The Entire Intestinal Tract Surveillance Using Capsule Endoscopy after Immune Checkpoint Inhibitor Administration: A Prospective Observational Study
Abstract
1. Background
2. Materials and Methods
2.1. Study Design
2.2. Assessment
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
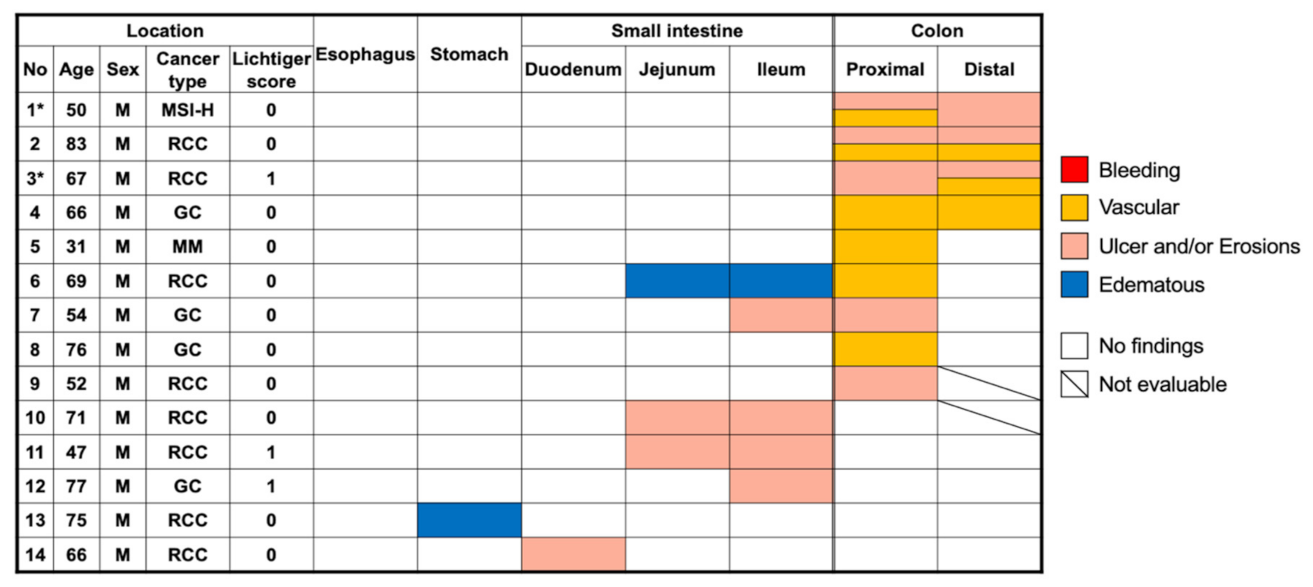
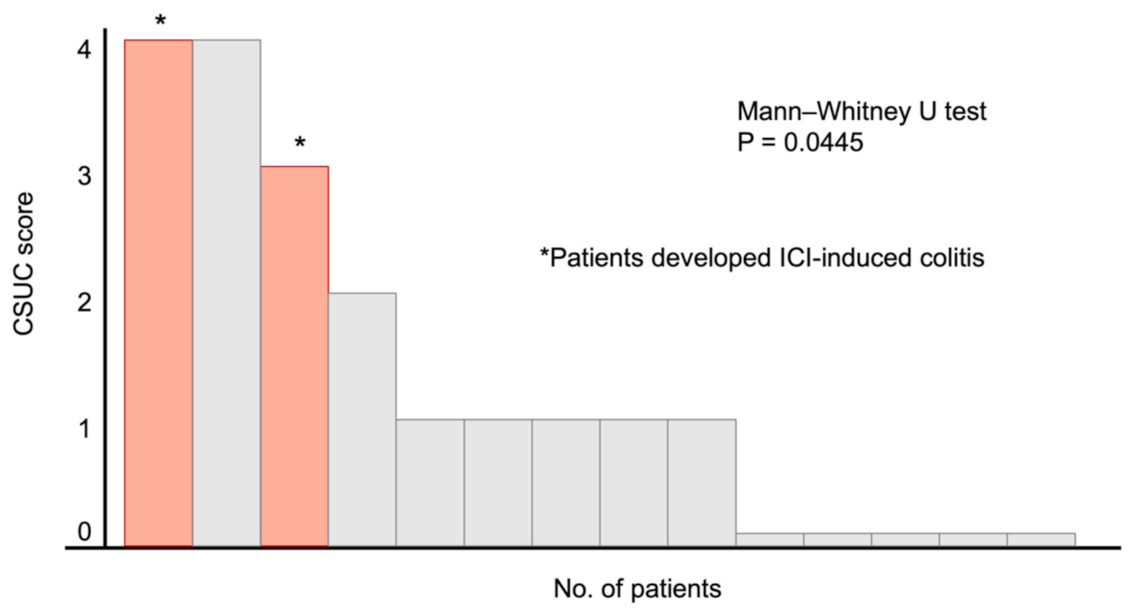
3.2. Positive Capsule Endoscopy Findings
3.3. Summary of the Two Cases Who Developed ICI-Induced Colitis
3.4. Survival
3.5. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crino, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.Y.; Chin, K.; Kadowaki, S.; Ahn, M.J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chavez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suarez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef]
- Iwama, S.; De Remigis, A.; Callahan, M.K.; Slovin, S.F.; Wolchok, J.D.; Caturegli, P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 2014, 6, 230ra245. [Google Scholar] [CrossRef]
- Luoma, A.M.; Suo, S.; Williams, H.L.; Sharova, T.; Sullivan, K.; Manos, M.; Bowling, P.; Hodi, F.S.; Rahma, O.; Sullivan, R.J.; et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 2020, 182, 655–671.e622. [Google Scholar] [CrossRef]
- Geukes Foppen, M.H.; Rozeman, E.A.; van Wilpe, S.; Postma, C.; Snaebjornsson, P.; van Thienen, J.V.; van Leerdam, M.E.; van den Heuvel, M.; Blank, C.U.; van Dieren, J.; et al. Immune checkpoint inhibition-related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open 2018, 3, e000278. [Google Scholar] [CrossRef]
- Gupta, A.; De Felice, K.M.; Loftus, E.V., Jr.; Khanna, S. Systematic review: Colitis associated with anti-CTLA-4 therapy. Aliment. Pharmacol. Ther. 2015, 42, 406–417. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Weber, J.S.; Dummer, R.; de Pril, V.; Lebbe, C.; Hodi, F.S.; Investigators, M.D.X. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: Detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013, 119, 1675–1682. [Google Scholar] [CrossRef]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; Committee, E.G. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Wang, Y.; Abu-Sbeih, H.; Mao, E.; Ali, N.; Ali, F.S.; Qiao, W.; Lum, P.; Raju, G.; Shuttlesworth, G.; Stroehlein, J.; et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: Retrospective review at MD Anderson. J. Immunother. Cancer 2018, 6, 37. [Google Scholar] [CrossRef]
- Zhang, M.L.; Neyaz, A.; Patil, D.; Chen, J.; Dougan, M.; Deshpande, V. Immune-related adverse events in the gastrointestinal tract: Diagnostic utility of upper gastrointestinal biopsies. Histopathology 2020, 76, 233–243. [Google Scholar] [CrossRef]
- Johncilla, M.; Grover, S.; Zhang, X.; Jain, D.; Srivastava, A. Morphological spectrum of immune check-point inhibitor therapy-associated gastritis. Histopathology 2020, 76, 531–539. [Google Scholar] [CrossRef]
- Ibraheim, H.; Baillie, S.; Samaan, M.A.; Abu-Sbeih, H.; Wang, Y.; Talley, N.J.; Jones, M.P.; Powell, N. Systematic review with meta-analysis: Effectiveness of anti-inflammatory therapy in immune checkpoint inhibitor-induced enterocolitis. Aliment. Pharmacol. Ther. 2020, 52, 1432–1452. [Google Scholar] [CrossRef]
- Bergqvist, V.; Hertervig, E.; Gedeon, P.; Kopljar, M.; Griph, H.; Kinhult, S.; Carneiro, A.; Marsal, J. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol. Immunother. 2017, 66, 581–592. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Shin, N.; Hoshino, T.; Kanai, T. Management of challenging immune-related gastrointestinal adverse events associated with immune checkpoint inhibitors. Future Oncol. 2018, 14, 3187–3198. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Soularue, E.; Lepage, P.; Colombel, J.F.; Coutzac, C.; Faleck, D.; Marthey, L.; Collins, M.; Chaput, N.; Robert, C.; Carbonnel, F. Enterocolitis due to immune checkpoint inhibitors: A systematic review. Gut 2018, 67, 2056–2067. [Google Scholar] [CrossRef]
- Wang, Y.; Abu-Sbeih, H.; Mao, E.; Ali, N.; Qiao, W.; Trinh, V.A.; Zobniw, C.; Johnson, D.H.; Samdani, R.; Lum, P.; et al. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm. Bowel Dis. 2018, 24, 1695–1705. [Google Scholar] [CrossRef]
- Hayashi, Y.; Hosoe, N.; Takabayashi, K.; Limpias Kamiya, K.J.L.; Tsugaru, K.; Shimozaki, K.; Hirata, K.; Fukuhara, K.; Fukuhara, S.; Mutaguchi, M.; et al. Clinical, Endoscopic, and Pathological Characteristics of Immune Checkpoint Inhibitor-Induced Gastroenterocolitis. Dig. Dis. Sci. 2020. [Google Scholar] [CrossRef]
- Marthey, L.; Mateus, C.; Mussini, C.; Nachury, M.; Nancey, S.; Grange, F.; Zallot, C.; Peyrin-Biroulet, L.; Rahier, J.F.; Bourdier de Beauregard, M.; et al. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J. Crohns Colitis 2016, 10, 395–401. [Google Scholar] [CrossRef]
- Otagiri, S.; Katsurada, T.; Yamanashi, K.; Sakurai, K.; Sato, M.T.; Sakakibara-Konishi, J.; Sakamoto, N. Immune checkpoint inhibitor-induced enteritis assessed using capsule endoscopy. JGH Open 2020, 4, 1231–1232. [Google Scholar] [CrossRef]
- Kroijer, R.; Kobaek-Larsen, M.; Qvist, N.; Knudsen, T.; Baatrup, G. Colon capsule endoscopy for colonic surveillance. Colorectal. Dis. 2019, 21, 532–537. [Google Scholar] [CrossRef]
- Spada, C.; Pasha, S.F.; Gross, S.A.; Leighton, J.A.; Schnoll-Sussman, F.; Correale, L.; Gonzalez Suarez, B.; Costamagna, G.; Hassan, C. Accuracy of First- and Second-Generation Colon Capsules in Endoscopic Detection of Colorectal Polyps: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1533–1543.e1538. [Google Scholar] [CrossRef]
- Spada, C.; Hassan, C.; Munoz-Navas, M.; Neuhaus, H.; Deviere, J.; Fockens, P.; Coron, E.; Gay, G.; Toth, E.; Riccioni, M.E.; et al. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest. Endosc. 2011, 74, 581–589.e581. [Google Scholar] [CrossRef]
- Pecere, S.; Senore, C.; Hassan, C.; Riggi, E.; Segnan, N.; Pennazio, M.; Sprujievnik, T.; Rondonotti, E.; Baccarin, A.; Quintero, E.; et al. Accuracy of colon capsule endoscopy for advanced neoplasia. Gastrointest. Endosc. 2020, 91, 406–414.e1. [Google Scholar] [CrossRef]
- Hausmann, J.; Schmelz, R.; Walldorf, J.; Filmann, N.; Zeuzem, S.; Albert, J.G. Pan-intestinal capsule endoscopy in patients with postoperative Crohn’s disease: A pilot study. Scand. J. Gastroenterol. 2017, 52, 840–845. [Google Scholar] [CrossRef]
- Boal Carvalho, P.; Rosa, B.; Dias de Castro, F.; Moreira, M.J.; Cotter, J. PillCam COLON 2 in Crohn’s disease: A new concept of pan-enteric mucosal healing assessment. World J. Gastroenterol. 2015, 21, 7233–7241. [Google Scholar] [CrossRef]
- Hosoe, N.; Nakano, M.; Takeuchi, K.; Endo, Y.; Matsuoka, K.; Abe, T.; Omori, T.; Hayashida, M.; Kobayashi, T.; Yoshida, A.; et al. Establishment of a Novel Scoring System for Colon Capsule Endoscopy to Assess the Severity of Ulcerative Colitis-Capsule Scoring of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 2641–2647. [Google Scholar] [CrossRef]
- Lichtiger, S.; Present, D.H.; Kornbluth, A.; Gelernt, I.; Bauer, J.; Galler, G.; Michelassi, F.; Hanauer, S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N. Engl. J. Med. 1994, 330, 1841–1845. [Google Scholar] [CrossRef]
- Sanders, D.; Webber, D.; Chatur, N. Enteritis with immune checkpoint inhibitor use. CMAJ 2019, 191, E1106. [Google Scholar] [CrossRef]
- Tandon, P.; Bourassa-Blanchette, S.; Bishay, K.; Parlow, S.; Laurie, S.A.; McCurdy, J.D. The Risk of Diarrhea and Colitis in Patients With Advanced Melanoma Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis. J. Immunother. 2018, 41, 101–108. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Del Vecchio, M.; Robert, C.; Mackiewicz, A.; Chiarion-Sileni, V.; Arance, A.; Lebbe, C.; Bastholt, L.; Hamid, O.; Rutkowski, P.; et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017, 18, 611–622. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Faleck, D.M.; Ricciuti, B.; Mendelsohn, R.B.; Naqash, A.R.; Cohen, J.V.; Sellers, M.C.; Balaji, A.; Ben-Betzalel, G.; Hajir, I.; et al. Immune Checkpoint Inhibitor Therapy in Patients With Preexisting Inflammatory Bowel Disease. J. Clin. Oncol. 2020, 38, 576–583. [Google Scholar] [CrossRef]
- Berman, D.; Parker, S.M.; Siegel, J.; Chasalow, S.D.; Weber, J.; Galbraith, S.; Targan, S.R.; Wang, H.L. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010, 10, 11. [Google Scholar]
- Matsubayashi, M.; Kobayashi, T.; Okabayashi, S.; Nakano, M.; Sagami, S.; Ozaki, R.; Kiyohara, H.; Morikubo, H.; Asonuma, K.; Miyatani, Y.; et al. Determining the usefulness of Capsule Scoring of Ulcerative Colitis in predicting relapse of inactive ulcerative colitis. J. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Kubo, K.; Kato, M.; Mabe, K. Nivolumab-Associated Colitis Mimicking Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2017, 15, A35–A36. [Google Scholar] [CrossRef][Green Version]
- Negreanu, L.; Babiuc, R.; Bengus, A.; Sadagurschi, R. PillCam Colon 2 capsule in patients unable or unwilling to undergo colonoscopy. World J. Gastrointest. Endosc. 2013, 5, 559–567. [Google Scholar] [CrossRef]
- Spada, C.; Hassan, C.; Barbaro, B.; Iafrate, F.; Cesaro, P.; Petruzziello, L.; Minelli Grazioli, L.; Senore, C.; Brizi, G.; Costamagna, I.; et al. Colon capsule versus CT colonography in patients with incomplete colonoscopy: A prospective, comparative trial. Gut 2015, 64, 272–281. [Google Scholar] [CrossRef]
- Cave, D.; Legnani, P.; de Franchis, R.; Lewis, B.S. ICCE consensus for capsule retention. Endoscopy 2005, 37, 1065–1067. [Google Scholar] [CrossRef]


| Total N = 23 | Positive Findings n = 14 | No Findings n = 9 | p-Value | ||
|---|---|---|---|---|---|
| Median age, range (years) | 67 (31–83) | 68 (31–83) | 66 (42–79) | 0.83 | |
| Male, no (%) | 21 (91%) | 14 (100%) | 7 (78%) | 0.044 * | |
| BMI, kg/m2, range | 19.6 (16–30.4) | 21.6 (17.6–30.4) | 17.9 (16–28.2) | 0.10 | |
| ECOG PS, no (%) | 0 | 8 (35%) | 6 (43%) | 2 (22%) | 0.30 |
| 1 | 15 (65%) | 8 (57%) | 7 (78%) | ||
| Primary tumor, no (%) | 0.28 | ||||
| Renal cell carcinoma | 12 (52%) | 8 (57%) | 4 (44%) | ||
| Gastric cancer | 6 (26%) | 4 (29%) | 2 (22%) | ||
| Esophageal cancer | 2 (9%) | 0 | 2 (22%) | ||
| Malignant melanoma | 2 (9%) | 1 (7%) | 1 (11%) | ||
| MSI-H solid tumor | 1 (4%) | 1 (7%) | 0 | ||
| Immune checkpoint inhibitors, no (%) | 0.32 | ||||
| Nivolumab | 20 (87%) | 11 (79%) | 9 (100%) | ||
| Pembrolizumab | 2 (9%) | 2 (14%) | 0 | ||
| Nivolumab plus ipilimumab | 1 (4%) | 1 (7%) | 0 | ||
| Median Lichtiger score, range | 0 (0–3) | 0 (0–1) | 0 (0–3) | 0.38 | |
| NSAIDs usage, no (%) | 6 (26%) | 3 (21%) | 3 (33%) | 0.37 | |
| PPI usage, no (%) | 8 (35%) | 5 (36%) | 3 (33%) | 0.90 | |
| Baseline laboratory findings | |||||
| WBC, range (/μL) | 6400 (3500–13,700) | 7000 (3500–13,700) | 6200 (4200–7300) | 0.20 | |
| Hb, range (g/dL) | 12.4 (7.7–15.1) | 12.6 (7.7–15.1) | 12.2 (8.8–14.5) | 0.70 | |
| CRP, range (mg/dL) | 0.25 (0.01–2.8) | 0.16 (0.01–2.8) | 0.1 (0.01–1.55) | 0.42 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimozaki, K.; Hirata, K.; Horie, S.; Chida, A.; Tsugaru, K.; Hayashi, Y.; Kawasaki, K.; Miyanaga, R.; Hayashi, H.; Mizuno, R.; et al. The Entire Intestinal Tract Surveillance Using Capsule Endoscopy after Immune Checkpoint Inhibitor Administration: A Prospective Observational Study. Diagnostics 2021, 11, 543. https://doi.org/10.3390/diagnostics11030543
Shimozaki K, Hirata K, Horie S, Chida A, Tsugaru K, Hayashi Y, Kawasaki K, Miyanaga R, Hayashi H, Mizuno R, et al. The Entire Intestinal Tract Surveillance Using Capsule Endoscopy after Immune Checkpoint Inhibitor Administration: A Prospective Observational Study. Diagnostics. 2021; 11(3):543. https://doi.org/10.3390/diagnostics11030543
Chicago/Turabian StyleShimozaki, Keitaro, Kenro Hirata, Sara Horie, Akihiko Chida, Kai Tsugaru, Yukie Hayashi, Kenta Kawasaki, Ryoichi Miyanaga, Hideyuki Hayashi, Ryuichi Mizuno, and et al. 2021. "The Entire Intestinal Tract Surveillance Using Capsule Endoscopy after Immune Checkpoint Inhibitor Administration: A Prospective Observational Study" Diagnostics 11, no. 3: 543. https://doi.org/10.3390/diagnostics11030543
APA StyleShimozaki, K., Hirata, K., Horie, S., Chida, A., Tsugaru, K., Hayashi, Y., Kawasaki, K., Miyanaga, R., Hayashi, H., Mizuno, R., Funakoshi, T., Hosoe, N., Hamamoto, Y., & Kanai, T. (2021). The Entire Intestinal Tract Surveillance Using Capsule Endoscopy after Immune Checkpoint Inhibitor Administration: A Prospective Observational Study. Diagnostics, 11(3), 543. https://doi.org/10.3390/diagnostics11030543






