LAMP in Neglected Tropical Diseases: A Focus on Parasites
Abstract
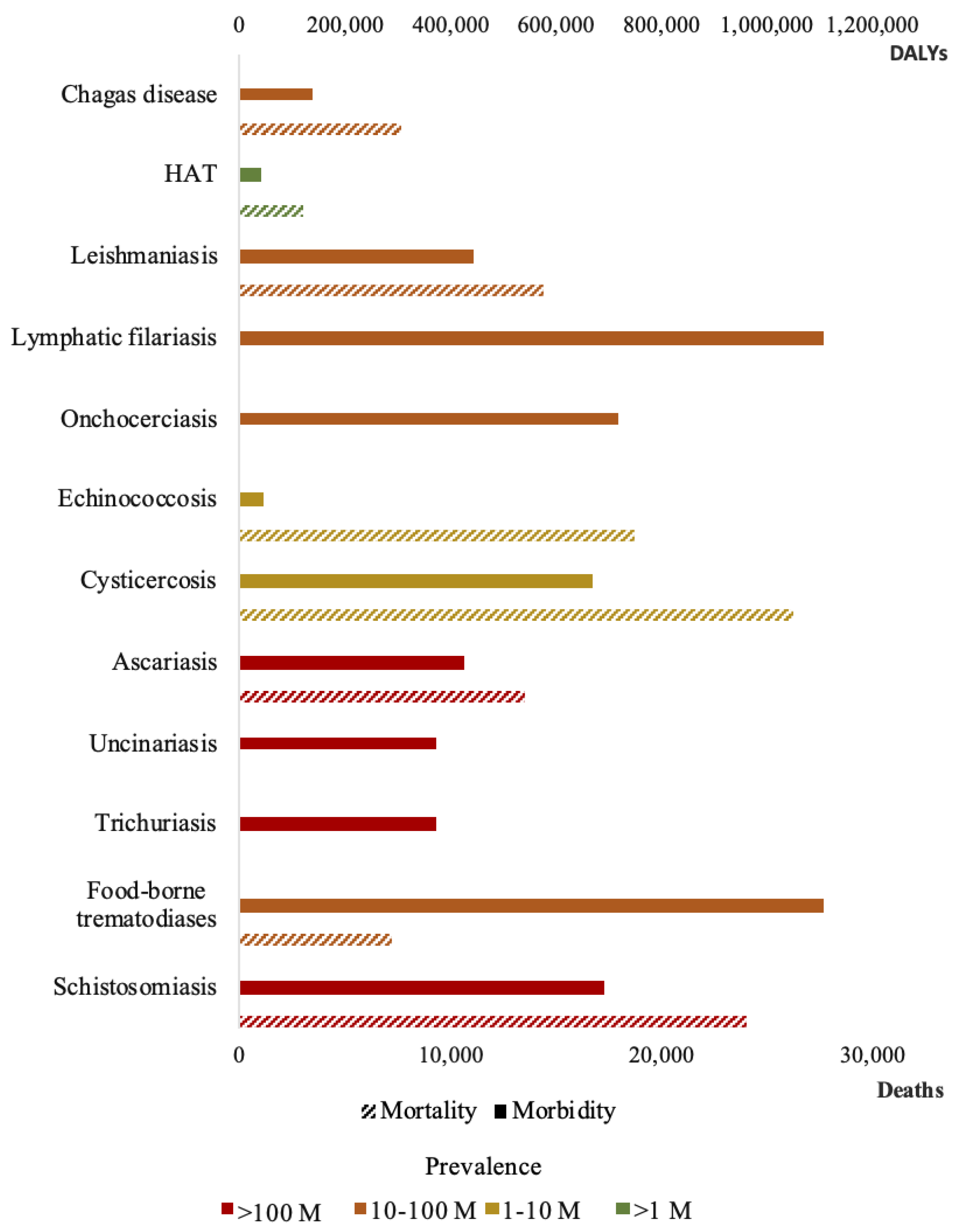
1. Neglected Tropical Diseases Caused by Parasites: The Diagnostic Limitation
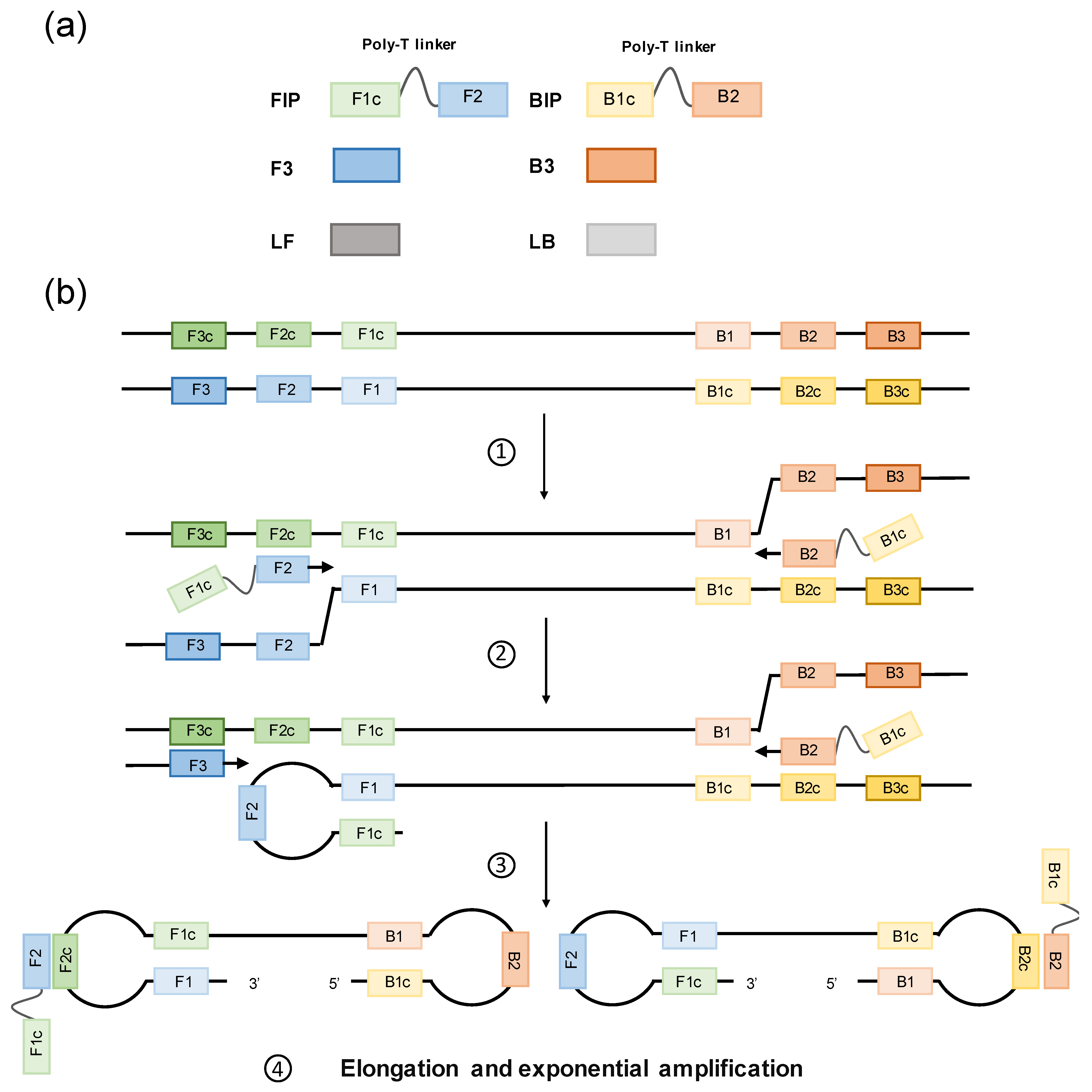
2. Loop-Mediated Isothermal Amplification
3. LAMP Development in Parasite-Caused NTDs
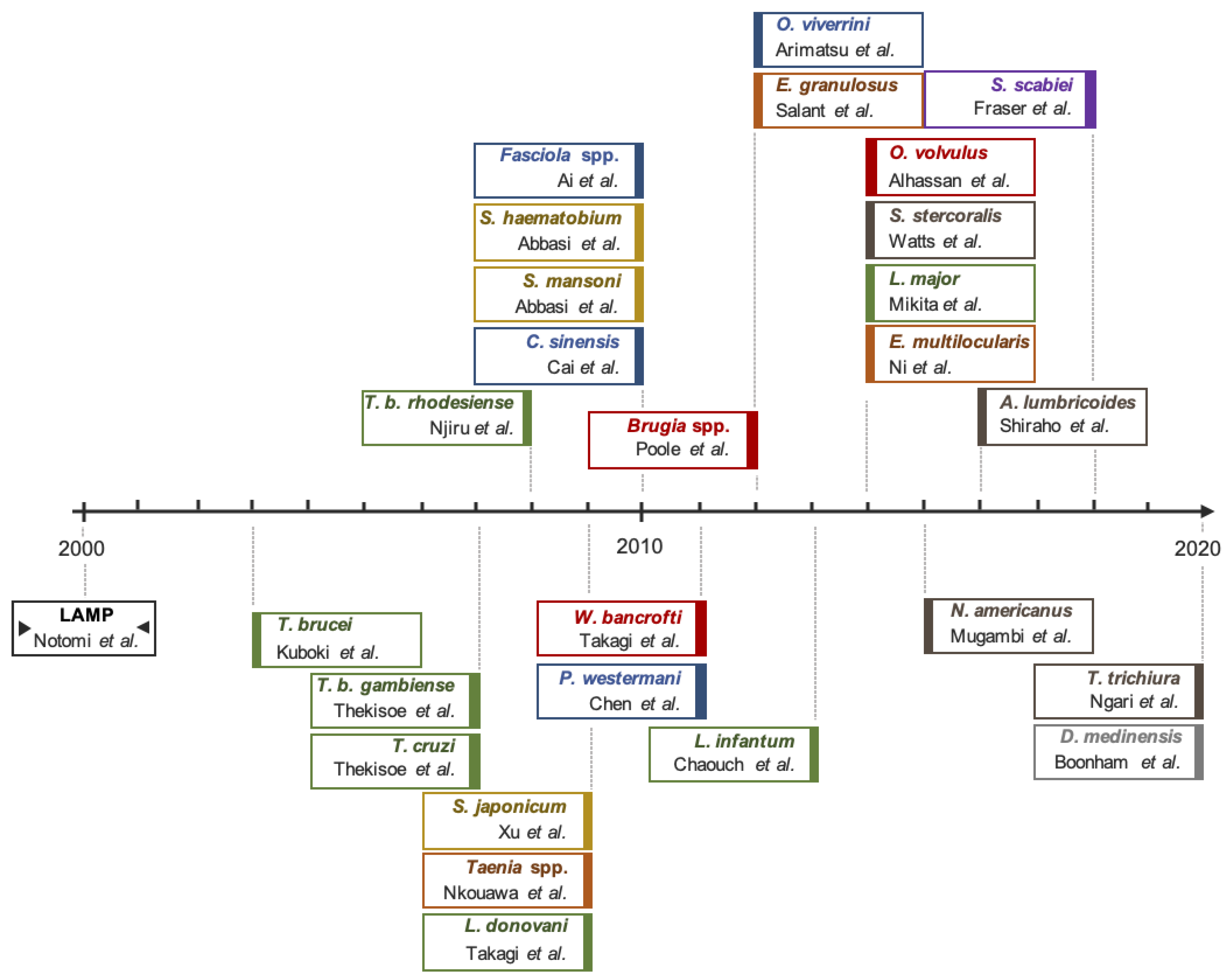
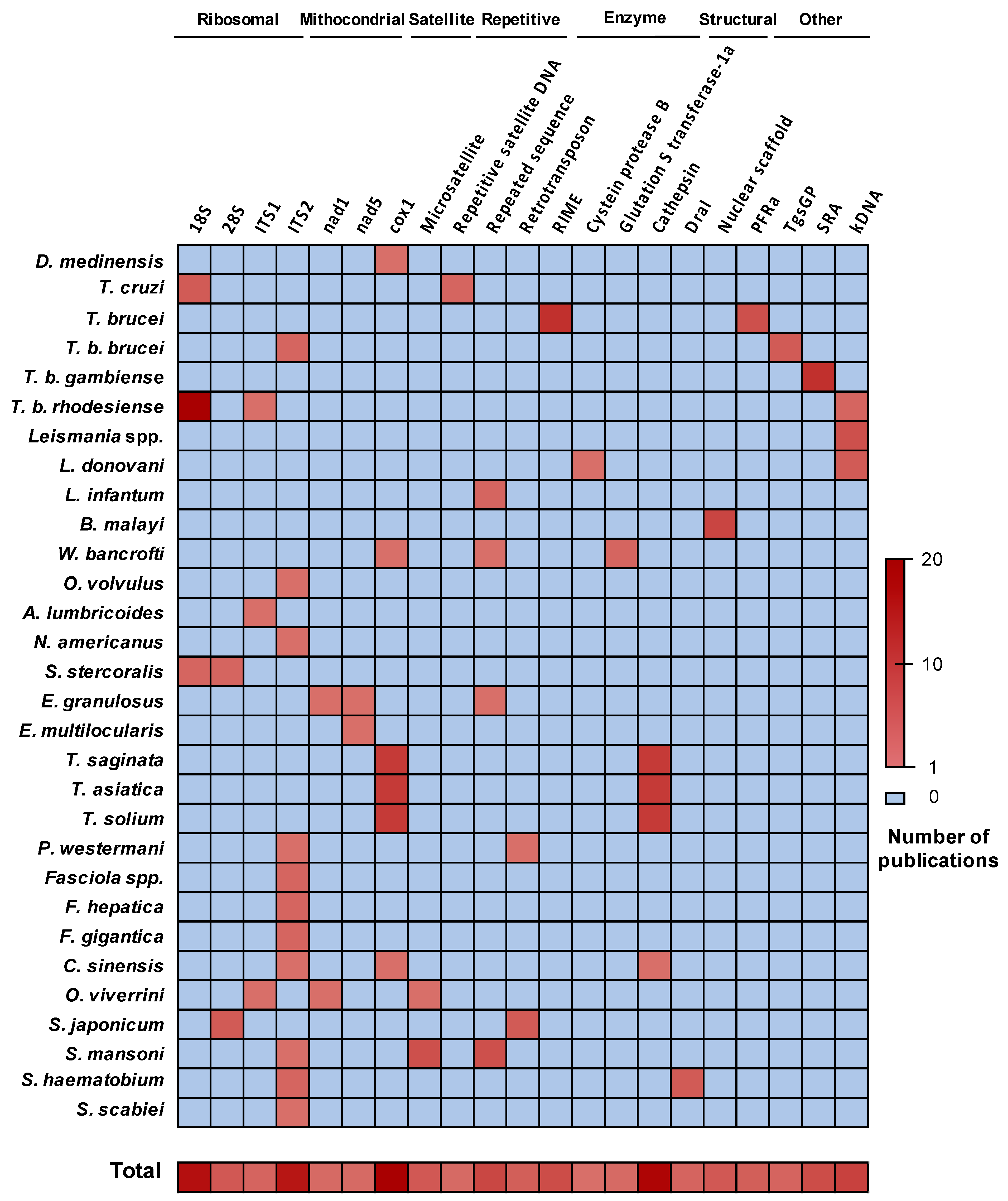
3.1. Genomic Target Selection and LAMP Optimization
3.2. LAMP in Molecular Xenomonitoring
3.3. LAMP in Experimental Infections
3.4. LAMP in Clinical Studies
3.5. LAMP in Post-Therapy Monitoring
| Disease | Application 1 | Specimen 2,3 | Clinical Studies | Key Points | |||||
|---|---|---|---|---|---|---|---|---|---|
| VE | AM | HS | PT | n 4 | Sensitivity | Specificity | |||
| Dracunculiasis | ✓ | ✓ | ✓ | ✕ | N/A [48] | N/A | N/A | N/A | Test applied in adult worms recovered from humans, not in human specimens. |
| Chagas | ✓ | ✓ | ✓ | ✕ | Blood [83,84,85] | 27 [83] 33 [84] 46 [85] | 100% 73.9% 93% | 100% 100% 100% | Accurate diagnosis in one test, regardless the clinical situation of the patient. |
| HAT | ✓ | ✓ | ✓ | ✕ | Blood [25,66,68,86] Buffy coat [87] CSF [87] Bone marrow [87] Sera [57] Saliva [57] Urine [57] | 128 [66] 355 [86] 181 [68] | 95.3–93.8% 87.3–93% 76.9% | N/T 92.8–96.4% 100% | Non-invasive samples such us saliva and urine useful substitutes of highly invasive CSF or bone marrow. Highly sensitive technique, fitting for the last stages of HAT control and elimination. |
| Leishmaniasis | Blood [70,71,72,73,74] Buffy coat [71] Saliva [75] Bone marrow [72] Skin [70,71,72] | One test can diagnose all presentations of leishmaniasis, in blood for VL and skin biopsies for CL or PKDL. However, invasive samples are still needed. Saliva might be a good alternative, but further studies are required. | |||||||
| VL | ✓ | ✓ | ✓ | ✕ | 186 [73] | 97.6–100% | 99.1% | ||
| 55 [72] | 96.4% | 98.5% | |||||||
| 30 [70] | 83% | 100% | |||||||
| 50 [71] | 92.3% | 100% | |||||||
| 267 [74] | 98.3% | 96.6% | |||||||
| CL | ✓ | ✓ | ✓ | ✕ | 43 [70] | 98% | 100% | ||
| 105 [71] | 95% | 86% | |||||||
| PKDL | ✓ | ✓ | ✓ | ✕ | 62 [72] | 96.2% | 98.5% | ||
| Lymphatic filariasis | ✓ | ✕ | ✓ | ✕ | Blood [34] | N/A | N/A | N/A | Valuable for molecular xenomonitoring in low-prevalence areas and epidemiological control post-MDA 5. |
| Onchocerciasis | ✓ | ✕ | ✓ | ✕ | Skin [88,89] | 70 [88] 146 [89] | 65.7% 88.2% | N/T 99.2% | |
| Trichuriasis | ✕ | ✓ | ✓ | ✕ | Stool [47,90] Urine [90] | 137 [47] | 77% | 88% | Urine might be a viable alternative to stool in epidemiological studies, but further evidence is needed. Ancylostoma duodenale does not have a specific LAMP designed yet. |
| Ascariasis | ✕ | ✕ | ✓ | ✕ | Stool [45] | 40 [45] | 96.3% | 61.5% | |
| Uncinariasis | ✕ | ✕ | ✓ | ✕ | Stool [44] | 106 [44] | 97% | 100% | |
| Strongyloidiasis | ✕ | ✓ | ✓ | ✕ | Stool [41,91,92] Serum [91] Broncho alveolar [91] Urine [92,93] | 28 [41] 396 [91] | 96.4% 77.4% | N/T 100% | |
| Echinococcosis | ✕ | ✓ | ✓ | ✕ | Stool [37,43,58] Hydatid cysts [94] | N/A | N/A | N/A | Good enough performance to avoid resource-demanding imaging techniques. Promising results in early infection detection, key in these diseases prognosis. |
| Taeniasis | ✕ | ✕ | ✓ | ✕ | Stool [95,96] Blood [79] | 43 [95] 100 [79] | 86% 74% | 100% 90.2% | |
| Paragonimiasis | ✓ | ✓ | ✓ | ✕ | Blood [59] Sputum [35] Pleural fluid [35] | N/A | N/A | N/A | Larger studies with human clinical samples are required. Highly variable analytical sensitivity and specificity results. |
| Fascioliasis | ✕ | ✓ | ✓ | ✕ | Stool [97] | N/A | N/A | N/A | |
| Clonorchiasis | ✓ | ✕ | ✓ | ✕ | Stool [80] | 120 [80] | 97.1% | 100% | |
| Opistorchiasis | ✓ | ✕ | ✓ | ✕ | Stool [36] | 50 [36] | 100% | 61.5% | |
| Schistosomiasis | Plasma [60] Serum [28,60,64] Urine [61,81,98,99] Stool [28,55,62,63] Blood [65] | 50 [28] 110 [64] 94 [98] 172 [81] 162 [55] 86 [99] 383 [63] | 96.7% 95.5% 100% 86.2% 92.9% 100% 97% | 100% 100% 86.7% N/T 80.1 100% 100% | Consistently shows similar or better performance than the other available diagnostic tools. Sufficient evidence in large clinical studies to start its implementation in public health of endemic and non-endemic regions | ||||
| S. japonicum | ✓ | ✓ | ✓ | ✓ | |||||
| S. haematobium | ✓ | ✓ | ✓ | ✕ | |||||
| S. mansoni | ✓ | ✓ | ✓ | ✕ | |||||
| Scabies | ✕ | ✓ | ✕ | ✕ | Skin [46] | N/A | N/A | N/A | - |
4. LAMP as Point-of-Care Test
4.1. Real-Time Connectivity
4.2. Ease of Specimen Collection
4.3. Affordable
4.4. User-Friendliness
4.5. Rapid and Robust
4.6. Equipment Free
4.7. Deliverable to End-Users
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Molyneux, D.H.; Hotez, P.J.; Fenwick, A. “Rapid-Impact Interventions”: How a Policy of Integrated Control for Africa ’ s Neglected Tropical Diseases Could Benefi t the Poor. PLoS Med. 2005, 2, e0020336. [Google Scholar] [CrossRef]
- Hotez, P.J.; Aksoy, S.; Brindley, P.J.; Kamhawi, S. World neglected tropical diseases day. PLoS Negl. Trop. Dis. 2020, 14, e0007999. [Google Scholar] [CrossRef]
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 1859–1922. [Google Scholar] [CrossRef]
- Engels, D.; Zhou, X.N. Neglected tropical diseases: An effective global response to local poverty-related disease priorities. Infect. Dis. Poverty 2020, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Bertino, A.M. Asymmetries of poverty: Why global burden of disease valuations underestimate the burden of neglected tropical diseases. PLoS Negl. Trop. Dis. 2008, 2, e209. [Google Scholar] [CrossRef]
- WHO Global Health Estimates (GHE). Available online: https://www.who.int/healthinfo/global_burden_disease/en/ (accessed on 21 January 2020).
- Hotez, P.; Aksoy, S. PLOS Neglected Tropical Diseases: Ten years of progress in neglected tropical disease control and elimination … More or less. PLoS Negl. Trop. Dis. 2017, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- WHO. Ending the Neglect to Attain the Sustainable Development Goals a Road Map for Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Hotez, P.J.; Pecoul, B.; Rijal, S.; Boehme, C.; Aksoy, S.; Malecela, M.; Tapia-Conyer, R.; Reeder, J.C. Eliminating the Neglected Tropical Diseases: Translational Science and New Technologies. PLoS Negl. Trop. Dis. 2016, 10, e0003895. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, F.; Li, Q.; Wang, L.; Fan, C. Isothermal Amplification of Nucleic Acids. Chem. Rev. 2015, 115, 12491–12545. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Wong, Y.P.; Othman, S.; Lau, Y.L.; Radu, S.; Chee, H.Y. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018, 124, 626–643. [Google Scholar] [CrossRef]
- Becherer, L.; Borst, N.; Bakheit, M.; Frischmann, S.; Zengerle, R.; Stetten, F. Von Loop-mediated isothermal amplification (LAMP)—Review and classification of methods for sequence-specific detection. Anal. Methods 2020, 12, 717–746. [Google Scholar] [CrossRef]
- Fu, S.; Qu, G.; Guo, S.; Ma, L.; Zhang, N.; Zhang, S.; Gao, S.; Shen, Z. Applications of loop-mediated isothermal DNA amplification. Appl. Biochem. Biotechnol. 2011, 163, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, P.; Zhou, S.; Zhang, L. Loop-mediated isothermal amplification (LAMP): A novel rapid detection platform for pathogens. Microb. Pathog. 2017, 107, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Y.; Fohlerova, Z.; Chang, H.; Iliescu, C. LAMP-on-a-chip: Revising microfluidic platforms for loop-mediated DNA amplification. TrAC Trends Anal. Chem. 2019, 113, 44–53. [Google Scholar] [CrossRef]
- Shang, Y.; Sun, J.; Ye, Y.; Zhang, J.; Zhang, Y.; Sun, X. Loop-mediated isothermal amplification-based microfluidic chip for pathogen detection. Crit. Rev. Food Sci. Nutr. 2020, 60, 201–224. [Google Scholar] [CrossRef]
- Ye, X.; Fang, X.; Li, X.; Kong, J. Gold nanoparticle-mediated nucleic acid isothermal amplification with enhanced specificity. Anal. Chim. Acta 2018, 1043, 150–157. [Google Scholar] [CrossRef]
- Ong, D.S.Y.; Poljak, M. Smartphones as mobile microbiological laboratories. Clin. Microbiol. Infect. 2020, 26, 421–424. [Google Scholar] [CrossRef]
- Garafutdinov, R.R.; Gilvanov, A.R.; Sakhabutdinova, A.R. The Influence of Reaction Conditions on DNA Multimerization During Isothermal Amplification with Bst exo− DNA Polymerase. Appl. Biochem. Biotechnol. 2020, 190, 758–771. [Google Scholar] [CrossRef]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef]
- Kuboki, N.; Inoue, N.; Sakurai, T.; Di Cello, F.; Grab, D.J.; Suzuki, H.; Sugimoto, C.; Igarashi, I. Loop-mediated isothermal amplification for detection of African trypanosomes. J. Clin. Microbiol. 2003, 41, 5517–5524. [Google Scholar] [CrossRef] [PubMed]
- Thekisoe, O.M.M.; Kuboki, N.; Nambota, A.; Fujisaki, K.; Sugimoto, C.; Igarashi, I.; Yasuda, J.; Inoue, N. Species-specific loop-mediated isothermal amplification (LAMP) for diagnosis of trypanosomosis. Acta Trop. 2007, 102, 182–189. [Google Scholar] [CrossRef]
- Njiru, Z.K.; Mikosza, A.S.J.; Armstrong, T.; Enyaru, J.C.; Ndung’u, J.M.; Thompson, A.R.C. Loop-mediated isothermal amplification (LAMP) method for rapid detection of Trypanosoma brucei rhodesiense. PLoS Negl. Trop. Dis. 2008, 2, e147. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Rong, R.; Zhang, H.Q.; Shi, C.J.; Zhu, X.Q.; Xia, C.M. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP). Int. J. Parasitol. 2010, 40, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Nkouawa, A.; Sako, Y.; Nakao, M.; Nakaya, K.; Ito, A. Loop-mediated isothermal amplification method for differentiation and rapid detection of Taenia species. J. Clin. Microbiol. 2009, 47, 168–174. [Google Scholar] [CrossRef]
- Ai, L.; Li, C.; Elsheikha, H.M.; Hong, S.J.; Chen, J.X.; Chen, S.H.; Li, X.; Cai, X.Q.; Chen, M.X.; Zhu, X.Q. Rapid identification and differentiation of Fasciola hepatica and Fasciola gigantica by a loop-mediated isothermal amplification (LAMP) assay. Vet. Parasitol. 2010, 174, 228–233. [Google Scholar] [CrossRef]
- Abbasi, I.; King, C.H.; Muchiri, E.M.; Hamburger, J. Detection of Schistosoma mansoni and Schistosoma haematobium DNA by loop-mediated isothermal amplification: Identification of infected snails from early prepatency. Am. J. Trop. Med. Hyg. 2010, 83, 427–432. [Google Scholar] [CrossRef]
- Cai, X.Q.; Xu, M.J.; Wang, Y.H.; Qiu, D.Y.; Liu, G.X.; Lin, A.; Tang, J.D.; Zhang, R.L.; Zhu, X.Q. Sensitive and rapid detection of Clonorchis sinensis infection in fish by loop-mediated isothermal amplification (LAMP). Parasitol. Res. 2010, 106, 1379–1383. [Google Scholar] [CrossRef]
- Takagi, H.; Itoh, M.; Islam, M.Z.; Razzaque, A.; Ekram, A.R.M.S.; Hashighuchi, Y.; Noiri, E.; Kimura, E. Sensitive, specific, and rapid detection of Leishmania donovani DNA by loop-mediated isothermal amplification. Am. J. Trop. Med. Hyg. 2009, 81, 578–582. [Google Scholar] [CrossRef]
- Takagi, H.; Itoh, M.; Kasai, S.; Yahathugoda, T.C.; Weerasooriya, M.V.; Kimura, E. Development of loop-mediated isothermal amplification method for detecting Wuchereria bancrofti DNA in human blood and vector mosquitoes. Parasitol. Int. 2011, 60, 493–497. [Google Scholar] [CrossRef]
- Chen, M.X.; Ai, L.; Zhang, R.L.; Xia, J.J.; Wang, K.; Chen, S.H.; Zhang, Y.N.; Xu, M.J.; Li, X.; Zhu, X.Q.; et al. Sensitive and rapid detection of Paragonimus westermani infection in humans and animals by loop-mediated isothermal amplification (LAMP). Parasitol. Res. 2011, 108, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Arimatsu, Y.; Kaewkes, S.; Laha, T.; Hong, S.J.; Sripa, B. Rapid detection of Opisthorchis viverrini copro-DNA using loop-mediated isothermal amplification (LAMP). Parasitol. Int. 2012, 61, 178–182. [Google Scholar] [CrossRef]
- Salant, H.; Abbasi, I.; Hamburger, J. The development of a loop-mediated isothermal amplification method (LAMP) for Echinococcus granulosis coprodetection. Am. J. Trop. Med. Hyg. 2012, 87, 883–887. [Google Scholar] [CrossRef]
- Poole, C.B.; Tanner, N.A.; Zhang, Y.; Evans, T.C.; Carlow, C.K.S. Diagnosis of Brugian Filariasis by Loop-Mediated Isothermal Amplification. PLoS Negl. Trop. Dis. 2012, 6, e1948. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, M.; Mhadhbi, M.; Adams, E.R.; Schoone, G.J.; Limam, S.; Gharbi, Z.; Darghouth, M.A.; Guizani, I.; BenAbderrazak, S. Development and evaluation of a loop-mediated isothermal amplification assay for rapid detection of Leishmania infantum in canine leishmaniasis based on cysteine protease B genes. Vet. Parasitol. 2013, 198, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, A.; Makepeace, B.L.; Lacourse, E.J.; Osei-Atweneboana, M.Y.; Carlow, C.K.S. A simple isothermal DNA amplification method to screen black flies for Onchocerca volvulus infection. PLoS ONE 2014, 9, e108927. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.R.; James, G.; Sultana, Y.; Ginn, A.N.; Outhred, A.C.; Kong, F.; Verweij, J.J.; Iredell, J.R.; Chen, S.C.A.; Lee, R. A loop-mediated isothermal amplification (LAMP) assay for Strongyloides stercoralis in stool that uses a visual detection method with SYTO-82 fluorescent dye. Am. J. Trop. Med. Hyg. 2014, 90, 306–311. [Google Scholar] [CrossRef]
- Mikita, K.; Maeda, T.; Yoshikawa, S.; Ono, T.; Miyahira, Y.; Kawana, A. The Direct Boil-LAMP method: A simple and rapid diagnostic method for cutaneous leishmaniasis. Parasitol. Int. 2014, 63, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; McManus, D.P.; Yan, H.; Yang, J.; Lou, Z.; Li, H.; Li, L.; Lei, M.; Cai, J.; Fan, Y.; et al. Loop-Mediated Isothermal Amplification (LAMP) assay for the identification of Echinococcus multilocularis infections in canine definitive hosts. Parasites Vectors 2014, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mugambi, R.M.; Agola, E.L.; Mwangi, I.N.; Kinyua, J.; Shiraho, E.A.; Mkoji, G.M. Development and evaluation of a Loop Mediated Isothermal Amplification (LAMP) technique for the detection of hookworm (Necator americanus) infection in fecal samples. Parasites Vectors 2015, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shiraho, E.A.; Eric, A.L.; Mwangi, I.N.; Maina, G.M.; Kinuthia, J.M.; Mutuku, M.W.; Mugambi, R.M.; Mwandi, J.M.; Mkoji, G.M. Development of a Loop Mediated Isothermal Amplification for Diagnosis of Ascaris lumbricoides in Fecal Samples. J. Parasitol. Res. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fraser, T.A.; Carver, S.; Martin, A.M.; Mounsey, K.; Polkinghorne, A.; Jelocnik, M. A Sarcoptes scabiei specific isothermal amplification assay for detection of this important ectoparasite of wombats and other animals. PeerJ 2018, 6, e5291. [Google Scholar] [CrossRef]
- Ngari, M.G.; Mwangi, I.N.; Njoroge, M.P.; Kinyua, J.; Osuna, F.A.; Kimeu, B.M.; Okanya, P.W.; Agola, E.L. Development and evaluation of a loop-mediated isothermal amplification (LAMP) diagnostic test for detection of whipworm, Trichuris trichiura, in faecal samples. J. Helminthol. 2020, 94, 1–7. [Google Scholar] [CrossRef]
- Boonham, N.; Tomlinson, J.; Ostoja-Starzewska, S.; McDonald, R.A. A pond-side test for Guinea worm: Development of a loop-mediated isothermal amplification (LAMP) assay for detection of Dracunculus medinensis. Exp. Parasitol. 2020, 217, 107960. [Google Scholar] [CrossRef]
- Kouassi, B.L.; De Souza, D.K.; Goepogui, A.; Narh, C.A.; King, S.A.; Mamadou, B.S.; Diakité, L.; Dadzie, S.K.; Boakye, D.A.; Utzinger, J.; et al. Assessing the presence of Wuchereria bancrofti in vector and human populations from urban communities in Conakry, Guinea. Parasites Vectors 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Pam, D.D.; de Souza, D.K.; D’Souza, S.; Opoku, M.; Sanda, S.; Nazaradden, I.; Anagbogu, I.N.; Okoronkwo, C.; Davies, E.; Elhassan, E.; et al. Is mass drug administration against lymphatic filariasis required in urban settings? The experience in Kano, Nigeria. PLoS Negl. Trop. Dis. 2017, 11, 1–15. [Google Scholar] [CrossRef]
- Gandasegui, J.; Fernández-Soto, P.; Hernández-Goenaga, J.; López-Abán, J.; Vicente, B.; Muro, A. Biompha-LAMP: A New Rapid Loop-Mediated Isothermal Amplification Assay for Detecting Schistosoma mansoni in Biomphalaria glabrata Snail Host. PLoS Negl. Trop. Dis. 2016, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, R.L.; Jannotti-Passos, L.K.; Dos Santos Carvalho, O. Use of Molecular Methods for the Rapid Mass Detection of Schistosoma mansoni (Platyhelminthes: Trematoda) in Biomphalaria spp. (Gastropoda: Planorbidae). J. Trop. Med. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.B.; Chen, R.; Zhang, Y.; Yang, G.J.; Kumagai, T.; Furushima-Shimogawara, R.; Lou, D.; Yang, K.; Wen, L.Y.; Lu, S.H.; et al. A new surveillance and response tool: Risk map of infected Oncomelania hupensis detected by Loop-mediated isothermal amplification (LAMP) from pooled samples. Acta Trop. 2015, 141, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, J.; Abbasi, I.; Kariuki, C.; Wanjala, A.; Mzungu, E.; Mungai, P.; Muchiri, E.; King, C.H. Evaluation of loop-mediated isothermal amplification suitable for molecular monitoring of schistosome-infected snails in field laboratories. Am. J. Trop. Med. Hyg. 2013, 88, 344–351. [Google Scholar] [CrossRef]
- Gandasegui, J.; Fernandez-Soto, P.; Muro, A.; Simoes Barbosa, C.; Lopes de Melo, F.; Loyo, R.; de Souza Gomes, E. A field survey using LAMP assay for detection of Schistosoma mansoni in a low-transmission area of schistosomiasis in Umbuzeiro, Brazil: Assessment in human and snail samples. PLoS Negl. Trop. Dis. 2018, 12, e0006314. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.-Q.; Xu, J.; Feng, T.; Lv, S.; Qian, Y.-J.; Zhang, L.-J.; Li, Y.-L.; Lv, C.; Bergquist, R.; Li, S.-Z.; et al. Field Evaluation of a Loop-Mediated Isothermal Amplification (LAMP) Platform for the Detection of Schistosoma japonicum Infection in Oncomelania hupensis Snails. Trop. Med. Infect. Dis. 2018, 3, 124. [Google Scholar] [CrossRef]
- Ngotho, M.; Kagira, J.M.; Gachie, B.M.; Karanja, S.M.; Waema, M.W.; Maranga, D.N.; Maina, N.W. Loop Mediated Isothermal Amplification for Detection of Trypanosoma brucei gambiense in Urine and Saliva Samples in Nonhuman Primate Model. Biomed. Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Ni, X.W.; McManus, D.P.; Lou, Z.Z.; Yang, J.F.; Yan, H.B.; Li, L.; Li, H.M.; Liu, Q.Y.; Li, C.H.; Shi, W.G.; et al. A comparison of loop-mediated isothermal amplification (LAMP) with other surveillance tools for Echinococcus granulosus diagnosis in canine definitive hosts. PLoS ONE 2014, 9, e0100877. [Google Scholar] [CrossRef] [PubMed]
- Xunhui, Z.; Qingming, K.; Qunbo, T.; Haojie, D.; Lesheng, Z.; Di, L.; Jianzu, D.; Bin, Z.; Rui, C.; Tianping, W.; et al. DNA detection of Paragonimus westermani: Diagnostic validity of a new assay based on loop-mediated isothermal amplification (LAMP) combined with a lateral flow dipstick. Acta Trop. 2019, 200, 105185. [Google Scholar] [CrossRef]
- Song, J.; Liu, C.; Bais, S.; Mauk, M.G.; Bau, H.H.; Greenberg, R.M. Molecular Detection of Schistosome Infections with a Disposable Microfluidic Cassette. PLoS Negl. Trop. Dis. 2015, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Soto, P.; Gandasegui, J.; Rodríguez, C.C.; Pérez-Arellano, J.L.; Crego-Vicente, B.; Diego, J.G.B.; López-Abán, J.; Vicente, B.; Muro, A. Detection of Schistosoma mansoni-derived DNA in human urine samples by loop-mediated isothermal amplification (LAMP). PLoS ONE 2019, 14, e0214125. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Gandasegui Arahuetes, J.; Sánchez Hernández, A.; López Abán, J.; Vicente Santiago, B.; Muro, A. A Loop-Mediated Isothermal Amplification (LAMP) Assay for Early Detection of Schistosoma mansoni in Stool Samples: A Diagnostic Approach in a Murine Model. PLoS Negl. Trop. Dis. 2014, 8, e3126. [Google Scholar] [CrossRef]
- Mwangi, I.N.; Agola, E.L.; Mugambi, R.M.; Shiraho, E.A.; Mkoji, G.M. Development and Evaluation of a Loop-Mediated Isothermal Amplification Assay for Diagnosis of Schistosoma mansoni Infection in Faecal Samples. J. Parasitol. Res. 2018, 2018, 1267826. [Google Scholar] [CrossRef]
- Xu, J.; Guan, Z.X.; Zhao, B.; Wang, Y.Y.; Cao, Y.; Zhang, H.Q.; Zhu, X.Q.; He, Y.K.; Xia, C.M. DNA Detection of Schistosoma japonicum: Diagnostic Validity of a LAMP Assay for Low-Intensity Infection and Effects of Chemotherapy in Humans. PLoS Negl. Trop. Dis. 2015, 9, e3668. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Yin, X.; Hua, W.; Hou, M.; Ji, M.; Yu, C.; Wu, G. Application of DNA-based diagnostics in detection of schistosomal DNA in early infection and after drug treatment. Parasites Vectors 2011, 4, 164. [Google Scholar] [CrossRef]
- Matovu, E.; Kuepfer, I.; Boobo, A.; Kibona, S.; Burri, C. Comparative detection of trypanosomal DNA by loop-mediated isothermal amplification and PCR from flinders technology associates cards spotted with patient blood. J. Clin. Microbiol. 2010, 48, 2087–2090. [Google Scholar] [CrossRef] [PubMed]
- Njiru, Z.K.; Mikosza, A.S.J.; Matovu, E.; Enyaru, J.C.K.; Ouma, J.O.; Kibona, S.N.; Thompson, R.C.A.; Ndung’u, J.M. African trypanosomiasis: Sensitive and rapid detection of the sub-genus Trypanozoon by loop-mediated isothermal amplification (LAMP) of parasite DNA. Int. J. Parasitol. 2008, 38, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Mugasa, C.M.; Katiti, D.; Boobo, A.; Lubega, G.W.; Schallig, H.D.F.H.; Matovu, E. Comparison of nucleic acid sequence-based amplification and loop-mediated isothermal amplification for diagnosis of human African trypanosomiasis. Diagn. Microbiol. Infect. Dis. 2014, 78, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Mugasa, C.M.; Schoone, G.J.; Ekangu, R.A.; Lubega, G.W.; Kager, P.A.; Schallig, H.D.F.H. Detection of Trypanosoma brucei parasites in blood samples using real-time nucleic acid sequence-based amplification. Diagn. Microbiol. Infect. Dis. 2008, 61, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.R.; Schoone, G.J.; Ageed, A.F.; El Safi, S.; Schallig, H.D.F.H. Development of a reverse transcriptase loop-mediated isothermal amplification (LAMP) assay for the sensitive detection of Leishmania parasites in clinical samples. Am. J. Trop. Med. Hyg. 2010, 82, 591–596. [Google Scholar] [CrossRef]
- Adams, E.R.; Schoone, G.; Versteeg, I.; Gomez, M.A.; Diro, E.; Mori, Y.; Perlee, D.; Downing, T.; Saravia, N.; Assaye, A.; et al. Development and evaluation of a novel LAMP assay for the diagnosis of Cutaneous and Visceral Leishmaniasis. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef]
- Verma, S.; Avishek, K.; Sharma, V.; Negi, N.S.; Ramesh, V.; Salotra, P. Application of loop-mediated isothermal amplification assay for the sensitive and rapid diagnosis of visceral leishmaniasis and post-kala-azar dermal leishmaniasis. Diagn. Microbiol. Infect. Dis. 2013, 75, 390–395. [Google Scholar] [CrossRef]
- Mukhtar, M.; Ali, S.S.; Boshara, S.A.; Albertini, A.; Monnerat, S.; Bessell, P.; Mori, Y.; Kubota, Y.; Ndung’u, J.M.; Cruz, I. Sensitive and less invasive confirmatory diagnosis of visceral leishmaniasis in Sudan using loop-mediated isothermal amplification (LAMP). PLoS Negl. Trop. Dis. 2018, 12, e6264. [Google Scholar] [CrossRef]
- Dixit, K.K.; Verma, S.; Singh, O.P.; Singh, D.; Singh, A.P.; Gupta, R.; Negi, N.S.; Das, P.; Sundar, S.; Singh, R.; et al. Validation of SYBR green I based closed tube loop mediated isothermal amplification (LAMP) assay and simplified direct-blood-lysis (DBL)-LAMP assay for diagnosis of visceral leishmaniasis (VL). PLoS Negl. Trop. Dis. 2018, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sriworarat, C.; Phumee, A.; Mungthin, M.; Leelayoova, S.; Siriyasatien, P. Development of loop-mediated isothermal amplification (LAMP) for simple detection of Leishmania infection. Parasites Vectors 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Nzelu, C.O.; Kato, H.; Peters, N.C. Loop-mediated isothermal amplification (LAMP): An advanced molecular point-of-care technique for the detection of Leishmania infection. PLoS Negl. Trop. Dis. 2019, 13, e7698. [Google Scholar] [CrossRef] [PubMed]
- Silva Nunes Bezerra, G.; Barbosa Júnior, W.L.; Virgínia Batista Vieira, A.; Xavier, A.T.; Sebastião Da Costa Lima Júnior, M.; Maria Xavier, E.; Da Silva, E.D.; Cintra Leal, N.; Medeiros, Z.M. De Loop-mediated isothermal amplification methods for diagnosis of visceral leishmaniasis (kala-azar)–a systematic review. Expert Rev. Mol. Diagn. 2020, 20, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Hirve, S.; Kroeger, A.; Matlashewski, G.; Mondal, D.; Banjara, M.R.; Das, P.; Be-Nazir, A.; Arana, B.; Olliaro, P. Towards elimination of visceral leishmaniasis in the Indian subcontinent—Translating research to practice to public health. PLoS Negl. Trop. Dis. 2017, 11, e5889. [Google Scholar] [CrossRef]
- Goyal, G.; Phukan, A.C.; Hussain, M.; Lal, V.; Modi, M.; Goyal, M.K.; Sehgal, R. Sorting out difficulties in immunological diagnosis of neurocysticercosis: Development and assessment of real time loop mediated isothermal amplification of cysticercal DNA in blood. J. Neurol. Sci. 2020, 408, 116544. [Google Scholar] [CrossRef]
- Rahman, S.M.M.; Song, H.B.; Jin, Y.; Oh, J.-K.; Lim, M.K.; Hong, S.-T.; Choi, M.-H. Application of a loop-mediated isothermal amplification (LAMP) assay targeting cox1 gene for the detection of Clonorchis sinensis in human fecal samples. PLoS Negl. Trop. Dis. 2017, 11, e5995. [Google Scholar] [CrossRef] [PubMed]
- Gandasegui, J.; Fernández-Soto, P.; Dacal, E.; Rodríguez, E.; Saugar, J.M.; Yepes, E.; Aznar-Ruiz-de-Alegría, M.L.; Espasa, M.; Ninda, A.; Bocanegra, C.; et al. Field and laboratory comparative evaluation of a LAMP assay for the diagnosis of urogenital schistosomiasis in Cubal, Central Angola. Trop. Med. Int. Heal. 2018, 23, 992–1001. [Google Scholar] [CrossRef]
- Bayoumi, A.; Al-Refai, S.; Badr, M.; Abd El-Aal, A.; El Akkad, D.; Saad, N.; KM, E.; Abdel Aziz, I. Loop-mediated isothermal amplification (LAMP): Sensitive and rapid detection of Schistosoma haematobium DNA in urine samples from Egyptian suspected cases. J. Egypt. Soc. Parasitol. 2016, 46, 299–308. [Google Scholar] [CrossRef]
- Rivero, R.; Bisio, M.; Velázquez, E.B.; Esteva, M.I.; Scollo, K.; González, N.L.; Altcheh, J.; Ruiz, A.M. Rapid detection of Trypanosoma cruzi by colorimetric loop-mediated isothermal amplification (LAMP): A potential novel tool for the detection of congenital Chagas infection. Diagn. Microbiol. Infect. Dis. 2017, 89, 26–28. [Google Scholar] [CrossRef]
- Besuschio, S.A.; Llano Murcia, M.; Benatar, A.F.; Monnerat, S.; Cruz Mata, I.; Picado de Puig, A.; de los Curto, M.Á.; Kubota, Y.; Wehrendt, D.P.; Pavia, P.; et al. Analytical sensitivity and specificity of a loop-mediated isothermal amplification (LAMP) kit prototype for detection of Trypanosoma cruzi DNA in human blood samples. PLoS Negl. Trop. Dis. 2017, 11, e5779. [Google Scholar] [CrossRef] [PubMed]
- Besuschio, S.A.; Picado, A.; Muñoz-Calderón, A.; Wehrendt, D.P.; Fernández, M.; Benatarid, A.; Diaz-Bello, Z.; Irurtia, C.; Cruz, I.; Ndung’u, J.M.; et al. Trypanosoma cruzi loop-mediated isothermal amplification (Trypanosoma cruzi loopamp) kit for detection of congenital, acute and chagas disease reactivation. PLoS Negl. Trop. Dis. 2020, 14, e8402. [Google Scholar] [CrossRef] [PubMed]
- Mitashi, P.; Hasker, E.; Ngoyi, D.M.; Pyana, P.P.; Lejon, V.; Van der Veken, W.; Lutumba, P.; Büscher, P.; Boelaert, M.; Deborggraeve, S. Diagnostic Accuracy of Loopamp Trypanosoma brucei Detection Kit for Diagnosis of Human African Trypanosomiasis in Clinical Samples. PLoS Negl. Trop. Dis. 2013, 7, e2504. [Google Scholar] [CrossRef] [PubMed]
- Njiru, Z.K.; Traub, R.; Ouma, J.O.; Enyaru, J.C.; Matovu, E. Detection of group 1 Trypanosoma brucei gambiense by loop-mediated isothermal amplification. J. Clin. Microbiol. 2011, 49, 1530–1536. [Google Scholar] [CrossRef]
- Alhassan, A.; Osei-Atweneboana, M.Y.; Kyeremeh, K.F.; Poole, C.B.; Li, Z.; Tettevi, E.; Tanner, N.A.; Carlow, C.K.S. Comparison of a new visual isothermal nucleic acid amplification test with PCR and skin snip analysis for diagnosis of onchocerciasis in humans. Mol. Biochem. Parasitol. 2016, 210, 10–12. [Google Scholar] [CrossRef]
- Lagatie, O.; Merino, M.; Batsa Debrah, L.; Debrah, A.Y.; Stuyver, L.J. An isothermal DNA amplification method for detection of Onchocerca volvulus infection in skin biopsies. Parasites Vectors 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Fernández-Medina, C.; Cruz-Fernández, S.; Crego-Vicente, B.; Febrer-Sendra, B.; García-Bernalt Diego, J.; Gorgojo-Galindo, Ó.; López-Abán, J.; Vicente Santiago, B.; Muro Álvarez, A. Whip-LAMP: A novel LAMP assay for the detection of Trichuris muris-derived DNA in stool and urine samples in a murine experimental infection model. Parasites Vectors 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Watts, M.R.; Kim, R.; Ahuja, V.; Robertson, G.J.; Sultana, Y.; Wehrhahn, M.; Bradbury, R.S.; Gilbert, G.L.; Lee, R. Comparison of loop-mediated isothermal amplification (LAMP) and real-time polymerase chain reaction (PCR) assays for the detection of Strongyloides in different specimen matrices. J. Clin. Microbiol. 2019, 57, e01173-18. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Sánchez-Hernández, A.; Gandasegui, J.; Bajo Santos, C.; López-Abán, J.; Saugar, J.M.; Rodríguez, E.; Vicente, B.; Muro, A. Strong-LAMP: A LAMP Assay for Strongyloides spp. Detection in Stool and Urine Samples. Towards the Diagnosis of Human Strongyloidiasis Starting from a Rodent Model. PLoS Negl. Trop. Dis. 2016, 10, e4836. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Celis-Giraldo, C.T.; Collar-Fernández, C.; Gorgojo, Ó.; Camargo, M.; Muñoz, J.; Salas-coronas, J.; Patarroyo, M.A.; Muro, A. Strong-LAMP Assay Based on a Strongyloides spp.—Derived Partial Sequence in the 18S rRNA as Potential Biomarker for Strongyloidiasis Diagnosis in Human Urine Samples. Dis Markers 2020, 2020, 5265198. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Eldigail, M.H.; Elamin, F.M.; Ali, I.A.; Grobusch, M.P.; Aradaib, I.E. Development and evaluation of real-time loop-mediated isothermal amplification assay for rapid detection of cystic echinococcosis. BMC Vet. Res. 2016, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nkouawa, A.; Sako, Y.; Li, T.; Chen, X.; Wandra, T.; Swastika, I.K.; Nakao, M.; Yanagida, T.; Nakaya, K.; Qiu, D.; et al. Evaluation of a loop-mediated isothermal amplification method using fecal specimens for differential detection of Taenia species from humans. J. Clin. Microbiol. 2010, 48, 3350–3352. [Google Scholar] [CrossRef] [PubMed]
- Sako, Y.; Nkouawa, A.; Yanagida, T.; Ito, A. Loop-Mediated Isothermal Amplifi cation Method for a Differential Identification of Human Taenia Tapeworms. In Nucleic Acid Detection: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2013; Volume 1039, pp. 109–120. ISBN 9781627035354. [Google Scholar]
- Ghodsian, S.; Rouhani, S.; Fallahi, S.; Seyyed-Tabaei, S.J.; Taghipour, N. Detection of spiked Fasciola hepatica eggs in stool specimens using LAMP technique. Iran. J. Parasitol. 2019, 14, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Gandasegui, J.; Fernández-Soto, P.; Carranza-Rodríguez, C.; Pérez-Arellano, J.L.; Vicente, B.; López-Abán, J.; Muro, A. The rapid-heat LAMPellet method: A potential diagnostic method for human urogenital schistosomiasis. PLoS Negl. Trop. Dis. 2015, 9, e3963. [Google Scholar] [CrossRef]
- Lodh, N.; Mikita, K.; Bosompem, K.M.; Anyan, W.K.; Quartey, J.K.; Otchere, J.; Shiff, C.J. Point of care diagnosis of multiple schistosome parasites: Species-specific DNA detection in urine by loop-mediated isothermal amplification (LAMP). Acta Trop. 2017, 173, 125–129. [Google Scholar] [CrossRef]
- Mabey, D.; Peeling, R.W.; Ustianowski, A.; Perkins, M.D. Diagnostics for the developing world. Nat. Rev. Microbiol. 2004, 2, 231–240. [Google Scholar] [CrossRef]
- Abou Tayoun, A.N.; Burchard, P.R.; Malik, I.; Scherer, A.; Tsongalis, G.J. Democratizing molecular diagnostics for the developing world. Am. J. Clin. Pathol. 2014, 141, 17–24. [Google Scholar] [CrossRef]
- Abdallah, S.D.; Thorsteindottir, H.; Douglas, K.M.; Alyna, C.S.; Shauna, N.; Singer, P.A. Top ten biotechnologies for improving health in developing countries. Nat. Genet. 2002, 32, 229–232. [Google Scholar]
- Pomari, E.; Piubelli, C.; Perandin, F.; Bisoffi, Z. Digital PCR: A new technology for diagnosis of parasitic infections. Clin. Microbiol. Infect. 2019, 25, 1510–1516. [Google Scholar] [CrossRef]
- Peeling, R.W.; Holmes, K.K.; Mabey, D.; Ronald, A. Rapid tests for sexually transmitted infections (STIs): The way forward. Sex. Transm. Infect. 2006, 82. [Google Scholar] [CrossRef] [PubMed]
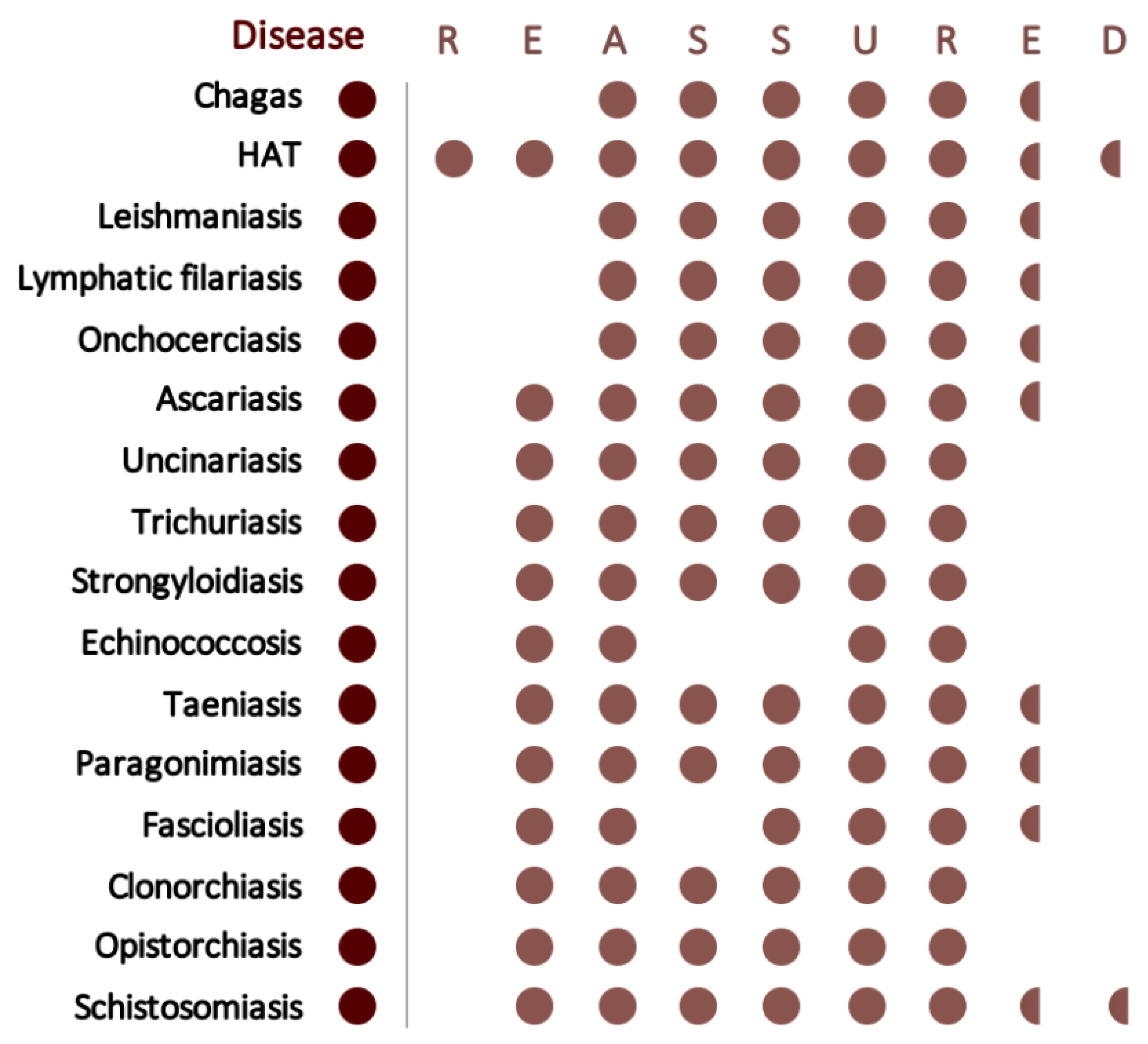
- Land, K.J.; Boeras, D.I.; Chen, X.S.; Ramsay, A.R.; Peeling, R.W. Reassured diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 2019, 4, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.S.; Thomas, M.R.; Budd, J.; Mashamba-Thompson, T.P.; Herbst, K.; Pillay, D.; Peeling, R.W.; Johnson, A.M.; McKendry, R.A.; Stevens, M.M. Taking connected mobile-health diagnostics of infectious diseases to the field. Nature 2019, 566, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Gao, J.; Chen, T.; Dong, C.; Li, H.; Wen, Y.Z.; Lun, Z.R.; Jia, Y.; Mak, P.I.; Martins, R.P. LampPort: A handheld digital microfluidic device for loop-mediated isothermal amplification (LAMP). Biomed. Microdevices 2019, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Priye, A.; Bird, S.W.; Light, Y.K.; Ball, C.S.; Negrete, O.A.; Meagher, R.J. A smartphone-based diagnostic platform for rapid detection of Zika, chikungunya, and dengue viruses. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Reboud, J.; Xu, G.; Garrett, A.; Adriko, M.; Yang, Z.; Tukahebwa, E.M.; Rowell, C.; Cooper, J.M. Paper-based microfluidics for DNA diagnostics of malaria in low resource underserved rural communities. Proc. Natl. Acad. Sci. USA 2019, 116, 4834–4842. [Google Scholar] [CrossRef]
- Chotiwan, N.; Brewster, C.D.; Magalhaes, T.; Weger-Lucarelli, J.; Duggal, N.K.; Rückert, C.; Nguyen, C.; Luna, S.M.G.; Fauver, J.R.; Andre, B.; et al. Rapid and specific detection of Asian- and African-lineage Zika viruses. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Mason, M.G.; Wang, Y.; Wee, E.; Turni, C.; Blackall, P.J.; Trau, M.; Botella, J.R. Nucleic acid purification from plants, animals and microbes in under 30 seconds. PLoS Biol. 2017, 15, e2003916. [Google Scholar] [CrossRef]
- Ibarra-Meneses, A.V.; Cruz, I.; Chicharro, C.; Sánchez, C.; Biéler, S.; Broger, T.; Moreno, J.; Carrillo, E. Evaluation of fluorimetry and direct visualization to interpret results of a loop-mediated isothermal amplification kit to detect Leishmania DNA. Parasites Vectors 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Wastling, S.L.; Picozzi, K.; Kakembo, A.S.L.; Welburn, S.C. LAMP for human African trypanosomiasis: A comparative study of detection formats. PLoS Negl. Trop. Dis. 2010, 4, e865. [Google Scholar] [CrossRef]
- Nkouawa, A.; Sako, Y.; Li, T.; Chen, X.; Nakao, M.; Yanagida, T.; Okamoto, M.; Giraudoux, P.; Raoul, F.; Nakaya, K.; et al. A loop-mediated isothermal amplification method for a differential identification of Taenia tapeworms from human: Application to a field survey. Parasitol. Int. 2012, 61, 723–725. [Google Scholar] [CrossRef]
- Song, J.; Liu, C.; Mauk, M.G.; Rankin, S.C.; Lok, J.B.; Greenberg, R.M.; Bau, H.H. Two-stage isothermal enzymatic amplification for concurrent multiplex molecular detection. Clin. Chem. 2017, 63, e263665. [Google Scholar] [CrossRef]
- Curtis, K.A.; Rudolph, D.L.; Nejad, I.; Singleton, J.; Beddoe, A.; Weigl, B.; LaBarre, P.; Owen, S.M. Isothermal amplification using a chemical heating device for point-of-care detection of HIV-1. PLoS ONE 2012, 7, e31432. [Google Scholar] [CrossRef]
- Nzelu, C.O.; Gomez, E.A.; Cáceres, A.G.; Sakurai, T.; Martini-Robles, L.; Uezato, H.; Mimori, T.; Katakura, K.; Hashiguchi, Y.; Kato, H. Development of a loop-mediated isothermal amplification method for rapid mass-screening of sand flies for Leishmania infection. Acta Trop. 2014, 132, 1–6. [Google Scholar] [CrossRef]
- Goto, M.; Honda, E.; Ogura, A.; Nomoto, A.; Hanaki, K.I. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques 2009, 46, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, O.; Gulumbe, B.; Eze, A.; Obishakin, E. Tools for Detection of Schistosomiasis in Resource Limited Settings. Med. Sci. 2018, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- García-Bernalt Diego, J.; Fernández-Soto, P.; Crego-Vicente, B.; Alonso-Castrillejo, S.; Febrer-Sendra, B.; Gómez-Sánchez, A.; Vicente, B.; López-Abán, J.; Muro, A. Progress in loop-mediated isothermal amplification assay for detection of Schistosoma mansoni DNA: Towards a ready-to-use test. Sci. Rep. 2019, 9, 14744. [Google Scholar] [CrossRef] [PubMed]
- Chander, Y.; Koelbl, J.; Puckett, J.; Moser, M.J.; Klingele, A.J.; Liles, M.R.; Carrias, A.; Mead, D.A.; Schoenfeld, T.W. A novel thermostable polymerase for RNA and DNA loop-mediated isothermal amplification (LAMP). Front. Microbiol. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Chen, T.; Gao, J.; Dong, C.; Wong, A.H.H.; Jia, Y.; Mak, P.I.; Deng, C.X.; Martins, R.P. A digital microfluidic system for loop-mediated isothermal amplification and sequence specific pathogen detection. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Njiru, Z.K. Rapid and sensitive detection of human African trypanosomiasis by loop-mediated isothermal amplification combined with a lateral-flow dipstick. Diagn. Microbiol. Infect. Dis. 2011, 69, 205–209. [Google Scholar] [CrossRef]
- Poole, C.B.; Li, Z.; Alhassan, A.; Guelig, D.; Diesburg, S.; Tanner, N.A.; Zhang, Y.; Evans, T.C.; LaBarre, P.; Wanji, S.; et al. Colorimetric tests for diagnosis of filarial infection and vector surveillance using noninstrumented Nucleic acid loop-mediated isothermal amplification (NINA-LAMP). PLoS ONE 2017, 12, e0169011. [Google Scholar] [CrossRef]
- WHO. Report of the First Meeting of the WHO Diagnostic Technical Advisory Group for Neglected Tropical Diseases, Geneva, Switzerland, 30–31 October 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Francois, P.; Tangomo, M.; Hibbs, J.; Bonetti, E.J.; Boehme, C.C.; Notomi, T.; Perkins, M.D.; Schrenzel, J. Robustness of a loop-mediated isothermal amplification reaction for diagnostic applications. FEMS Immunol. Med. Microbiol. 2011, 62, 41–48. [Google Scholar] [CrossRef]
- Hollingsworth, T.D.; Langley, I.; Nokes, D.J.; Macpherson, E.E.; McGivern, G.; Adams, E.R.; Bockarie, M.J.; Mortimer, K.; Reimer, L.J.; Squire, B.; et al. Infectious disease and health systems modelling for local decision making to control neglected tropical diseases. BMC Proc. 2015, 9, 1–6. [Google Scholar] [CrossRef]
- Nkouawa, A.; Sako, Y.; Okamoto, M.; Ito, A. Simple identification of human taenia species by multiplex loop-mediated isothermal amplification in combination with dot enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 2016, 94, 1318–1323. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Bernalt Diego, J.; Fernández-Soto, P.; Muro, A. LAMP in Neglected Tropical Diseases: A Focus on Parasites. Diagnostics 2021, 11, 521. https://doi.org/10.3390/diagnostics11030521
García-Bernalt Diego J, Fernández-Soto P, Muro A. LAMP in Neglected Tropical Diseases: A Focus on Parasites. Diagnostics. 2021; 11(3):521. https://doi.org/10.3390/diagnostics11030521
Chicago/Turabian StyleGarcía-Bernalt Diego, Juan, Pedro Fernández-Soto, and Antonio Muro. 2021. "LAMP in Neglected Tropical Diseases: A Focus on Parasites" Diagnostics 11, no. 3: 521. https://doi.org/10.3390/diagnostics11030521
APA StyleGarcía-Bernalt Diego, J., Fernández-Soto, P., & Muro, A. (2021). LAMP in Neglected Tropical Diseases: A Focus on Parasites. Diagnostics, 11(3), 521. https://doi.org/10.3390/diagnostics11030521







