MDM2 Amplified Sarcomas: A Literature Review
Abstract
1. Introduction
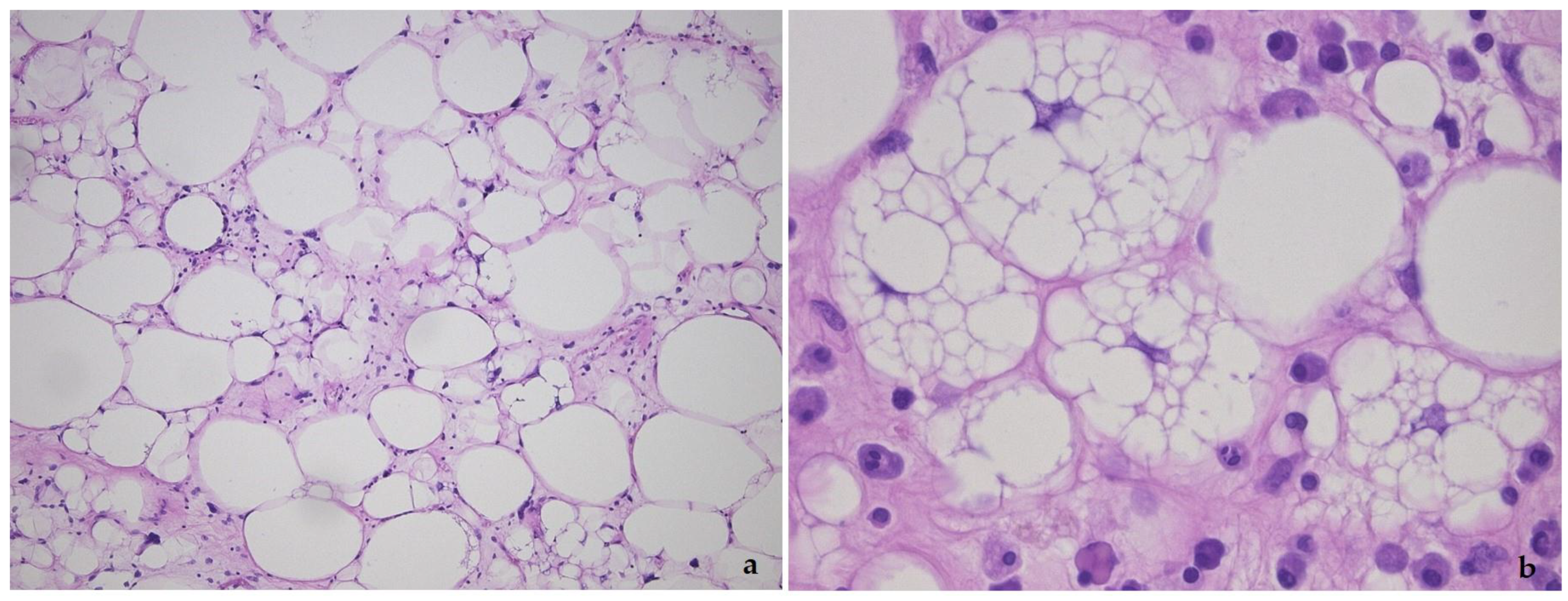
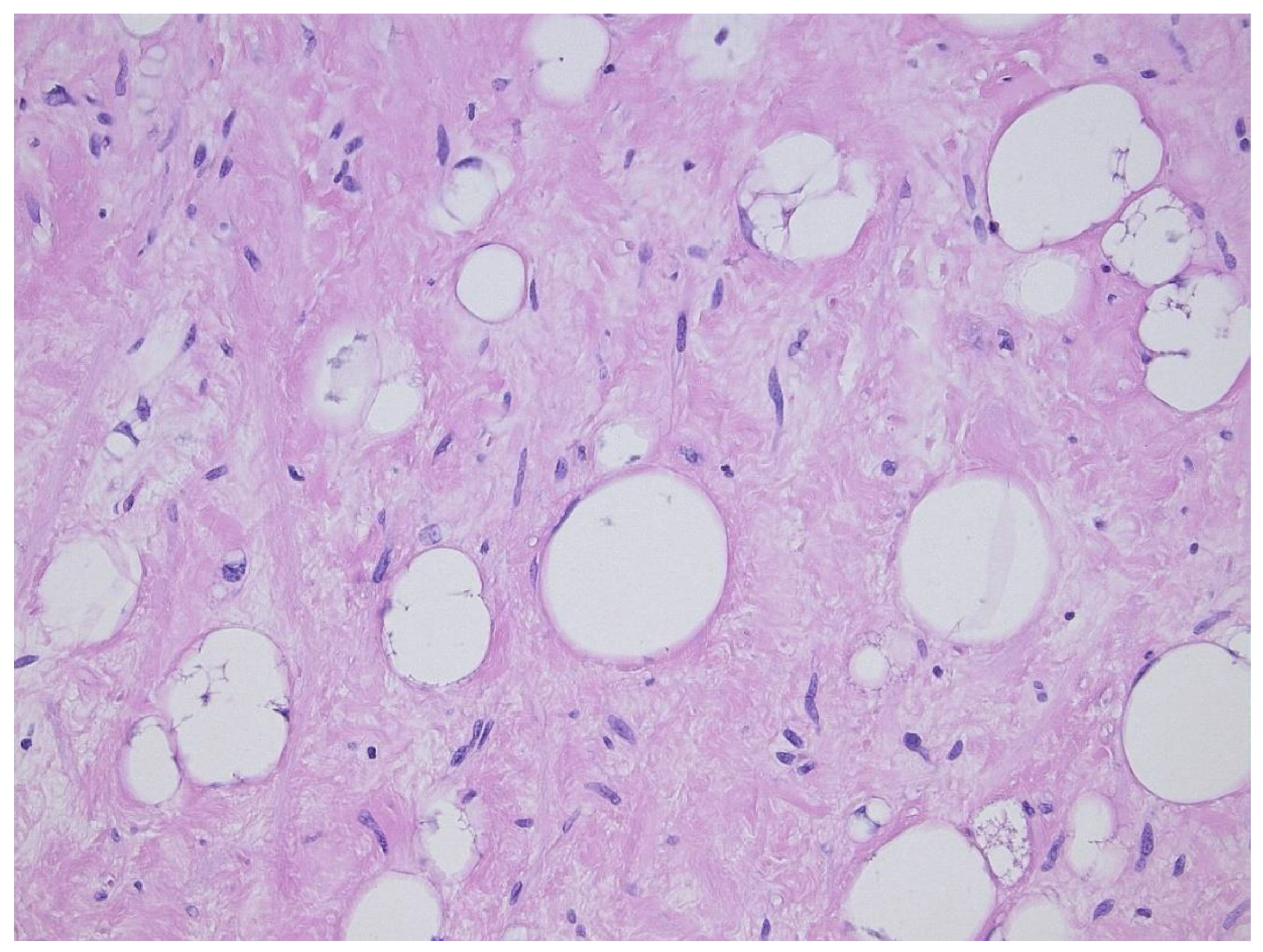
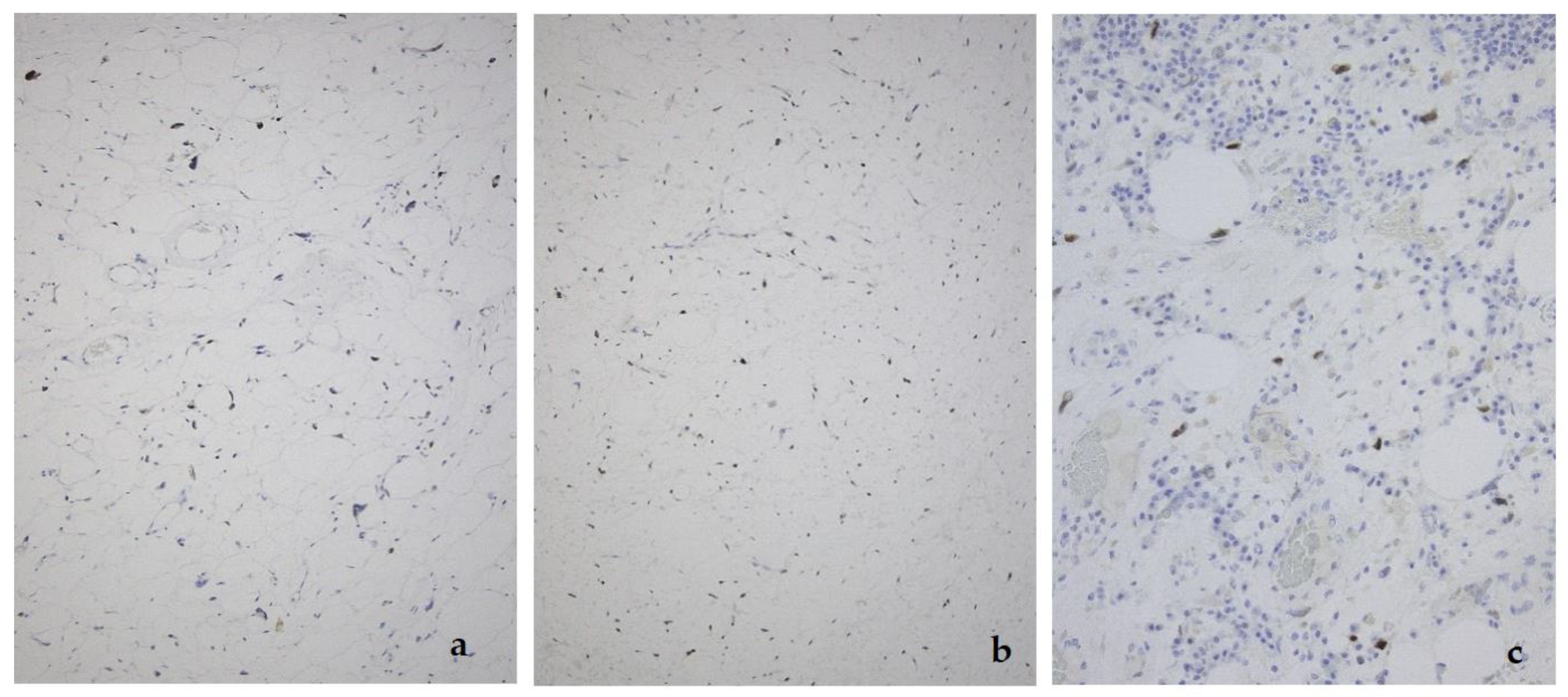
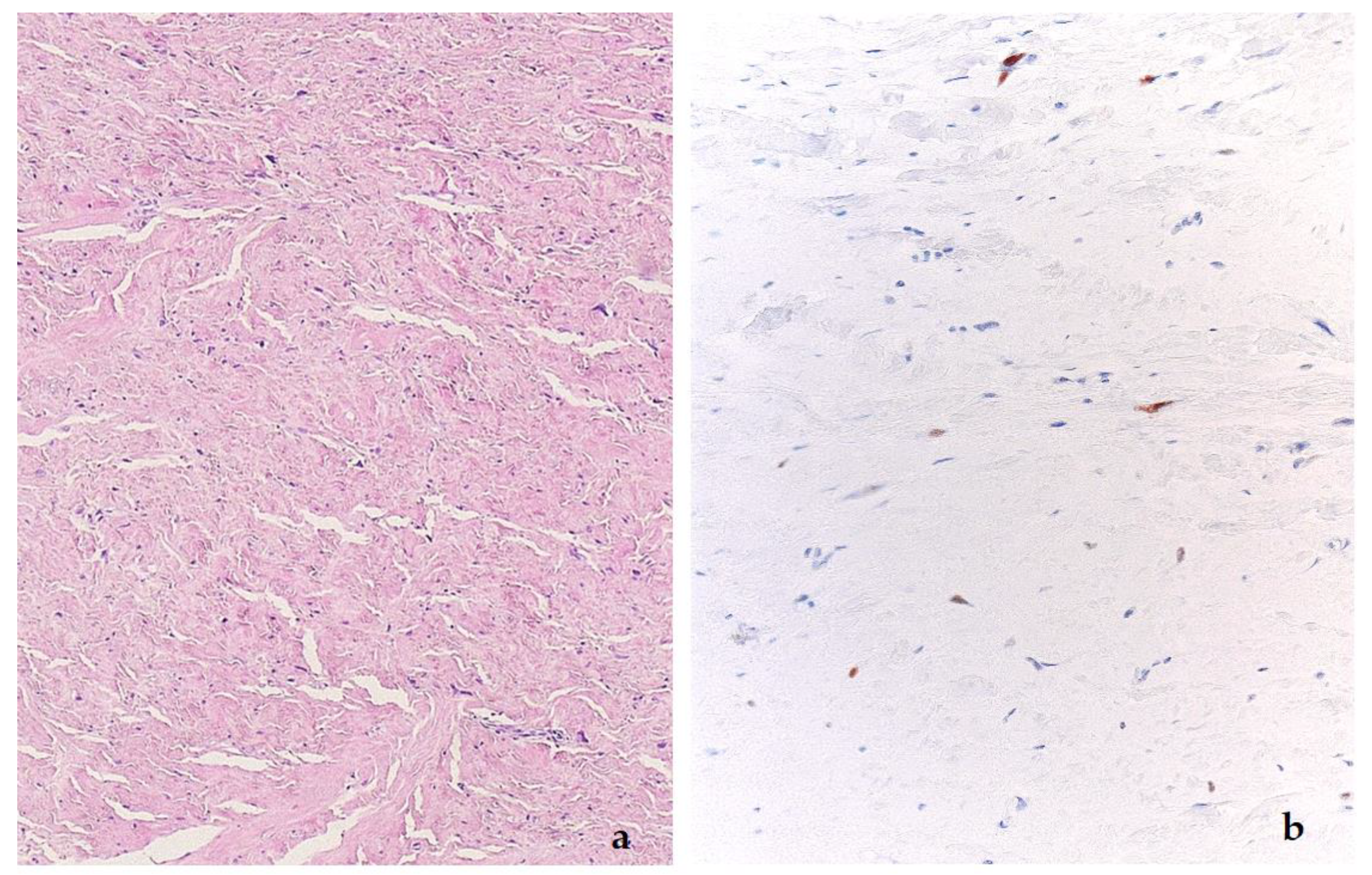
2. Well-Differentiated Liposarcoma/Atypical Lipomatous Tumor (WDL/ATL)
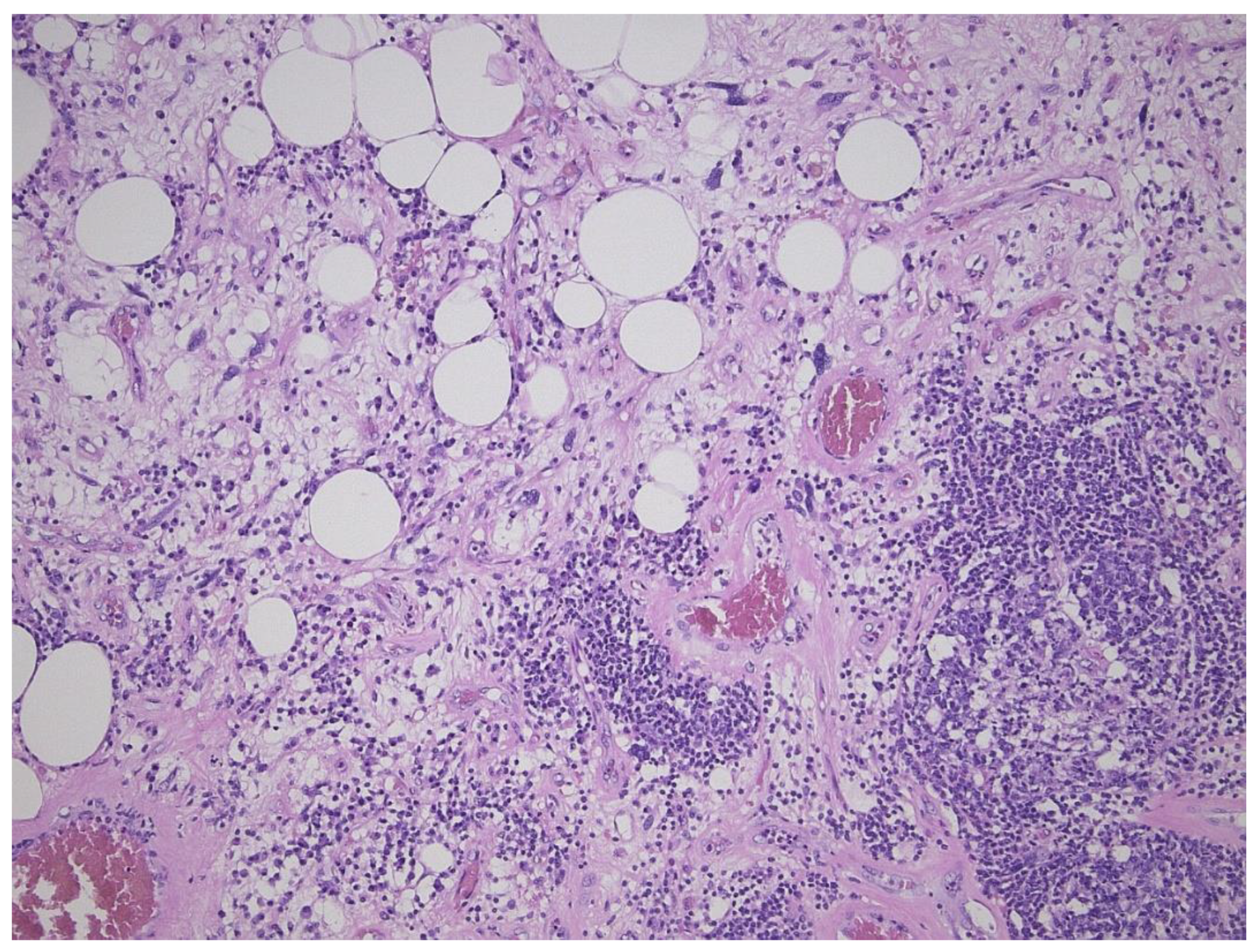
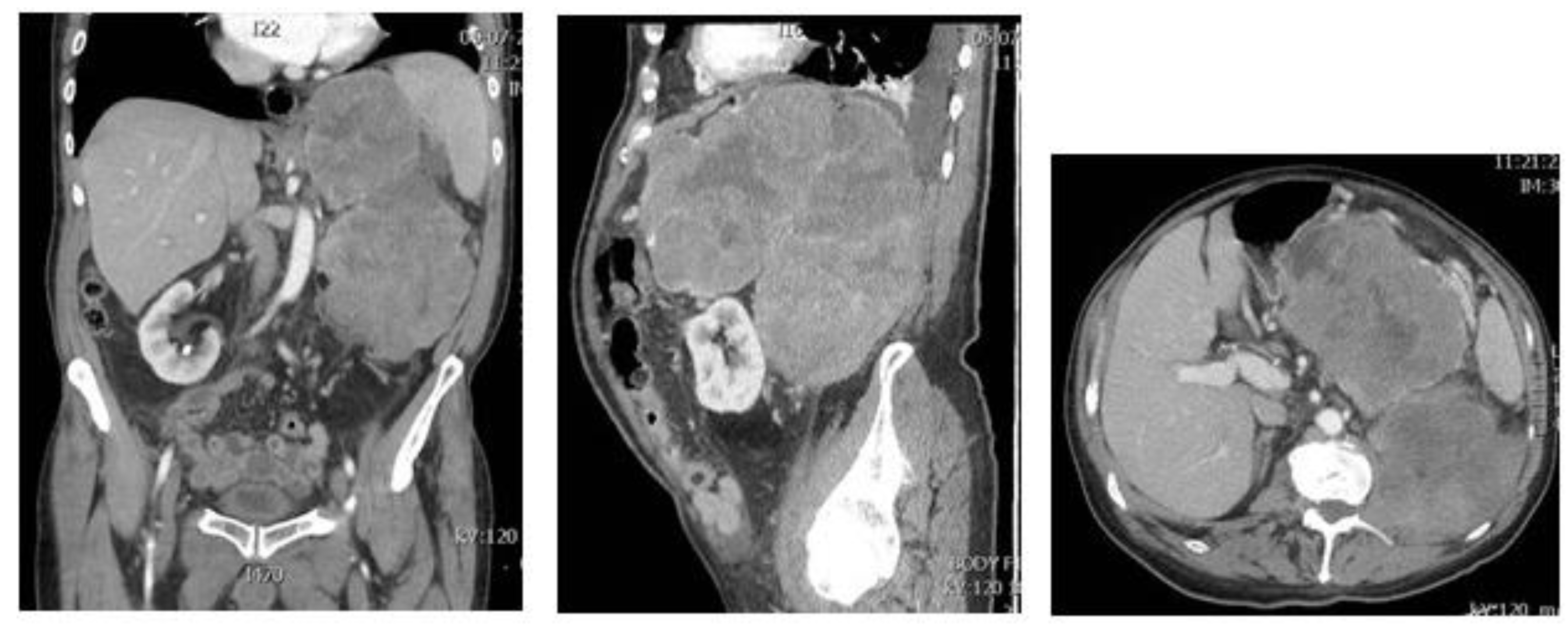
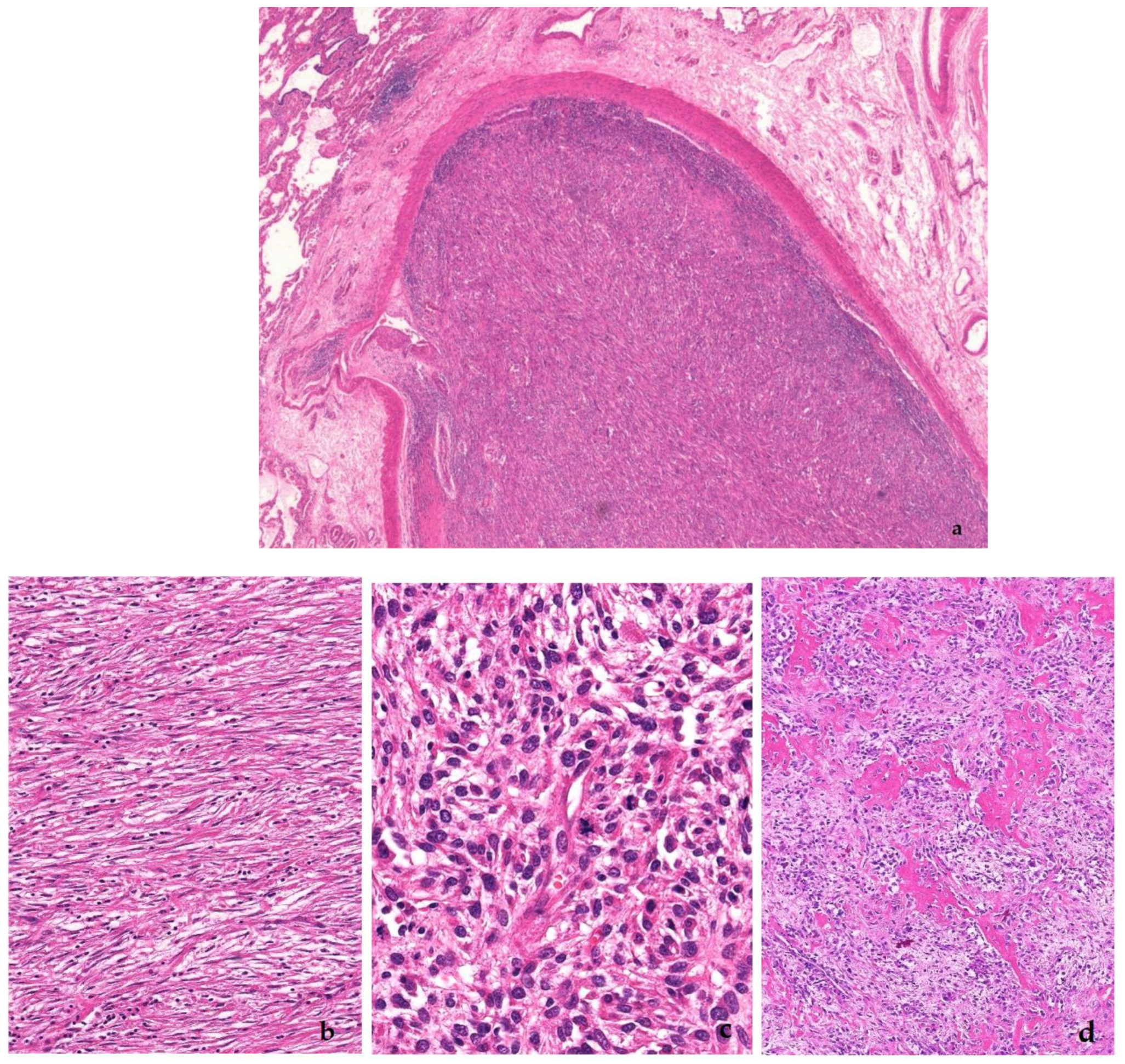
3. Dedifferentiated Liposarcoma
4. Intimal Sarcoma
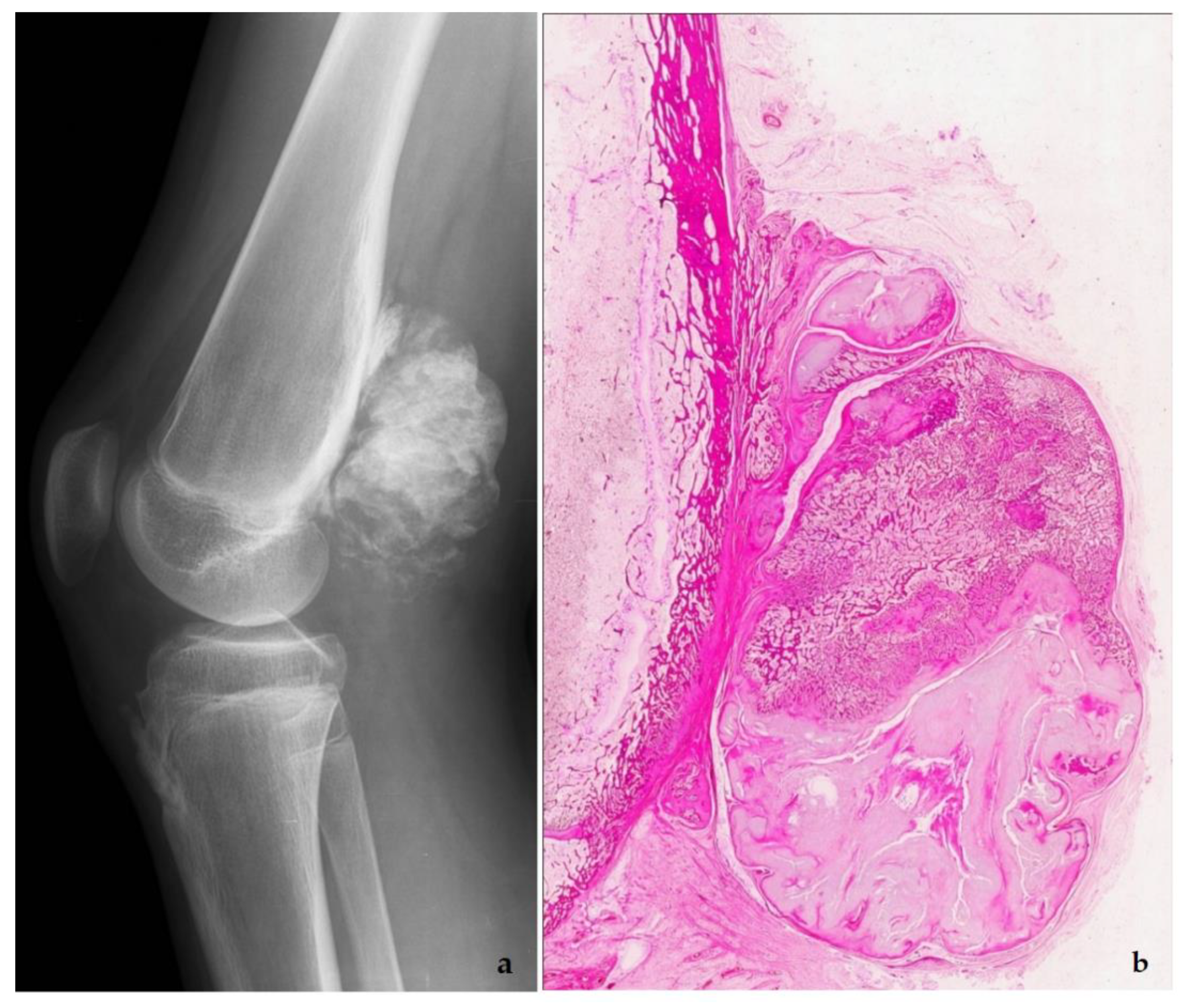
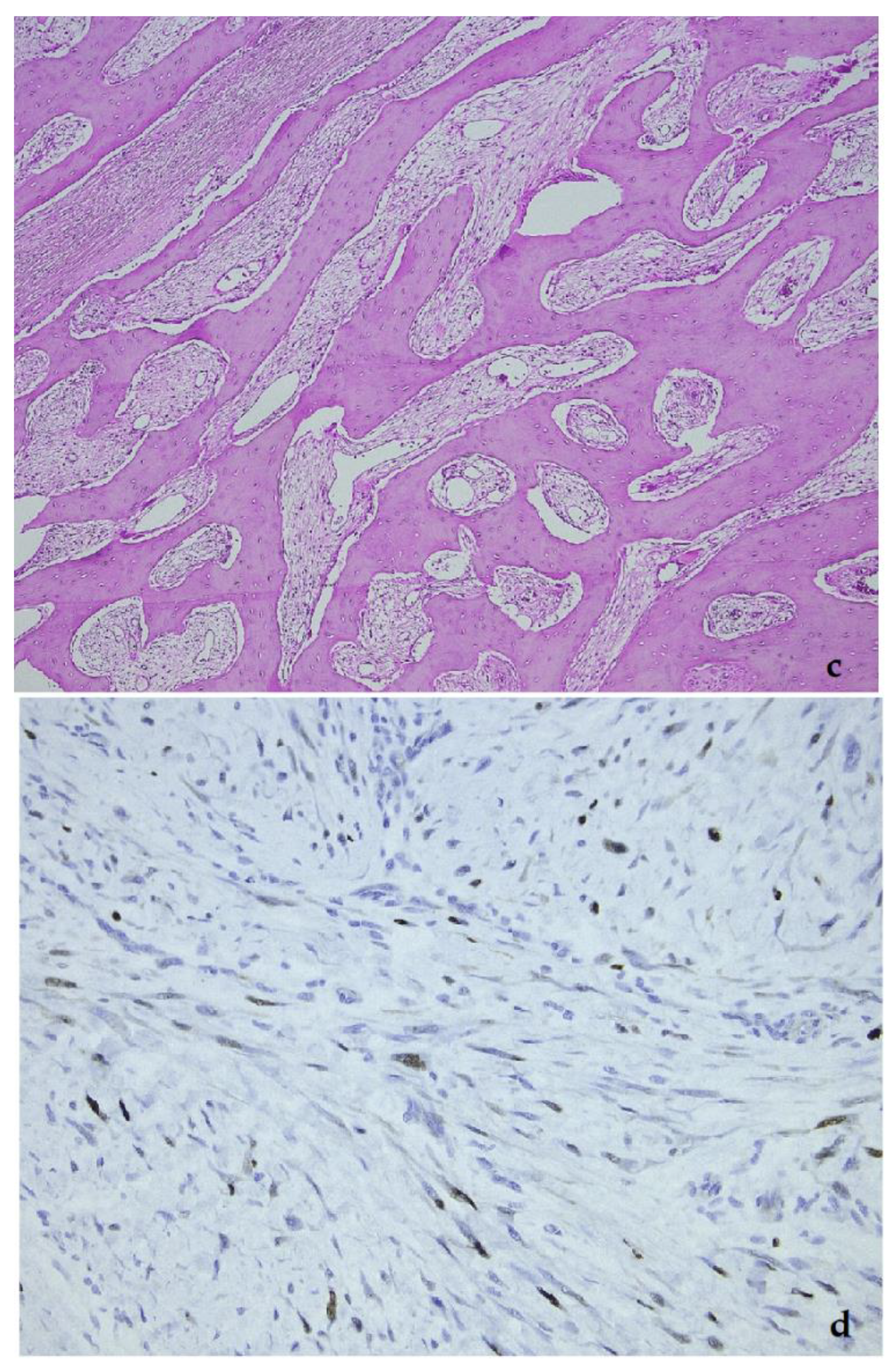
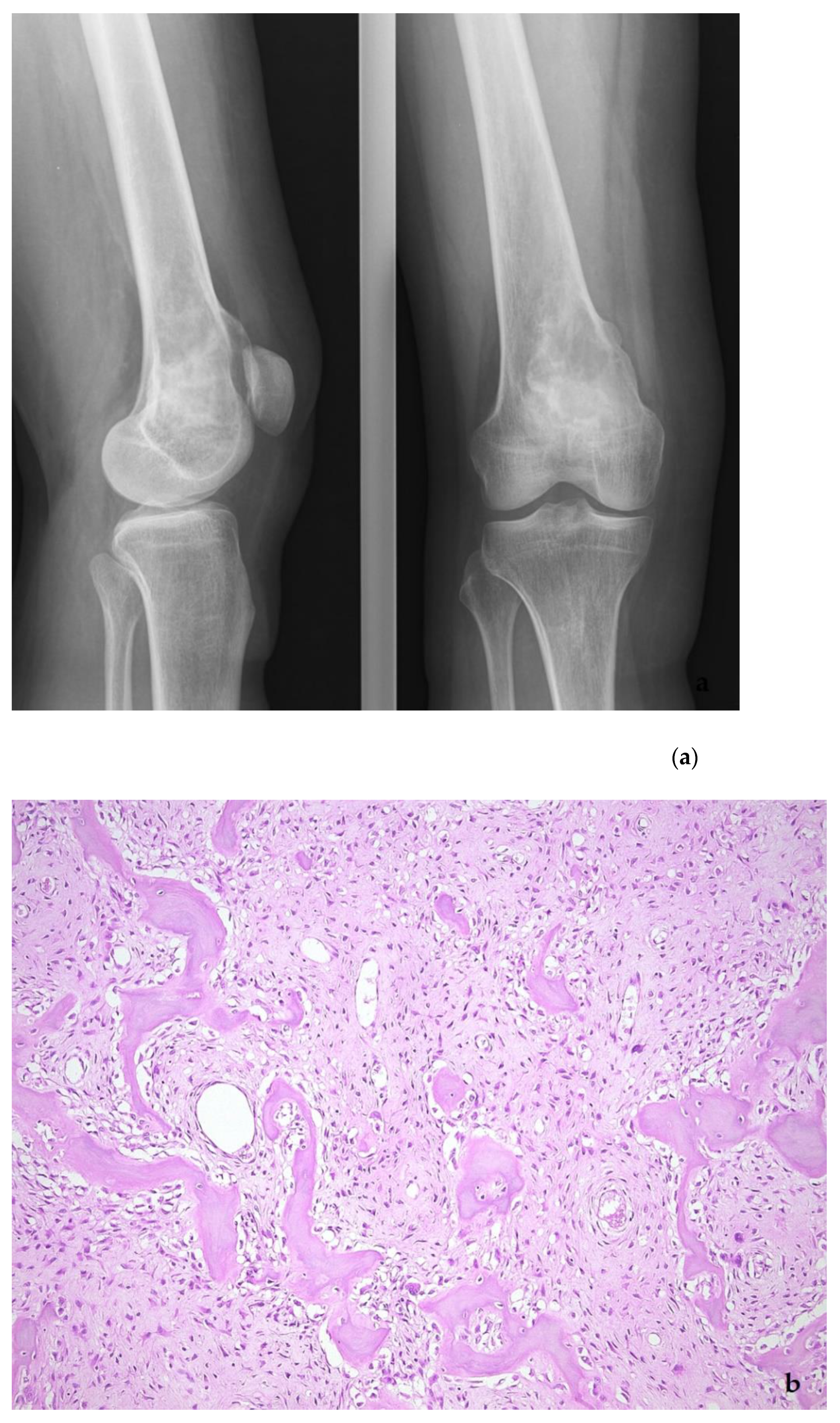
5. Low-Grade Osteosarcoma
Funding
Acknowledgments
Conflicts of Interest
References
- Momand, J.; Wu, H.H.; Dasgupta, G. MDM2-master regulator of the p53 tumor suppressosofier protein. Gene 2000, 242, 15–29. [Google Scholar] [CrossRef]
- Cissé, M.Y.; Pyrdziak, S.; Firmin, N.; Gayte, L.; Heuillet, M.; Bellvert, F.; Fuentes Delpech, H.; Riscal, R.; Arena, G.; Chibon, F.; et al. Targeting MDM2-dependent serine metabolism as a therapeutic strategy for liposarcoma. Sci. Transl. Med. 2020, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dembla, V.; Somaiah, N.; Barata, P.; Hess, K.; Fu, S.; Janku, F.; Krap, D.D.; Naing, A.; Piha-Paul, S.A.; Subbiah, V.; et al. Prevalence of MDM2 amplification and coalterations in 523 advanced cancer patients in the MD Anderson phase 1 clinic. Oncotarget 2018, 9, 33232–33243. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Ross, J.S.; Gay, L.; Dayyani, F.; Roszik, J.; Subbiah, V.; Kurzrock, R. Analysis of MDM2 Amplification: Next-Generation Sequencing of Patients With Diverse Malignancies. JCO Precis Oncol. 2018, 2, 1–14. [Google Scholar] [CrossRef]
- Kommoss, F.E.K.F.; Chang, K.T.E.; Stichel, D.; Banito, A.; Jones, D.T.W.; Heilig, C.E.; Fröhling, S.; Sahm, F.; Stenzinger, A.; Hartmann, W.; et al. Endometrial stromal sarcomas with BCOR-rearrangement harbor MDM2 amplifications. J. Pathol. Clin. Res. 2020, 6, 178–184. [Google Scholar] [CrossRef]
- Biswas, S.; Killick, E.; Jochemsen, A.G.; Lunec, J. The clinical development of p53-reactivating drugs in sarcomas-charting future therapeutic approaches and understanding the clinical molecular toxicology of Nutlins. Expert Opin. Investig. Drugs 2014, 23, 629–645. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; El-Naggar, A.; Xiong, S.; Yang, P.; Jackson, J.G.; Chau, G.; Lozano, G. Therapeutic Efficacy of p53 Restoration in Mdm25-Overexpressing Tumors. Mol Cancer Res. 2014, 12, 901–911. [Google Scholar] [CrossRef]
- Obrador-Hevia, A.; Martinez-Font, E.; Felipe-Abrio, I.; Calabuig-Fariňas Serra-Sitjar, M.; Lόpez-Guerrero, J.A.; Ramos, R.; Alemany, R.; Martĭn-Broto, J. RG7112, a Small-Molecule Inhibitor of MDM2, Enhances Trabectedin Response in Soft Tissue Sarcomas. Cancer Investig. 2015, 33, 440–450. [Google Scholar] [CrossRef]
- Bill, K.L.; Garnett, J.; Meaux, I.; Ma, X.Y.; Bolshakov, S.; Creighton, C.J.; Barriere, C.; Dubusche, L.; Lazar, A.; Prudner, B.C.; et al. SAR405838: A novel and potent inhibitor of the MDM2:p53 axis for the treatment of dedifferentiated liposarcoma. Clin. Cancer Res. 2015. epub ahead of print. [Google Scholar] [CrossRef]
- Cornillie, J.; Wozniak, A.; Li, H.; Gebreyohannes, Y.K.; Wellens, J.; Hompes, D.; Debiec-Rychter, M.; Sciot, R.; Schöffski, P. Anti-tumor activity of the MDM2-TP53 inhibitor BI-907828 in dedifferentiated liposarcoma patient-derived xenograft models harboring MDM2 amplification. Clin. Transl. Oncol. 2020, 22, 546–554. [Google Scholar] [CrossRef]
- Konopleva, M.; Martinelli, G.; Daver, N.; Papayannidis, C.; Wei, A.; Higgins, B.; Ott, M.; Mascarenhas, J.; Andreeff, M. MDM2 inhibation: an important step forward in cancer therapy. Leukemia 2020, 34, 2858–2874. [Google Scholar] [CrossRef] [PubMed]
- Aydin, G.; Paksoy, M.N.; Orhan, M.D.; Avsar, T.; Yurtsever, M.; Durdagi, S. Proposing novel MDM2 inhibitors: combined physics-driven high-throughput virtual screening and in vitro studies. Chem. Biol. Drug Des. 2020, 96, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qin, J.-J.; Rajaei, M.; Li, X.; Yu, X.; Hunt, C.; Zang, R. Targetting MDM2 for novel molecular therapy: Beyond oncology. Med Res Rev. 2020, 40, 856–880. [Google Scholar] [CrossRef] [PubMed]
- Sbaraglia, M.; Dei Tos, A.P.; Pedeutour, F. Atypical lipomatous tumor/well-differentiated liposarcoma. In WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumors; International Agency for Research on Cancer: Lyon, France, 2020; pp. 36–38. [Google Scholar]
- Weaver, J.; Rao, P.; Goldblum, J.R.; Joyce, M.J.; Turner, S.L.; Lazar, A.J.F.; Lόpez-Terada, D.; Tubbs, R.R.; Rubin, B.P. Can MDM2 analytical tests performed on core needle biopsy be relied upon to diagnose well-differentiated liposarcoma? Mod. Pathol. 2010, 23, 1301–1306. [Google Scholar] [CrossRef]
- Kimura, H.; Dobashi, Y.; Nojima, T.; Nakamura, H.; Yamamoto, N.; Tsuchiya, H.; Ikeda, H.; Sawada-Kitamura, S.; Oyama, T.; Ooi, A. Utility of fluorescence in situ hybridization to detect MDM2 amplification in liposarcomas and their morphological mimics. Int. J. Clin. Exp. Pathol. 2013, 6, 1306–1316. [Google Scholar]
- Kulkarni, A.S.; Wojcik, J.B.; Chougule, A.; Arora, K.; Chittampalli, Y.; Kurzawa, P.; Mullen, J.T.; Chebib, I.; Nielsen, G.P.; Rivera, N.M.; et al. MDM2 RNA In Situ Hybridization for the Diagnosis of Atypical Lipomatous Tumor, A Study Evaluating DNA, RNA, and Protein Expression. AM J. Surg. Pathol. 2019, 43, 446–454. [Google Scholar] [CrossRef]
- Clay, M.R.; Martinez, A.P.; Weiss, S.W.; Edgar, M.A. MDM2 Amplification in Problematic Lipomatous Tumors. Analysis of FISH Testing Criteria. Am. J. Surg. Pathol. 2015, 39, 1433–1439. [Google Scholar] [CrossRef]
- Deblenko, L.V.; Perez-Atayde, A.R.; Dubois, S.G.; Grier, H.E.; Pai, S.-Y.; Shamberger, R.C.; Kozakewich, H.P.W. p53+/mdm2- Atypical Lipolatous Tumor/Well-differentiated Lipomasarcoma in Young Children: An Early Expression of Li-Fraumeni Syndrome. Pediatric Dev. Pathol. 2010, 13, 218–224. [Google Scholar] [CrossRef]
- Mentzel, T.; Palmedo, G.; Kuhnen, C. Well-differentiated spindle cell liposarcoma (‘atypical spindle cell lipomatous tumor’) does not belong to the spectrum of atypical lipomatous tumor but has a close relationship to spindle cell lipoma: clinicopathologic, immunohistochemical and molecular analysis of six cases. Mod. Pathol. 2010, 23, 729–736. [Google Scholar]
- Creytens, D. What’s new in adipocytic neoplasia? Virchows Archiv. 2020, 476, 29–39. [Google Scholar] [CrossRef]
- Hung, Y.P.; Michal, M.; Dubuc, A.M.; Rosenberg, A.E.; Nielsen, G.P. Dysplastic lipoma: potential diagnostic pitfall of using MDM2 RNA in situ hybridization to distinguish between lipoma and atypical lipomatous tumor. Hum. Pthology 2020, 101, 53–57. [Google Scholar] [CrossRef]
- Agaimy, A. Anisometric cel lipoma: Insight from a case series and review of the literature on adipocytic neoplasms in survivors of retinoblastoma suggest a role for RB1 loss and possible relationship to fat-predominant (“fat-only”) spindle cell lipoma. Ann. Diagn. Pathol. 2017, 29, 52–56. [Google Scholar] [CrossRef]
- den Bakker, M.A. Response to “‘Dysplastic Lipoma’ Is Probably Not a Separate Entity but Rather Belongs to the Morphological Spectrum of Atypical Spindle Cell/Pleomorphic Lipomatous Tumor”. Int. J. Surg. Pathol. 2020, 28, 931. [Google Scholar] [CrossRef]
- Thway, K.; Jones, R.L.; Noujaim, J.; Zaidi, S.; Miah, A.B.; Fisher, C. Dedifferentiated Liposarcoma: Updates on Morphology, Genetics and Therapeutic Strategies. Adv. Anat. Pathol. 2016, 23, 30–40. [Google Scholar] [CrossRef]
- Dei Tos, A.P.; Marino-Enriquez, A.; Pedeutour, F. Dedifferentiated liposarcoma. In WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumors; International Agency for Research on Cancer: Lyon, France, 2020; pp. 39–41. [Google Scholar]
- Thway, K.; Robertson, D.; Thway, Y.; Fisher, C. Dedifferentiated Liposarcoma With Meningothelial-like Whorls, Metaplastic Bone Formation, and CDK4, MDM2, and p16 Expression: A Morphologic and Immunohistochemical Study. Am. J. Surg. Pathol. 2011, 35, 356–363. [Google Scholar] [CrossRef]
- Le Guellec, S.; Chibon, F.; Ouali, M.; Perot, G.; Decouvelaere, A.-V.; Robin, Y.-M.; Larousserie, F.; Terrier, P.; Coindre, J.-M.; Neuville, A. Are Peripheral Purely Undifferentiated Pleomorphic Sarcomas with MDM2 Amplification Dedifferentiated Liposarcomas? Am. J. Surg. Pathol. 2014, 38, 293–304. [Google Scholar] [CrossRef]
- Marino-Enriquez, A.; Fletcher, C.D.M.; Cin, P.D.; Hornick, J.L. Dedifferentiated Liposarcoma with “Homologous” Lipoblastic (Pleomorphic Liposarcoma-like) Differentiation: Clinicopathologic and Molecular Analysis of a Series Suggesting Revised Diagnostic Criteria. Am. J. Surg. Path 2010, 34, 1122–1131. [Google Scholar] [CrossRef]
- Gronchi, A.; Collini, P.; Miceli, R.; Valeri, B.; Renne, S.L.; Dagrada, G.; Fiore, M.; Sanfilippo, R.; Barisella, M.; Colombo, C.; et al. Myogenic Differentation and Histologic Grading Are Major Prognostic Determinants in Retroperitoneal Lipocarcoma. Am. J. Surg. Pathol. 2015, 39, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Riciotti, R.W.; Baraff, A.J.; Jour, G.; Kyriss, M.C.K.; Wu, Y.; Liu, Y.; Li, S.-C.; Hoch, B.; Liu, Y.J. High amplification levels of MDM2 and CDK4 correlate with poor outcome in patients with dedifferentiated liposarcoma: A cytogenomic microarray analysis of 47 cases. Cancer Genet. 2017, 218–219, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Bill, K.L.J.; Seligson, N.D.; Hays, J.L.; Awasthi, A.; Demoret, B.; Stets, C.W.; Duggan, M.C.; Bupathi, M.; Brock, G.N.; Millis, S.Z.; et al. Degree of MDM2 Amplification Affects Clinical Outcomes in Dedifferentiated Liposarcoma. Oncologist 2019, 24, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Saâda-Bouzid, E.; Burel-Vandenbos, F.; Ranchère-Vince, D.; Birtwisle-Peyrottes, I.; Chetaille, B.; Bouvier, C.; Château, M.-C.; Peoc’h, M.; Battistella, M.; Bazin, A.; et al. Prognostic value of HMGA2, CDK4, and JUN amplification in well-differentiated and dedifferentiated liposarcomas. Mod. Pathol. 2015, 28, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Asano, N.; Katayama, K.; Yoshida, A.; Tsuda, Y.; Sekimizu, M.; Mitani, S.; Kobayashi, E.; Komiyama, M.; Fujimoto, H.; et al. Integrated exome and RNA sequencing of dedifferentiated liposarcoma. Nat. Commun. 2019, 10, 5683. [Google Scholar] [CrossRef]
- Dewaele, B.; Floris, G.; Finalet-Ferreiro, J.; Fletcher, C.D.; Coindre, J.M.; Gillou, L.; Hogendoorn, P.C.W.; Wozniak, A.; Vanspauwen, V.; Schöffski, P.; et al. Coactivated Platelet-Derived Growth Factor Receptor α and Epidermal Growth Factor Receptor Are Potential Therapeutic Targets in Intimal Sarcoma. Cancer Res. 2010, 70, 7304–7314. [Google Scholar] [CrossRef]
- Neuville, A.; Collin, F.; Bruneval, P.; Parrens, M.; Thivolet, F.; Gomez-Brouchet, A.; Terrier, P.; de Montpreville, V.T.; Le Gall, F.; Hostein, I.; et al. Intimal sarcoma in the most frequent primary cardiac sarcoma: clinicopathologic and molecular retrospective analysis of 100 primary cardiac sarcomas. Am. J. Surg. Pathol. 2014, 38, 461–469. [Google Scholar] [CrossRef]
- Jimbo, N.; Komatsu, M.; Itoh, T.; Hirose, T. MDM2 dual-color in situ hybridization (DISH) aids the diagnosis of intimal sarcomas. Cardiovac. Pathol. 2019, 43, 107142. [Google Scholar] [CrossRef]
- Van Dievel, J.; Sciot, R.; Delcroix, M.; Vandeweyer, R.O.; Debiec-Rychter, M.; Dewaele, B.; Cornillie, J.; Van Cann, T.; Meyns, B.; Schöffski, P. Single-Center Experience with Intimal Sarcoma, an Ultra-Orphan, Commonly Fatal Mesenchymal Malignancy. Oncol. Res. Treat. 2017, 40, 353–359. [Google Scholar] [CrossRef]
- Bode-Lesnieuwska, B.; Debiec-Rychter, M.; Toavora, F. Intimal sarcoma. In WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumors; International Agency for Research on Cancer: Lyon, France, 2020; pp. 315–317. [Google Scholar]
- Wang, J.; Nord, K.H.; O’Donnell, P.G.; Yoshida, A. Parosteal osteosarcoma. In WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumors; International Agency for Research on Cancer: Lyon, France, 2020; pp. 410–413. [Google Scholar]
- Yoshida, A.; Bredella, M.A.; Gambarotti, M.; Sumathi, V.P. Low-grade central osteosarcoma. In WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumors; International Agency for Research on Cancer: Lyon, France, 2020; pp. 400–402. [Google Scholar]
- Dujardin, F.; Bui Nhuyen Binh, M.; Bouvier, C.; Gomez-Brouchet, A.; Larousserie, F.; de Muret, A.; Louis-Brennetot, C.; Aurias, A.; Coindre, J.M.; Guillou, L.; et al. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod. Pathol. 2011, 24, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Ushiku, T.; Motoi, T.; Beppu, Y.; Fukayama, M.; Tsuda, H.; Shibata, T. MDM2 and CDK4 Immunohistochemical Coexpression in High-grade Osteosarcoma: Correlation with a Dedifferentiated Subtype. Am. J. Surg. Pathol. 2012, 36, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Lott Limbach, A.; Lingen, M.W.; McElherne, J.; Mashek, H.; Fitzpatrick, C.; Hyjek, E.; Mostofi, R.; Cipriani, N.A. The Utility of MDM2 and CDK4 Immunohistochemistry and MDM2 FISH in Craniofacial Osteosarcoma. Head Neck Pathol. 2020, 14, 889–898. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciot, R. MDM2 Amplified Sarcomas: A Literature Review. Diagnostics 2021, 11, 496. https://doi.org/10.3390/diagnostics11030496
Sciot R. MDM2 Amplified Sarcomas: A Literature Review. Diagnostics. 2021; 11(3):496. https://doi.org/10.3390/diagnostics11030496
Chicago/Turabian StyleSciot, Raf. 2021. "MDM2 Amplified Sarcomas: A Literature Review" Diagnostics 11, no. 3: 496. https://doi.org/10.3390/diagnostics11030496
APA StyleSciot, R. (2021). MDM2 Amplified Sarcomas: A Literature Review. Diagnostics, 11(3), 496. https://doi.org/10.3390/diagnostics11030496





