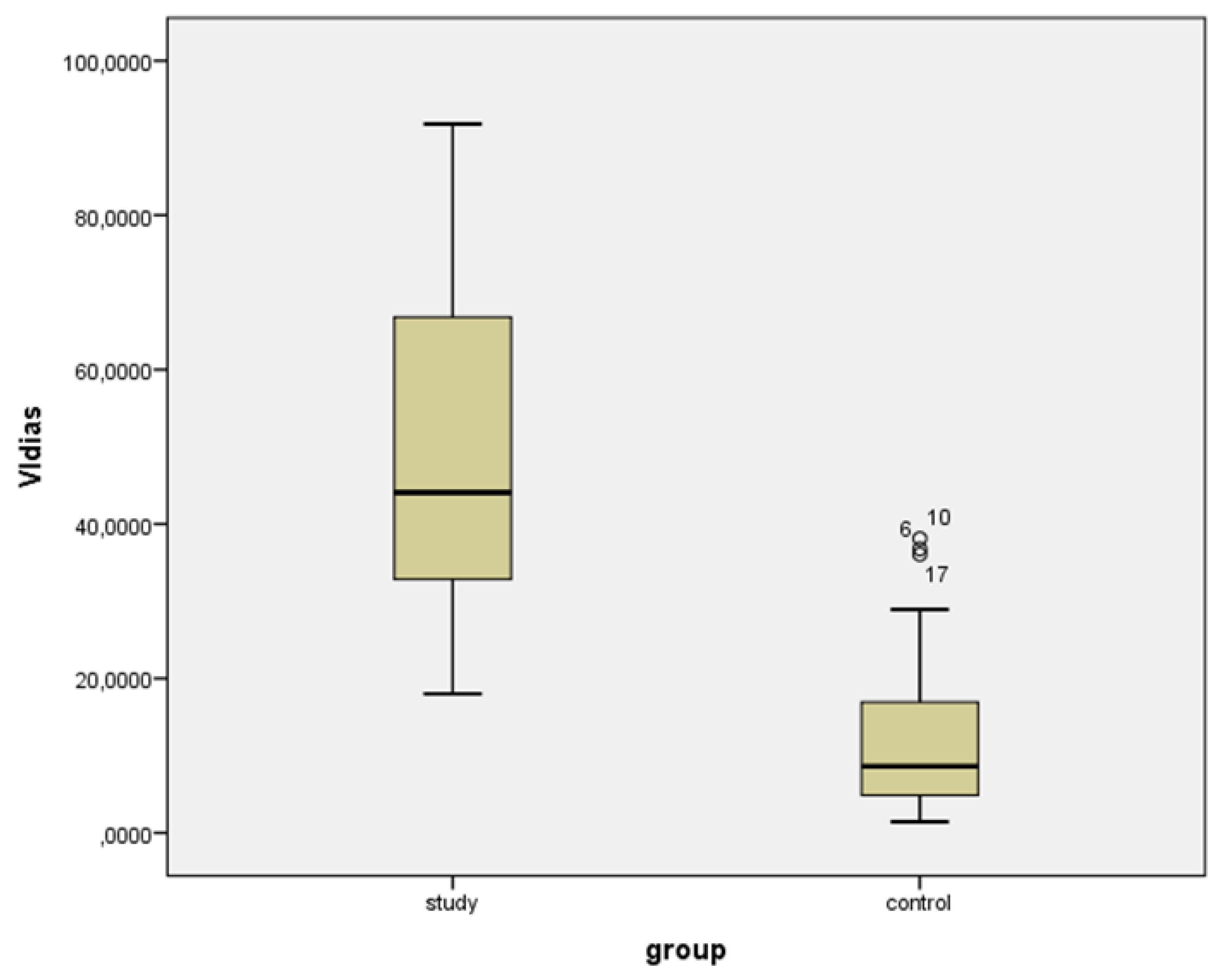
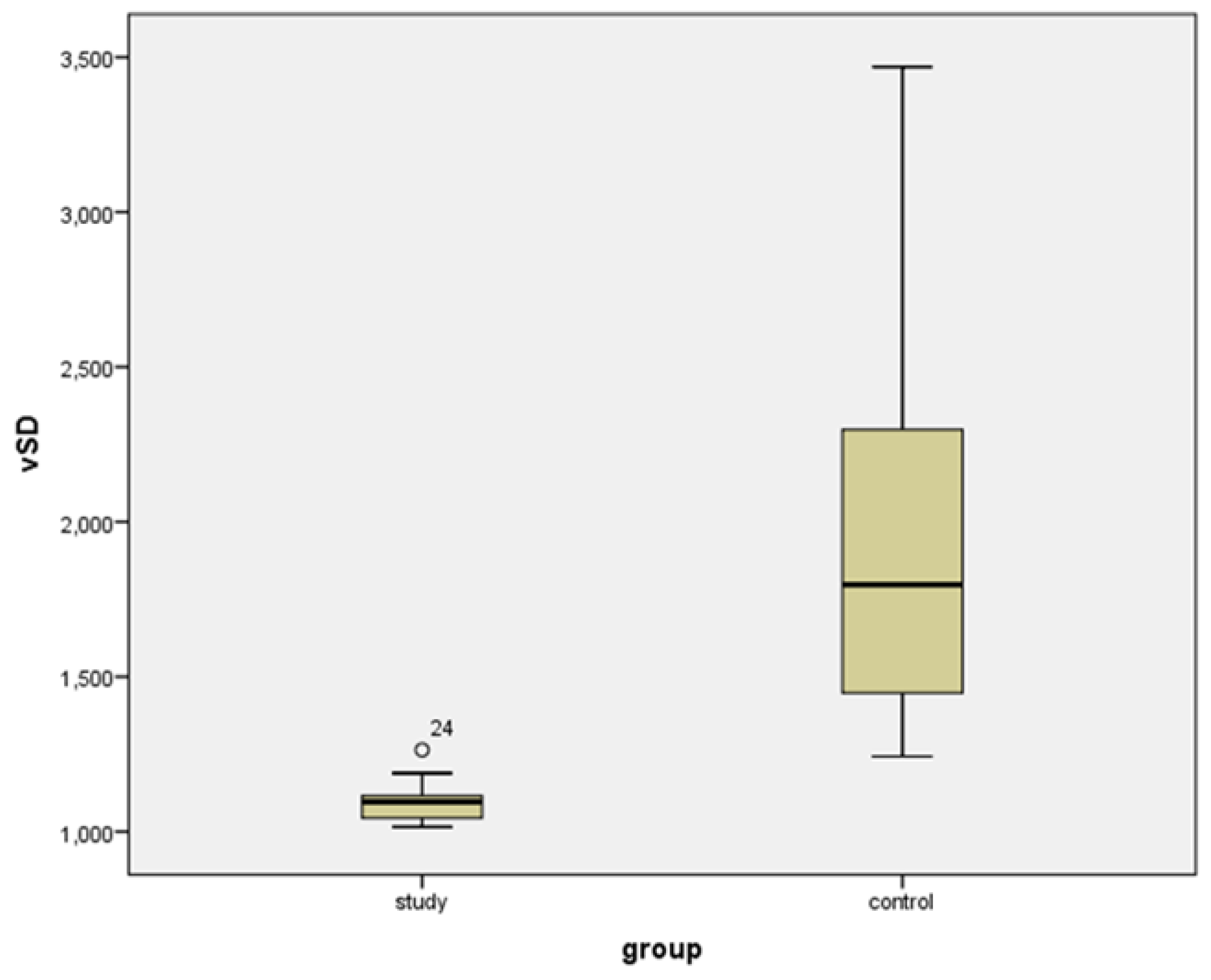
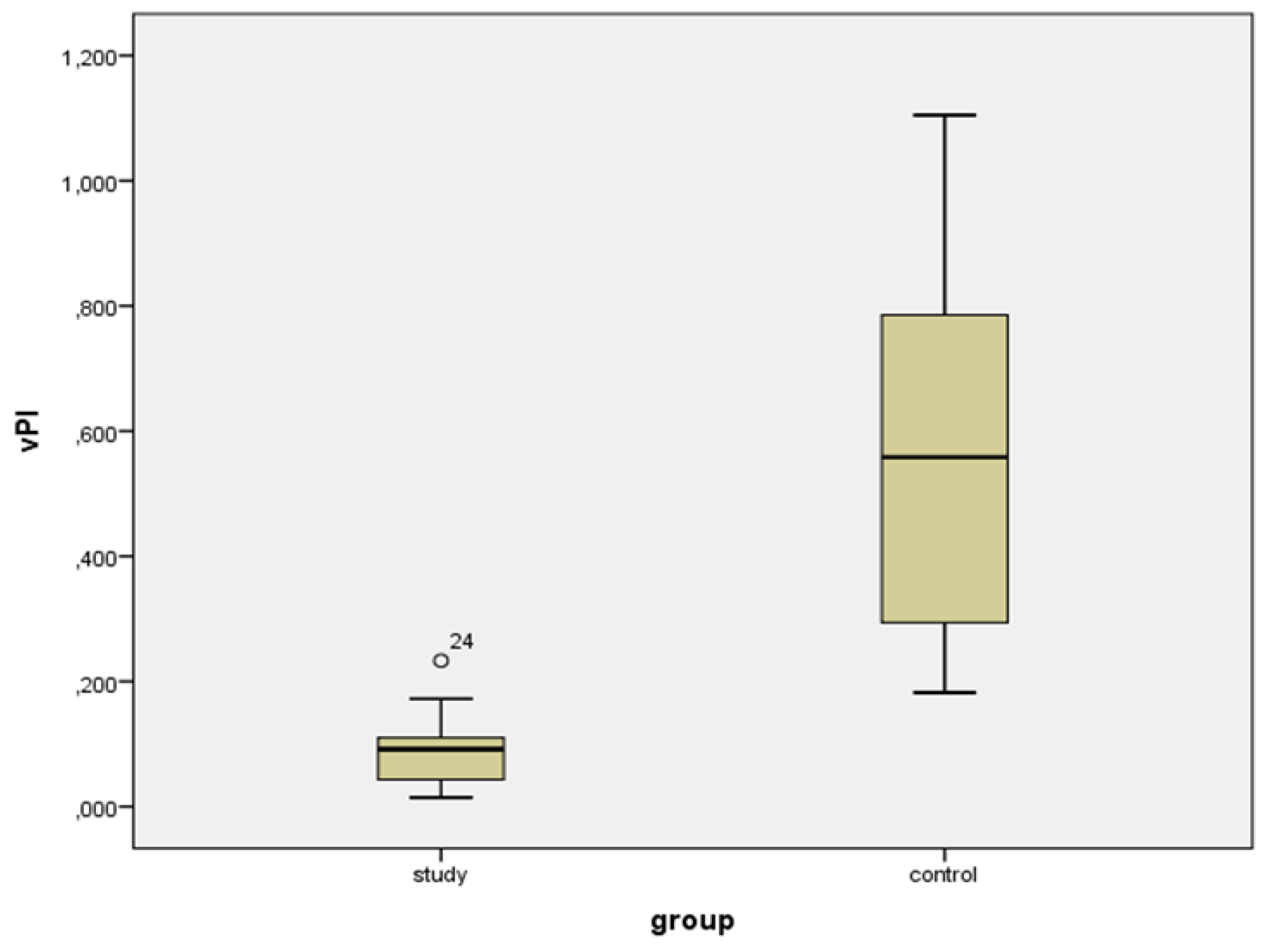
| VIsys * | VIdias * | vS/D + | vPI + | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (%) (SD) | Range | Mean (%) (SD) | Range | Median (IQR) | Range | Median (IQR) | Range | |
| Study group | 53.729 (22.104) | 19.737 to 93.359 | 49.169 (21.827) | 18.508 to 91.800 | 1.096 (0.770) | 1.015 to 1.204 | 0.092 (0.071) | 0.015 to 0.233 |
| Controls | 22.201 (15.989) | 2.876 to 57.912 | 13.447 (11.835) | 1.440 to 38.116 | 1.794 (0.994) | 1.243 to 3.469 | 0.558 (0.581) | 0.182 to 1.105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudla, M.J.; Zikan, M.; Fischerova, D.; Stolecki, M.; Alcazar, J.L. 4D Doppler Ultrasound in High Grade Serous Ovarian Cancer Vascularity Evaluation—Preliminary Study. Diagnostics 2021, 11, 582. https://doi.org/10.3390/diagnostics11040582
Kudla MJ, Zikan M, Fischerova D, Stolecki M, Alcazar JL. 4D Doppler Ultrasound in High Grade Serous Ovarian Cancer Vascularity Evaluation—Preliminary Study. Diagnostics. 2021; 11(4):582. https://doi.org/10.3390/diagnostics11040582
Chicago/Turabian StyleKudla, Marek Jerzy, Michal Zikan, Daniela Fischerova, Mateusz Stolecki, and Juan Luis Alcazar. 2021. "4D Doppler Ultrasound in High Grade Serous Ovarian Cancer Vascularity Evaluation—Preliminary Study" Diagnostics 11, no. 4: 582. https://doi.org/10.3390/diagnostics11040582
APA StyleKudla, M. J., Zikan, M., Fischerova, D., Stolecki, M., & Alcazar, J. L. (2021). 4D Doppler Ultrasound in High Grade Serous Ovarian Cancer Vascularity Evaluation—Preliminary Study. Diagnostics, 11(4), 582. https://doi.org/10.3390/diagnostics11040582







