Microstructural and Strength Changes in Trabecular Bone in Elderly Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
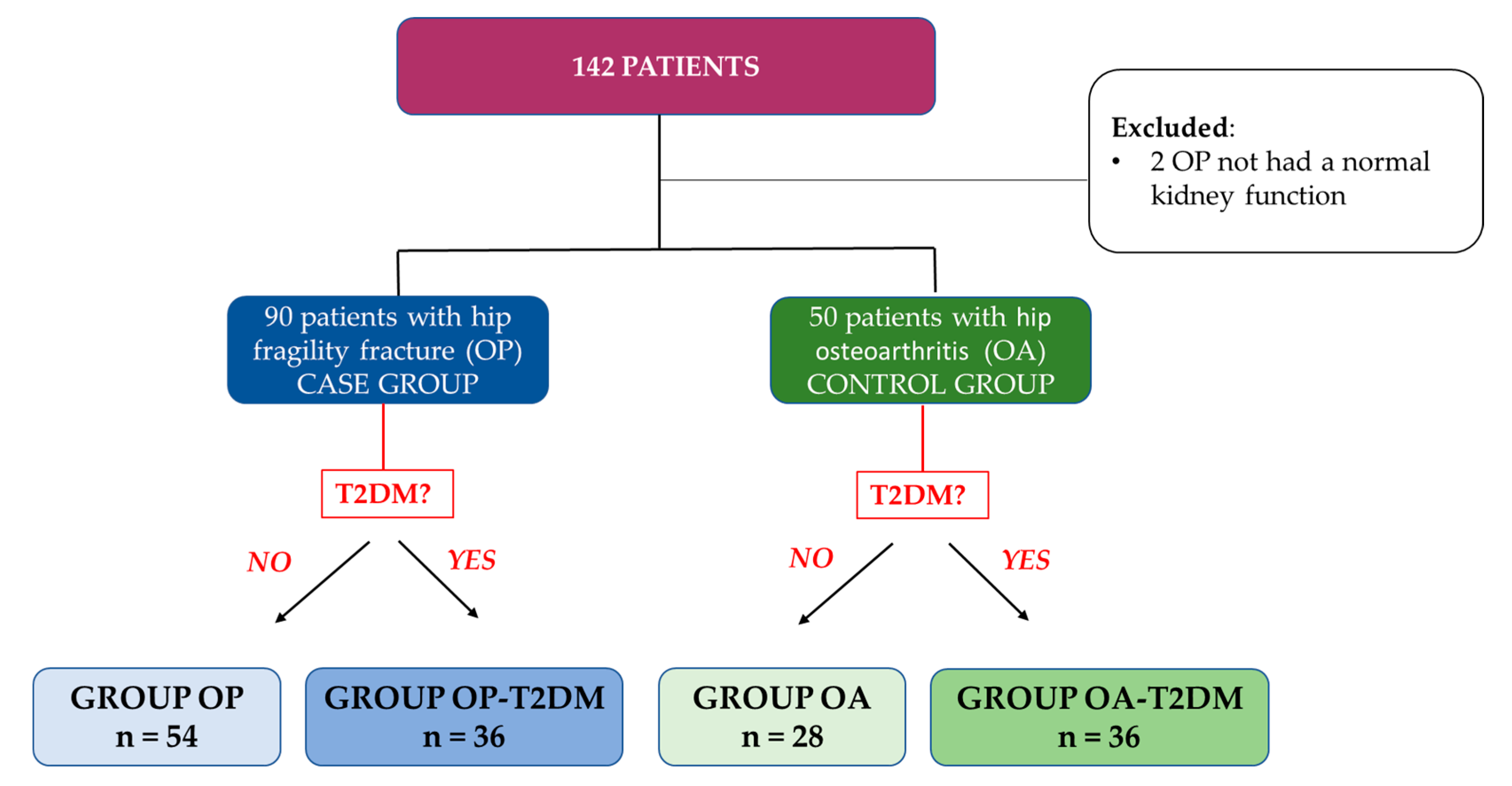
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Biochemical Measurements
2.3. Bone Mineral Density
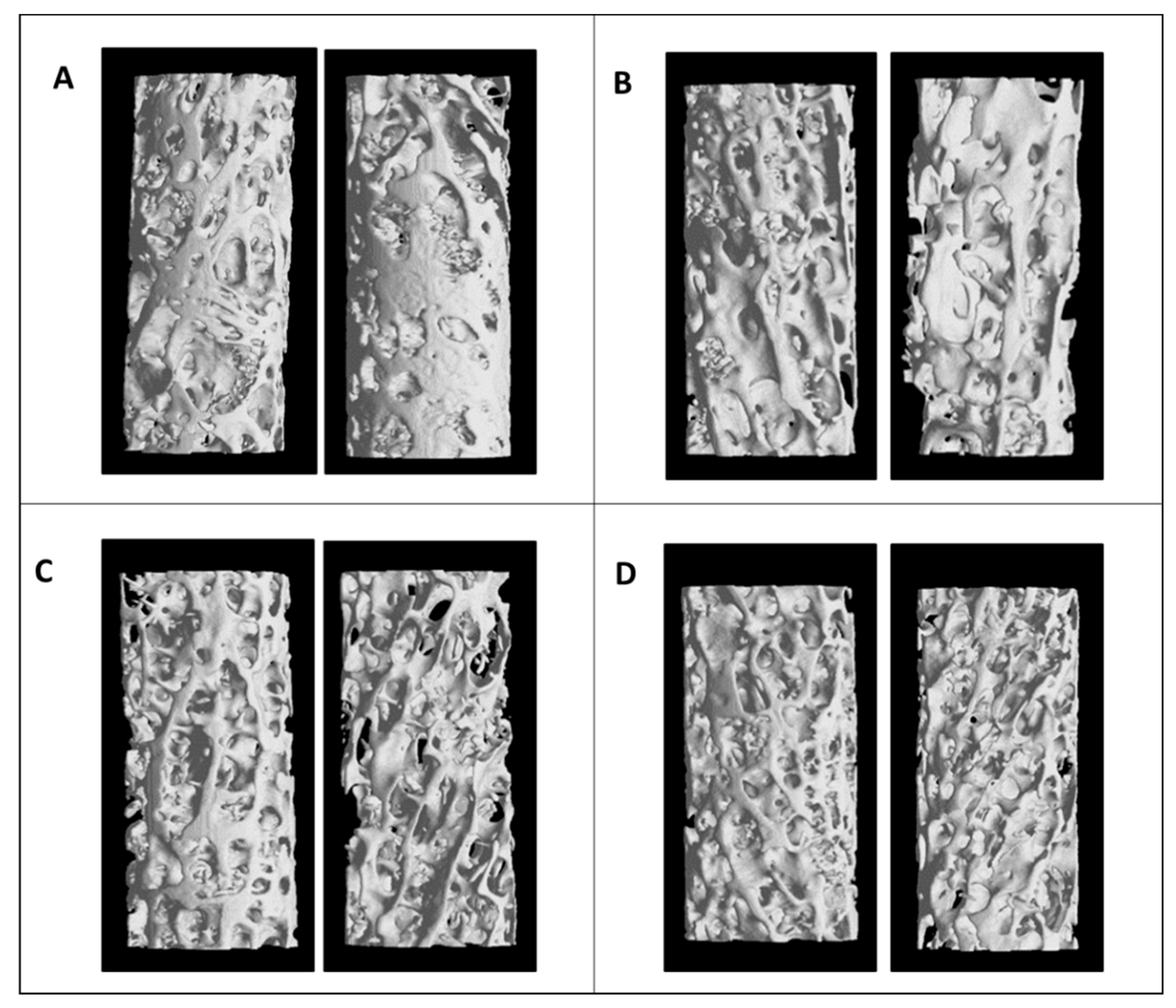
2.4. Microstructural and Bone Material Strength
2.5. Statistical Analysis
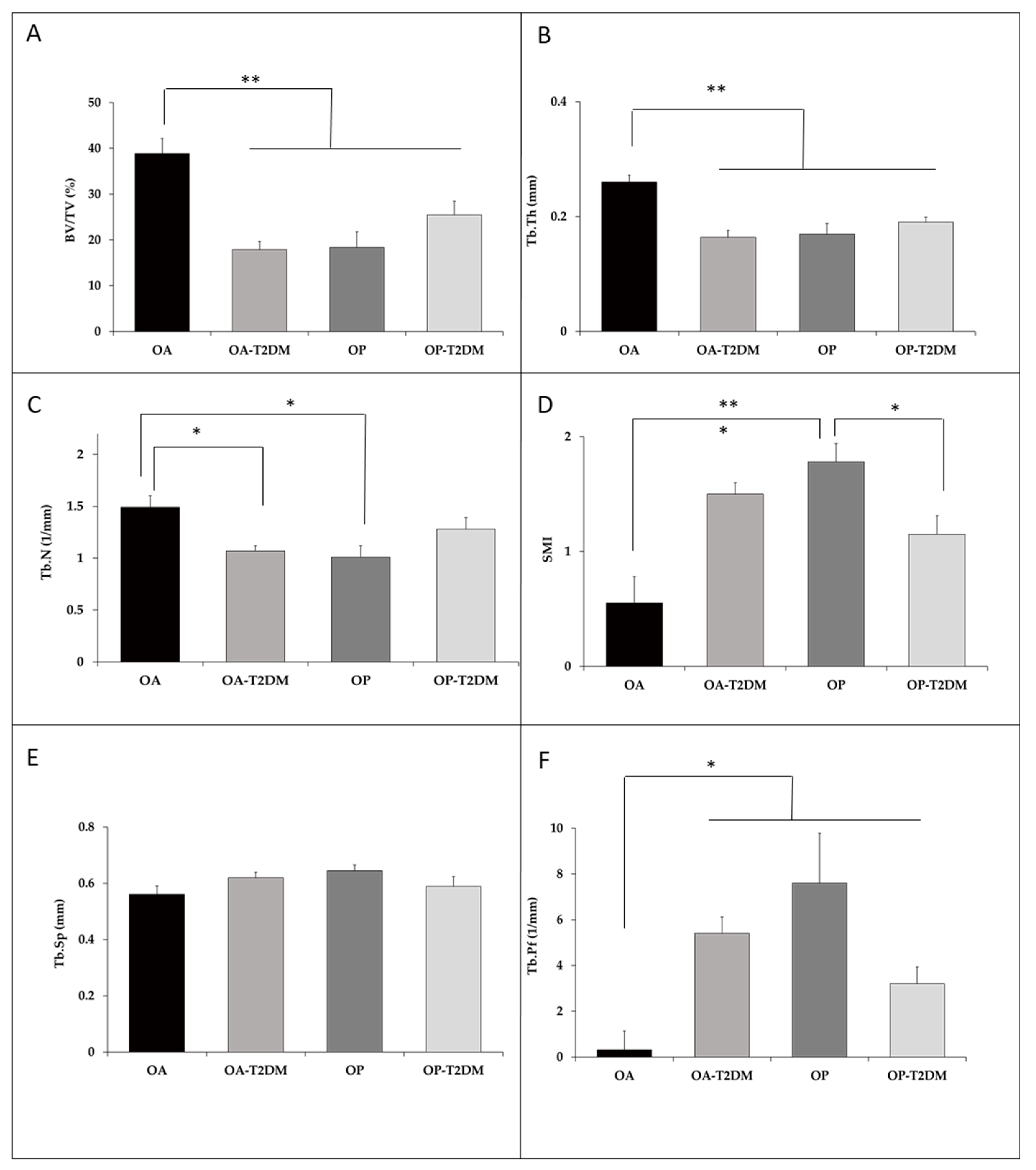
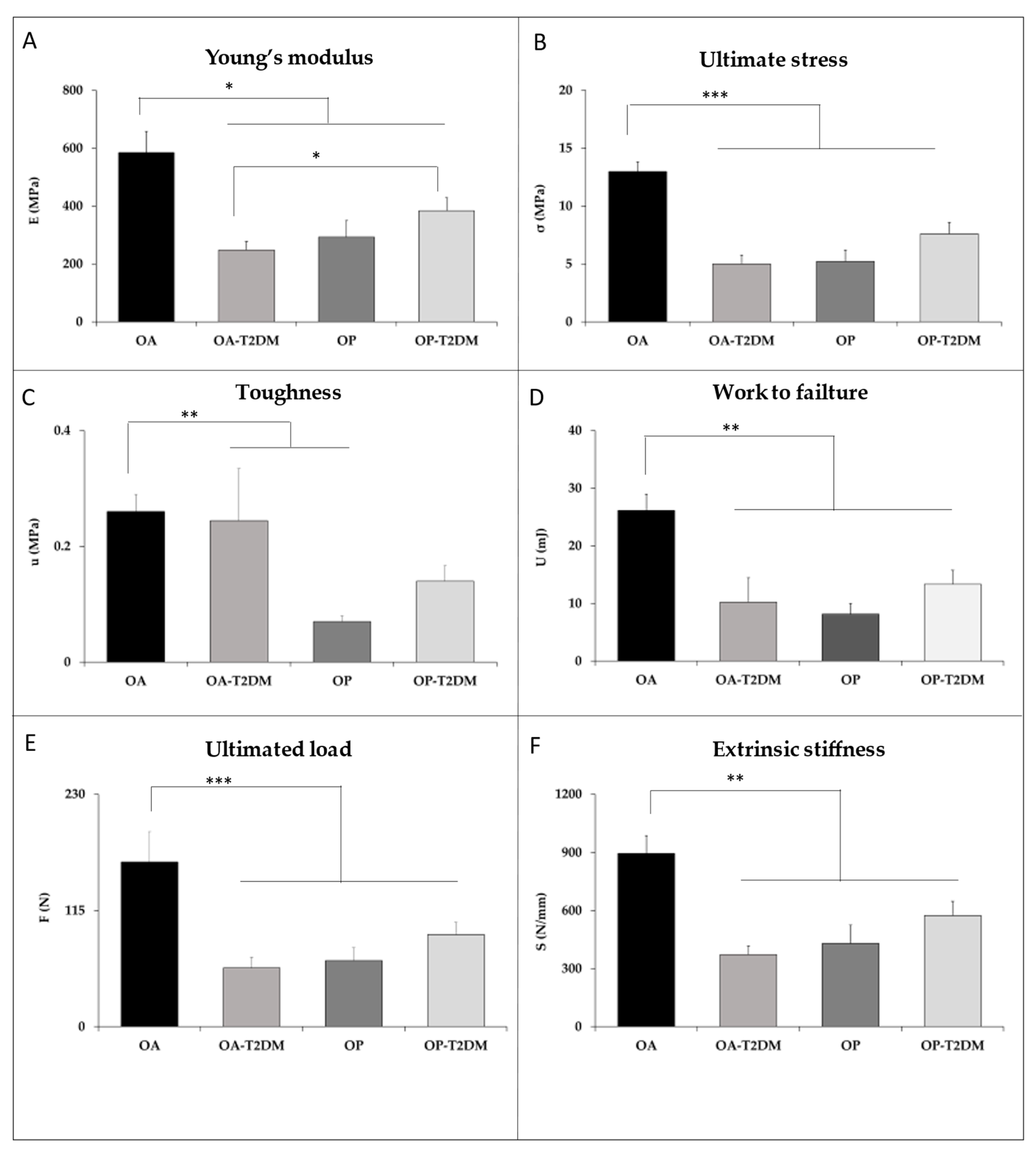
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khazai, N.B.; Beck, G.R.; Umpierrez, G.E. Diabetes and fractures: An overshadowed association. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 435–445. [Google Scholar] [CrossRef]
- Hamann, C.; Kirschner, S.; Günther, K.-P.; Hofbauer, L.C. Bone, sweet bone—Osteoporotic fractures in diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Jackuliak, P.; Payer, J. Osteoporosis, Fractures, and Diabetes. Int. J. Endocrinol. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Johnston, C.C.; Slemenda, C.W. Pathogenesis of osteoporosis. Bone 1995, 17, 19S–22S. [Google Scholar] [CrossRef]
- Hinton, R.; Moody, R.L.; Davis, A.W.; Thomas, S.F. Osteoarthritis: Diagnosis and therapeutic considerations. Am. Fam. Physician 2002, 65, 841–848. [Google Scholar] [PubMed]
- Dequeker, J.; Aerssens, J.; Luyten, F.P. Osteoarthritis and osteoporosis: Clinical and research evidence of inverse relationship. Aging Clin. Exp. Res. 2003, 15, 426–439. [Google Scholar] [CrossRef]
- Adami, S. Bone health in diabetes: Considerations for clinical management. Curr. Med. Res. Opin. 2009, 25, 1057–1072. [Google Scholar] [CrossRef]
- Oei, L.; Rivadeneira, F.; Zillikens, M.C.; Oei, E.H.G. Diabetes, diabetic complications, and fracture risk. Curr. Osteoporos. Rep. 2015, 13, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Bonds, D.E.; Larson, J.C.; Schwartz, A.V.; Strotmeyer, E.S.; Robbins, J.; Rodriguez, B.L.; Johnson, K.C.; Margolis, K.L. Risk of Fracture in Women with Type 2 Diabetes: The Women’s Health Initiative Observational Study. J. Clin. Endocrinol. Metab. 2006, 91, 3404–3410. [Google Scholar] [CrossRef]
- de Liefde, I.I.; van der Klift, M.; de Laet, C.E.D.H.; van Daele, P.L.A.; Hofman, A.; Pols, H.A.P. Bone mineral density and fracture risk in type-2 diabetes mellitus: The Rotterdam Study. Osteoporos. Int. 2005, 16, 1713–1720. [Google Scholar] [CrossRef]
- Vestergaard, P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos. Int. 2007, 18, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Yamaguchi, T.; Yamauchi, M.; Sugimoto, T. Low Serum Level of the Endogenous Secretory Receptor for Advanced Glycation End Products (esRAGE) Is a Risk Factor for Prevalent Vertebral Fractures Independent of Bone Mineral Density in Patients With Type 2 Diabetes. Diabetes Care 2009, 32, 2263–2268. [Google Scholar] [CrossRef]
- Janghorbani, M.; Van Dam, R.M.; Willett, W.C.; Hu, F.B. Systematic Review of Type 1 and Type 2 Diabetes Mellitus and Risk of Fracture. Am. J. Epidemiol. 2007, 166, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, A.; Morin, S.; Cheung, A.M.; Atkinson, S.; Brown, J.P.; Feldman, S.; Hanley, D.A.; Hodsman, A.; Jamal, S.A.; Kaiser, S.M.; et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: Summary. CMAJ 2010, 182, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Montoya, M.J.; Giner, M.; Miranda, C.; Vázquez, M.A.; Caeiro, J.R.; Guede, D.; Pérez-Cano, R. Microstructural trabecular bone from patients with osteoporotic hip fracture or osteoarthritis: Its relationship with bone mineral density and bone remodelling markers. Maturitas 2014, 79, 299–305. [Google Scholar] [CrossRef]
- Yan, W.; Li, X. Impact of diabetes and its treatments on skeletal diseases. Front. Med. 2013, 7, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Sosa, M.; Saavedra, P.; Jódar, E.; Lozano-Tonkin, C.; Quesada, J.M.; Torrijos, A.; Pérez-Cano, R.; Nogués, X.; Díaz-Curiel, M.; Moro, M.J.; et al. Bone mineral density and risk of fractures in aging, obese post-menopausal women with type 2 diabetes. The GIUMO Study. Aging Clin. Exp. Res. 2009, 21, 27–32. [Google Scholar] [CrossRef]
- Farr, J.N.; Drake, M.T.; Amin, S.; Melton, L.J.; McCready, L.K.; Khosla, S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J. Bone Miner. Res. 2014, 29, 787–795. [Google Scholar] [CrossRef]
- Jiajue, R.; Jiang, Y.; Wang, O.; Li, M.; Xing, X.; Cui, L.; Yin, J.; Xu, L.; Xia, W. Suppressed bone turnover was associated with increased osteoporotic fracture risks in non-obese postmenopausal Chinese women with type 2 diabetes mellitus. Osteoporos. Int. 2014, 25, 1999–2005. [Google Scholar] [CrossRef]
- Giangregorio, L.M.; Leslie, W.D.; Lix, L.M.; Johansson, H.; Oden, A.; McCloskey, E.; Kanis, J.A. FRAX underestimates fracture risk in patients with diabetes. J. Bone Miner. Res. 2012, 27, 301–308. [Google Scholar] [CrossRef]
- Hart, D.J.; Mootoosamy, I.; Doyle, D.V.; Spector, T.D. The relationship between osteoarthritis and osteoporosis in the general population: The Chingford Study. Ann. Rheum. Dis. 1994, 53, 158–162. [Google Scholar] [CrossRef]
- Sambrook, P.; Naganathan, V. What is the relationship between osteoarthritis and osteoporosis? Baillieres. Clin. Rheumatol. 1997, 11, 695–710. [Google Scholar] [CrossRef]
- Sainz-Aja Guerra, J.A.; Alonso, M.A.; Ferreño Blanco, D.; Pérez-Núñez, M.I.; Ruiz Martínez, E.; García-Ibarbia, C.; Casado del Prado, J.A.; Gutiérrez-Solana, F.R.J. Study of the microstructure of femoral patients with hip osteoarthritis and hip fracture by microCT. Rev. Osteoporos Metab Miner. 2016, 8, 75–81. [Google Scholar] [CrossRef]
- Stewart, A.; Black, A.J. Bone mineral density in osteoarthritis. Curr. Opin. Rheumatol. 2000, 12, 464–467. [Google Scholar] [CrossRef]
- Burghardt, A.J.; Issever, A.S.; Schwartz, A.V.; Davis, K.A.; Masharani, U.; Majumdar, S.; Link, T.M. High-Resolution Peripheral Quantitative Computed Tomographic Imaging of Cortical and Trabecular Bone Microarchitecture in Patients with Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2010, 95, 5045–5055. [Google Scholar] [CrossRef]
- Seeman, E.; Delmas, P.D. Bone Quality—The Material and Structural Basis of Bone Strength and Fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Chavassieux, P.; Seeman, E.; Delmas, P.D. Insights into material and structural basis of bone fragility from diseases associated with fractures: How determinants of the biomechanical properties of bone are compromised by disease. Endocr. Rev. 2007, 28, 151–164. [Google Scholar] [CrossRef]
- Patsch, J.M.; Burghardt, A.J.; Yap, S.P.; Baum, T.; Schwartz, A.V.; Joseph, G.B.; Link, T.M. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J. Bone Miner. Res. 2013, 28, 313–324. [Google Scholar] [CrossRef]
- Montoya, M.J.; Vázquez, M.A.; Miranda, C.; Miranda, M.J.; Pérez-Cano, R.G.M. Influence of vitamin D on biomechanical microstructure and properties of patients with hip fracture. Rev. Osteoporos. Metab. Min. 2017, 9, 121–129. [Google Scholar]
- Starup-Linde, J.; Vestergaard, P. Biochemical bone turnover markers in diabetes mellitus—A systematic review. Bone 2016, 82, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, J.S.; Cremers, S.; Dempster, D.W.; Zhou, H.; Dworakowski, E.; Kode, A.; Kousteni, S.; Rubin, M.R. Circulating Osteogenic Precursor Cells in Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2012, 97, 3240–3250. [Google Scholar] [CrossRef] [PubMed]
- Oei, L.; Zillikens, M.C.; Dehghan, A.; Buitendijk, G.H.S.; Castaño-Betancourt, M.C.; Estrada, K.; Stolk, L.; Oei, E.H.G.; van Meurs, J.B.J.; Janssen, J.A.M.J.L.; et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: The Rotterdam Study. Diabetes Care 2013, 36, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V.; Margolis, K.L.; Sellmeyer, D.E.; Vittinghoff, E.; Ambrosius, W.T.; Bonds, D.E.; Josse, R.G.; Schnall, A.M.; Simmons, D.L.; Hue, T.F.; et al. Intensive Glycemic Control Is Not Associated With Fractures or Falls in the ACCORD Randomized Trial. Diabetes Care 2012, 35, 1525–1531. [Google Scholar] [CrossRef]
- Moayeri, A.; Mohamadpour, M.; Mousavi, S.F.; Shirzadpour, E.; Mohamadpour, S.; Amraei, M. Fracture risk in patients with type 2 diabetes mellitus and possible risk factors: A systematic review and meta-analysis. Ther. Clin. Risk Manag. 2017, 13, 455–468. [Google Scholar] [CrossRef] [PubMed]
| OA | OA-T2DM | p | |
|---|---|---|---|
| Gender (Male/Female) | 28 (10/18) | 22 (8/14) | - |
| Age (years) Female Male | 73.4 ± 6.2 73.4 ± 7.4 73.4 ± 5.7 | 74.5 ± 6.4 75.1 ± 6.7 73.5 ± 6.3 | 0.528 |
| BMI (kg/m2) Female Male | 30.7 ± 3.8 29.5 ± 3.5 32.8 ± 3.5 | 31.7 ± 5.1 32.3 ± 4.1 30.5 ± 7.0 | 0.459 |
| Femoral neck BMD (gHA/cm2) Female Male | 0.685 ± 0.12 0.654 ± 0.12 0.746 ± 0.09 | 0.756 ± 0.15 0.712 ± 0.15 0.843 ± 0.07 | 0.080 |
| Hip BMD (gHA/cm2) Female Male | 0.909 ± 0.13 0.868 ± 0.12 0.991 ± 0.09 | 0.944 ± 0.16 0.896 ± 0.16 1.04 ± 0.10 | 0.405 |
| 10-year risk of major fracture (FRAX® tool) | 9.3 ± 6.8 | 6.3 ± 5.4 | 0.183 |
| 10-year risk of hip fracture (FRAX® tool) | 3.5 ± 3.2 | 4.1 ± 7.1 | 0.772 |
| 25-hydroxyvitamin D (ng/mL) | 21.8 ± 15.5 | 15.3 ± 6.6 | 0.076 |
| PTH (pg/mL) | 54.5 ± 23.4 | 59.5 ± 28.1 | 0.545 |
| β-CrossLaps (µg/mL) | 0.34 ± 0.17 | 0.33 ± 0.21 | 0.869 |
| P1NP (ng/mL) | 43.28 ± 20.3 | 49.2 ± 31.7 | 0.468 |
| IGF-1 (ng/mL) | 82.4 ± 34.4 | 88.8 ± 42.4 | 0.592 |
| HbA1c (%) | 5.5 ± 0.3 | 7.2 ± 1.4 | 0.000 |
| OP | OP-T2DM | p | |
|---|---|---|---|
| Gender (Male/Female) | 54 (9/45) | 36 (8/28) | - |
| Age (years) Female Male | 78.2 ± 6.7 78.3 ± 647 77.6 ± 8.4 | 79.1 ± 6.2 79.3 ± 5.7 78.2 ± 8.2 | 0.538 |
| BMI (kg/m2) Female Male | 28.3 ± 4.3 28.8 ± 4.4 26.6 ± 2.6 | 29.6 ± 5.2 29.4 ± 6.0 30.1 ± 2.9 | 0.256 |
| Femoral neck BMD (gHA/cm2) Female Male | 0.569 ± 0.09 0.566 ± 0.08 0.588 ± 0.11 | 0.596 ± 0.09 0.572 ± 0.09 0.681 ± 0.06 | 0.183 |
| Hip BMD (gHA/cm2) Female Male | 0.758 ± 0.10 0.747 ± 0.09 0.817 ± 0.10 | 0.794 ± 0.11 0.777 ± 0.09 0.884 ± 0.10 | 0.122 |
| 10-year risk of major fracture (FRAX® tool) | 13.7 ± 8.8 | 12.4 ± 8.7 | 0.501 |
| 10-year risk of hip fracture (FRAX® tool) | 6.3 ± 6.3 | 5.8 ± 6.4 | 0.702 |
| PTH (pg/mL) | 68.1 ± 41.1 | 71.3 ± 41.5 | 0.746 |
| β-CrossLaps (µg/mL) | 0.66 ± 0.37 | 0.51 ± 0.26 | 0.060 |
| P1NP (ng/mL) | 63.8 ± 41.7 | 46.3 ± 25.7 | 0.055 |
| IGF-1 (ng/mL) | 78.4 ± 34.7 | 65.9 ± 30.9 | 0.163 |
| HbA1c (%) | 5.4 ± 0.3 | 6.7 ± 1.4 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giner, M.; Miranda, C.; Vázquez-Gámez, M.A.; Altea-Manzano, P.; Miranda, M.-J.; Casado-Díaz, A.; Pérez-Cano, R.; Montoya-García, M.-J. Microstructural and Strength Changes in Trabecular Bone in Elderly Patients with Type 2 Diabetes Mellitus. Diagnostics 2021, 11, 577. https://doi.org/10.3390/diagnostics11030577
Giner M, Miranda C, Vázquez-Gámez MA, Altea-Manzano P, Miranda M-J, Casado-Díaz A, Pérez-Cano R, Montoya-García M-J. Microstructural and Strength Changes in Trabecular Bone in Elderly Patients with Type 2 Diabetes Mellitus. Diagnostics. 2021; 11(3):577. https://doi.org/10.3390/diagnostics11030577
Chicago/Turabian StyleGiner, Mercè, Cristina Miranda, María Angeles Vázquez-Gámez, Patricia Altea-Manzano, María-José Miranda, Antonio Casado-Díaz, Ramón Pérez-Cano, and María-José Montoya-García. 2021. "Microstructural and Strength Changes in Trabecular Bone in Elderly Patients with Type 2 Diabetes Mellitus" Diagnostics 11, no. 3: 577. https://doi.org/10.3390/diagnostics11030577
APA StyleGiner, M., Miranda, C., Vázquez-Gámez, M. A., Altea-Manzano, P., Miranda, M.-J., Casado-Díaz, A., Pérez-Cano, R., & Montoya-García, M.-J. (2021). Microstructural and Strength Changes in Trabecular Bone in Elderly Patients with Type 2 Diabetes Mellitus. Diagnostics, 11(3), 577. https://doi.org/10.3390/diagnostics11030577






