Acute Pulmonary Embolism Severity Assessment Evaluated with Dual Energy CT Perfusion Compared to Conventional CT Angiographic Measurements
Abstract
1. Introduction
2. Materials and Methods
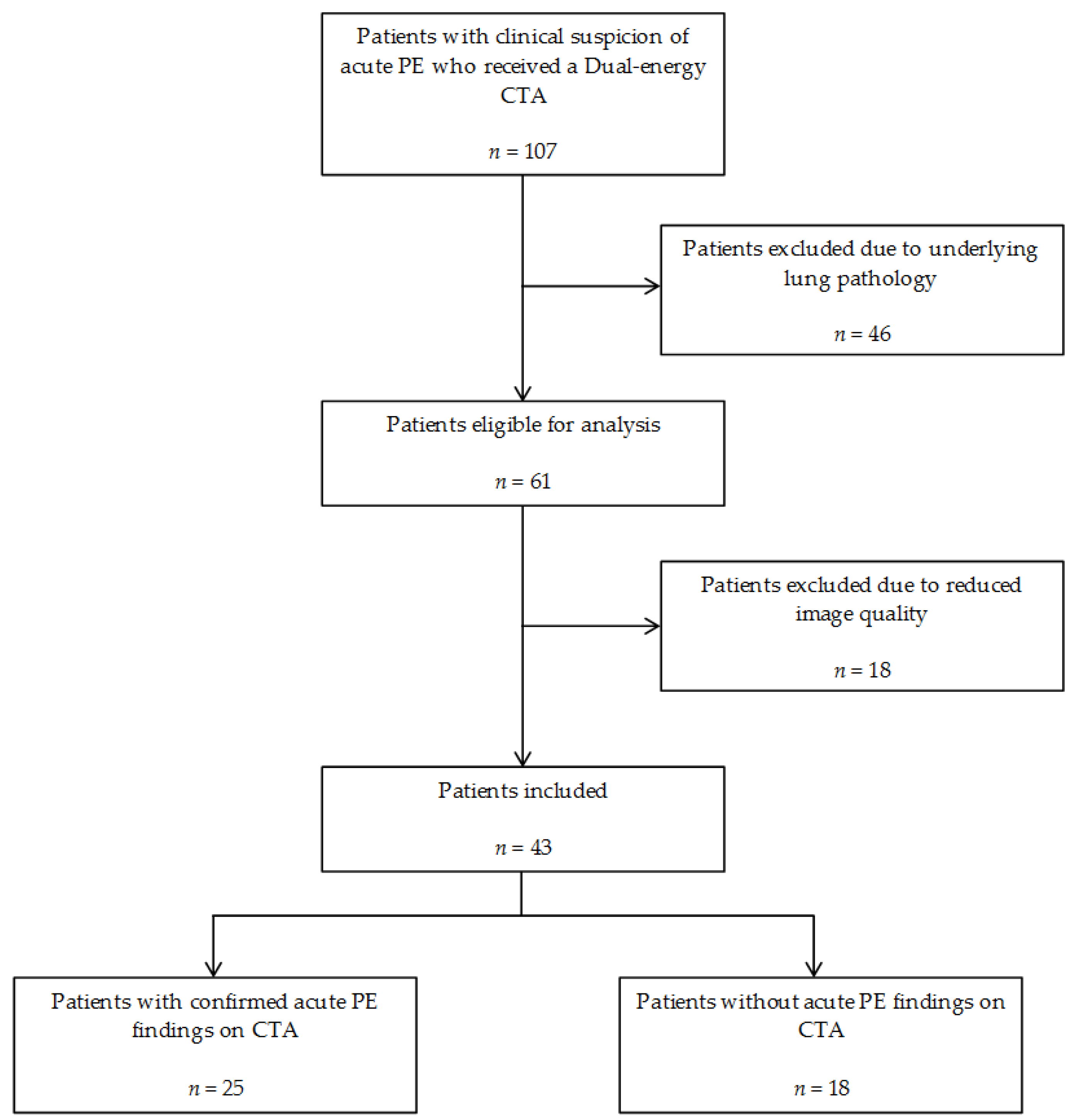
2.1. Patients
2.2. CT Acquisition
2.3. CTA Interpretation
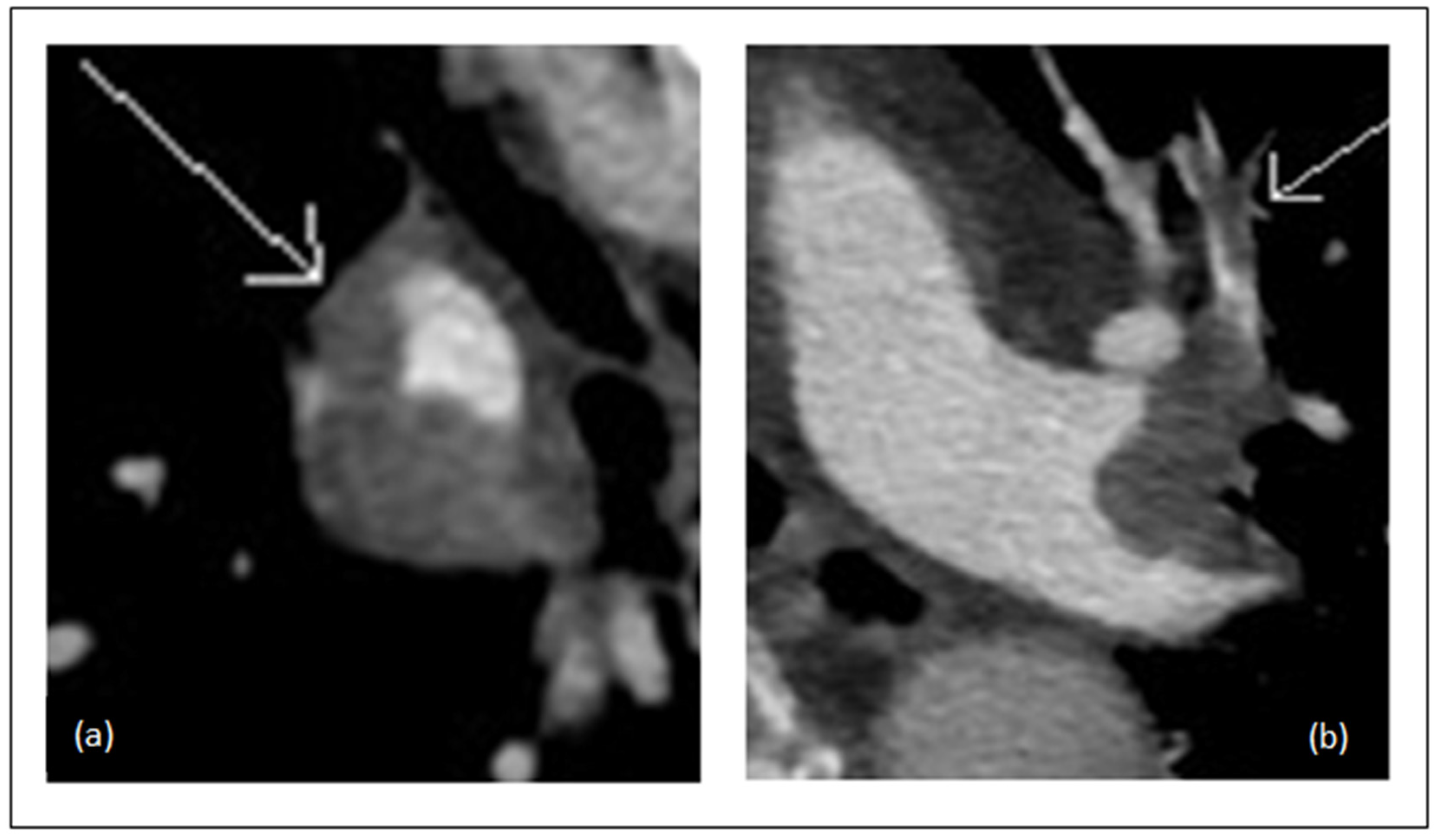
2.4. CTA Obstruction Score
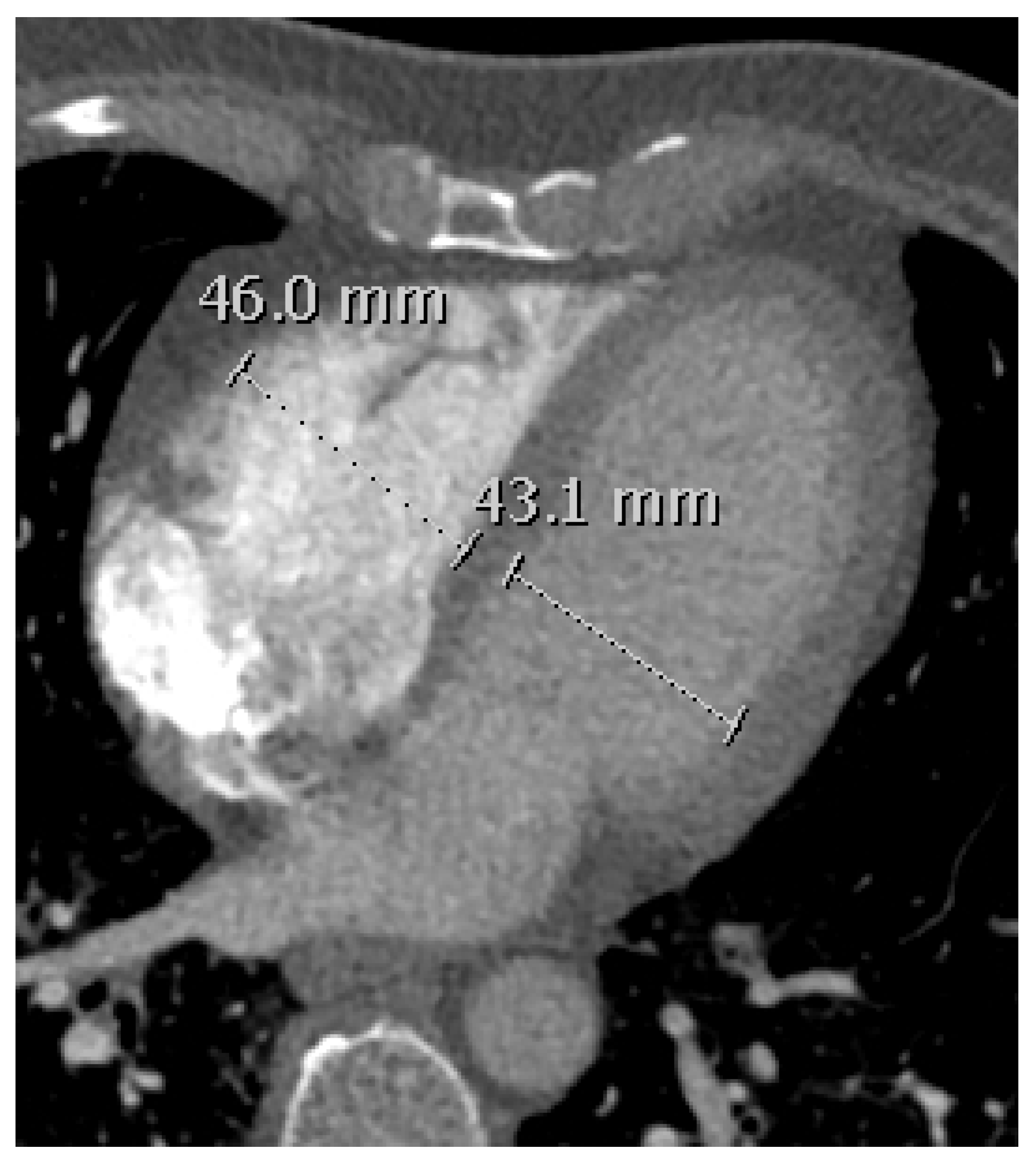
2.5. RVD Assessment
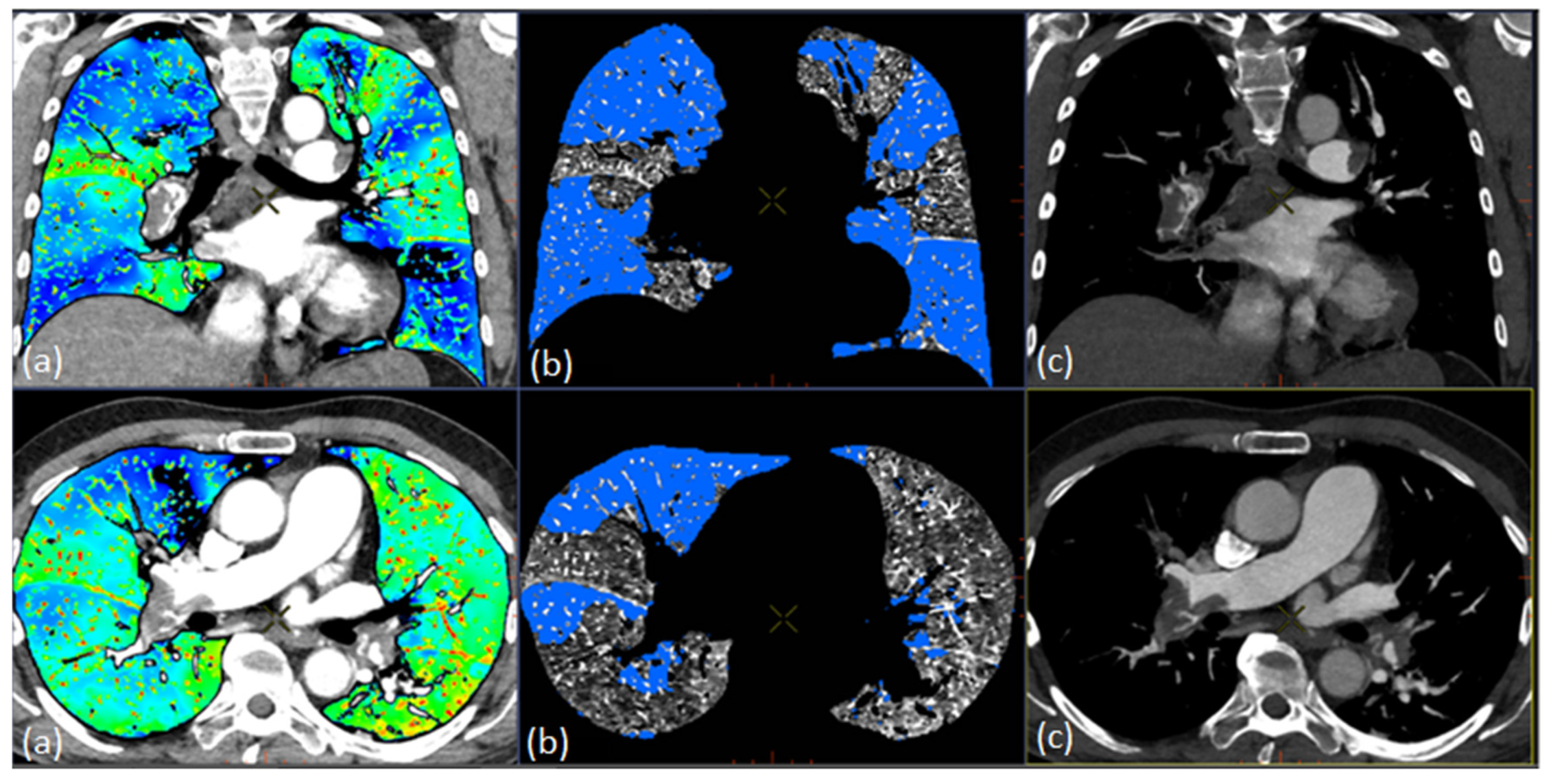
2.6. Quantification of Total Lung Volume and PDvol
2.7. Statistical Analysis
3. Results
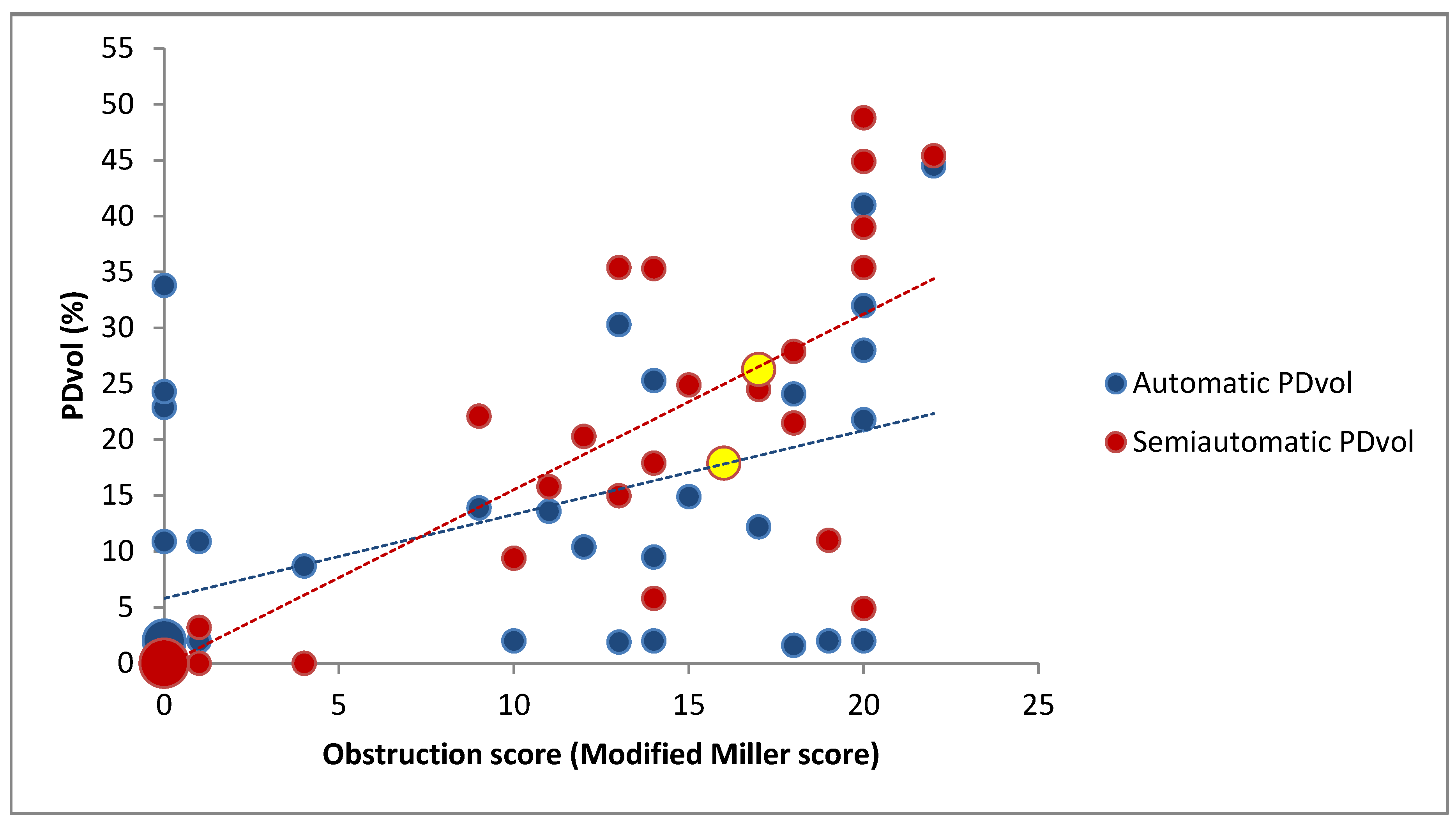
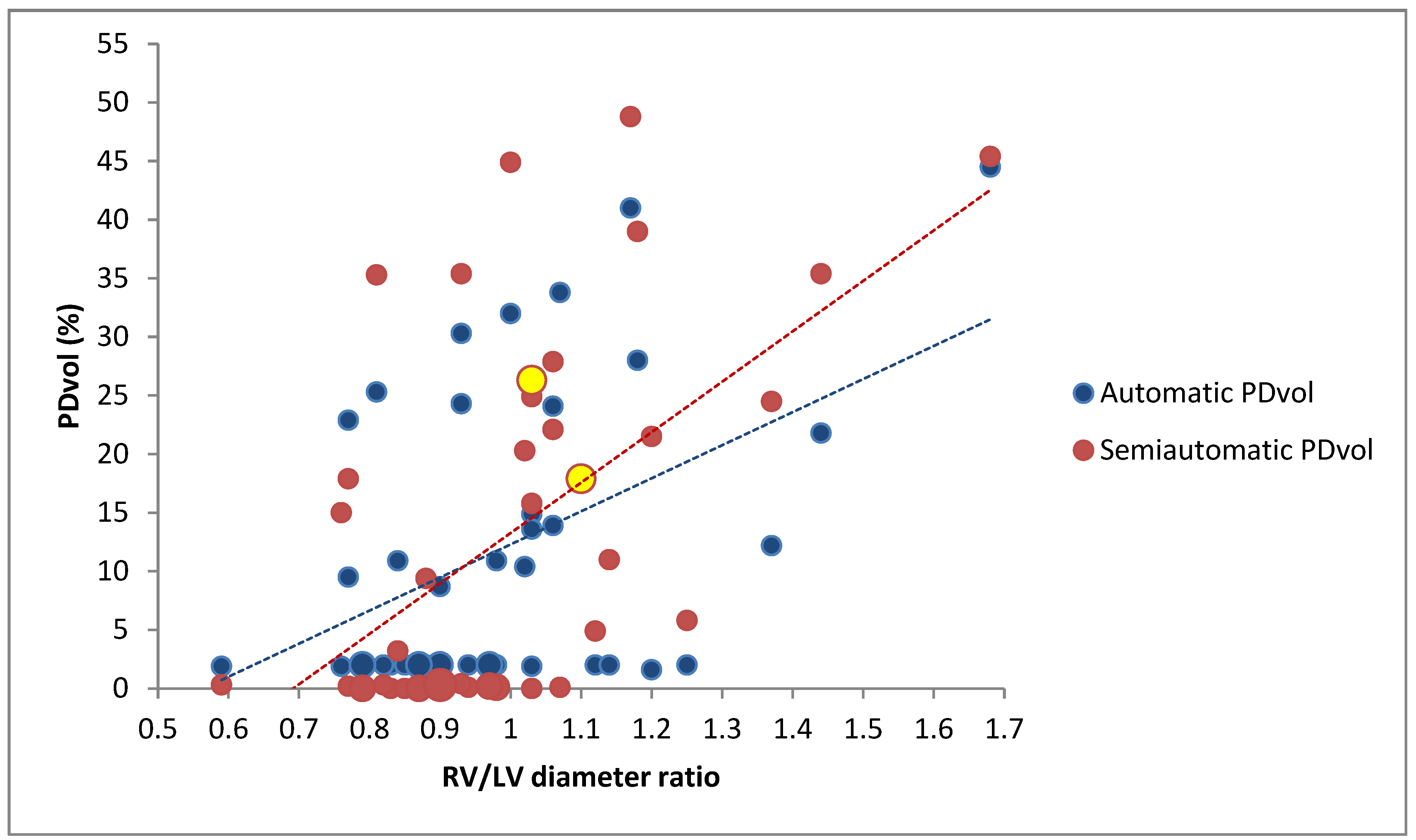
3.1. Correlation Between PDvol, RV/LV Diameter and CTA Obstruction Score in all Patients (n = 43)
3.2. Correlation Between PDvol, RV/LV Diameter and CTA Obstruction Score in Patients with Confirmed Acute PE (n = 25)
3.3. Difference Between the Group with Confirmed Acute PE (n = 25) and the Group without Acute PE (n = 18)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giri, J.; Sista, A.K.; Weinberg, I.; Kearon, C.; Kumbhani, D.J.; Desai, N.D.; Piazza, G.; Gladwin, M.T.; Chatterjee, S.; Kobayashi, T.; et al. Interventional Therapies for Acute Pulmonary Embolism: Current Status and Principles for the Development of Novel Evidence: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e774–e801. [Google Scholar] [CrossRef] [PubMed]
- Meinel, F.G.; Graef, A.; Bamberg, F.; Thieme, S.F.; Schwarz, F.; Sommer, W.H.; Neurohr, C.; Kupatt, C.; Reiser, M.F.; Johnson, T.R.C. Effectiveness of Automated Quantification of Pulmonary Perfused Blood Volume Using Dual-Energy CTPA for the Severity Assessment of Acute Pulmonary Embolism. Investig. Radiol. 2013, 48, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galiè, N.; Gibbs, J.S.R.; Huisman, M.V.; Humbert, M.; Kucher, N.; et al. 2014 ESC Guidelines on the Diagnosis and Management of Acute Pulmonary EmbolismThe Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)Endorsed by the European Respiratory Society (ERS). Eur. Heart J. 2014, 35, 3033–3080. [Google Scholar] [CrossRef] [PubMed]
- Remy-Jardin, M.; Pistolesi, M.; Goodman, L.R.; Gefter, W.B.; Gottschalk, A.; Mayo, J.R.; Sostman, H.D. Management of Suspected Acute Pulmonary Embolism in the Era of CT Angiography: A Statement from the Fleischner Society. Radiology 2007, 245, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Alis, J.; Latson, L.A.; Haramati, L.B.; Shmukler, A. Navigating the Pulmonary Perfusion Map: Dual-Energy Computed Tomography in Acute Pulmonary Embolism. J. Comput. Assist. Tomogr. 2018, 42, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Ghaye, B.; Ghuysen, A.; Willems, V.; Lambermont, B.; Gerard, P.; D’Orio, V.; Gevenois, P.A.; Dondelinger, R.F. Severe Pulmonary Embolism:Pulmonary Artery Clot Load Scores and Cardiovascular Parameters as Predictors of Mortality. Radiology 2006, 239, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Araoz, P.A.; Gotway, M.B.; Trowbridge, R.L.; Bailey, R.A.; Auerbach, A.D.; Reddy, G.P.; Dawn, S.K.; Webb, W.R.; Higgins, C.B. Helical CT Pulmonary Angiography Predictors of In-Hospital Morbidity and Mortality in Patients with Acute Pulmonary Embolism. J. Thorac. Imaging 2003, 18, 207–216. [Google Scholar] [CrossRef]
- Pontana, F.; Faivre, J.-B.; Remy-Jardin, M.; Flohr, T.; Schmidt, B.; Tacelli, N.; Pansini, V.; Remy, J. Lung Perfusion with Dual-Energy Multidetector-Row CT (MDCT). Acad. Radiol. 2008, 15, 1494–1504. [Google Scholar] [CrossRef]
- Fink, C.; Johnson, T.; Michaely, H.; Morhard, D.; Becker, C.; Reiser, M.; Nikolaou, K. Dual-Energy CT Angiography of the Lung in Patients with Suspected Pulmonary Embolism: Initial Results. Fortschr. Röntgenstr. 2008, 180, 879–883. [Google Scholar] [CrossRef]
- Thieme, S.F.; Becker, C.R.; Hacker, M.; Nikolaou, K.; Reiser, M.F.; Johnson, T.R.C. Dual Energy CT for the Assessment of Lung Perfusion—Correlation to Scintigraphy. Eur. J. Radiol. 2008, 68, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Chae, E.J.; Seo, J.B.; Jang, Y.M.; Krauss, B.; Lee, C.W.; Lee, H.J.; Song, K.-S. Dual-Energy CT for Assessment of the Severity of Acute Pulmonary Embolism: Pulmonary Perfusion Defect Score Compared With CT Angiographic Obstruction Score and Right Ventricular/Left Ventricular Diameter Ratio. AJR Am. J. Roentgenol. 2010, 194, 604–610. [Google Scholar] [CrossRef]
- Thieme, S.F.; Ashoori, N.; Bamberg, F.; Sommer, W.H.; Johnson, T.R.C.; Leuchte, H.; Becker, A.; Maxien, D.; Helck, A.D.; Behr, J.; et al. Severity Assessment of Pulmonary Embolism Using Dual Energy CT—Correlation of a Pulmonary Perfusion Defect Score with Clinical and Morphological Parameters of Blood Oxygenation and Right Ventricular Failure. Eur. Radiol. 2012, 22, 269–278. [Google Scholar] [CrossRef]
- Qanadli, S.D.; El Hajjam, M.; Vieillard-Baron, A.; Joseph, T.; Mesurolle, B.; Oliva, V.L.; Barré, O.; Bruckert, F.; Dubourg, O.; Lacombe, P. New CT Index to Quantify Arterial Obstruction in Pulmonary Embolism: Comparison with Angiographic Index and Echocardiography. AJR Am. J. Roentgenol. 2001, 176, 1415–1420. [Google Scholar] [CrossRef]
- Quiroz, R.; Kucher, N.; Schoepf, U.J.; Kipfmueller, F.; Solomon, S.D.; Costello, P.; Goldhaber, S.Z. Right Ventricular Enlargement on Chest Computed Tomography: Prognostic Role in Acute Pulmonary Embolism. Circulation 2004, 109, 2401–2404. [Google Scholar] [CrossRef] [PubMed]
- Kamel, E.M.; Schmidt, S.; Doenz, F.; Adler-Etechami, G.; Schnyder, P.; Qanadli, S.D. Computed Tomographic Angiography in Acute Pulmonary Embolism: Do We Need Multiplanar Reconstructions to Evaluate the Right Ventricular Dysfunction? J. Comput. Assist. Tomogr. 2008, 32, 438–443. [Google Scholar] [CrossRef]
- Wittenberg, R.; van Vliet, J.W.; Ghaye, B.; Peters, J.F.; Schaefer-Prokop, C.M.; Coche, E. Comparison of Automated 4-Chamber Cardiac Views versus Axial Views for Measuring Right Ventricular Enlargement in Patients with Suspected Pulmonary Embolism. Eur. J. Radiol. 2012, 81, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.T.; Demehri, S.; Cai, T.; Parast, L.; Hunsaker, A.R.; Goldhaber, S.Z.; Rybicki, F.J. Axial and Reformatted Four-Chamber Right Ventricle-to-Left Ventricle Diameter Ratios on Pulmonary CT Angiography as Predictors of Death after Acute Pulmonary Embolism. AJR Am. J. Roentgenol. 2012, 198, 1353–1360. [Google Scholar] [CrossRef]
- Thoracic VCAR User Guide, Revision 1, General Electric Company. 2018. Available online: Https://172.31.186.201/Index2.Jsp?Lang=EN (accessed on 1 August 2019).
- Zou, K.H.; Tuncali, K.; Silverman, S.G. Correlation and Simple Linear Regression. Radiology 2003, 227, 617–628. [Google Scholar] [CrossRef]
- Apfaltrer, P.; Bachmann, V.; Meyer, M.; Henzler, T.; Barraza, J.M.; Gruettner, J.; Walter, T.; Schoepf, U.J.; Schoenberg, S.O.; Fink, C. Prognostic Value of Perfusion Defect Volume at Dual Energy CTA in Patients with Pulmonary Embolism: Correlation with CTA Obstruction Scores, CT Parameters of Right Ventricular Dysfunction and Adverse Clinical Outcome. Eur. J. Radiol. 2012, 81, 3592–3597. [Google Scholar] [CrossRef]
- Kong, W.-F.; Wang, Y.-T.; Yin, L.-L.; Pu, H.; Tao, K.-Y. Clinical Risk Stratification of Acute Pulmonary Embolism: Comparing the Usefulness of CTA Obstruction Score and Pulmonary Perfusion Defect Score with Dual-Energy CT. Int. J. Card Imaging 2017, 33, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shi, H.; Wang, Y.; Kumar, A.R.; Chi, B.; Han, P. Assessment of Correlation between CT Angiographic Clot Load Score, Pulmonary Perfusion Defect Score and Global Right Ventricular Function with Dual-Source CT for Acute Pulmonary Embolism. Br. J. Radiol. 2012, 85, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Perez-Johnston, R.; Plodkowski, A.J.; Halpenny, D.F.; Hayes, S.A.; Capanu, M.; Araujo-Filho, J.A.B.; Weinsaft, J.W.; Ginsberg, M.S. Perfusion Defects on Dual-Energy CTA in Patients with Suspected Pulmonary Embolism Correlate with Right Heart Strain and Lower Survival. Eur. Radiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sharma, A.; McDermott, S.; Homayounieh, F.; Rastogi, S.; Flores, E.J.; Shepard, J.A.O.; Gilman, M.D.; Digumarthy, S.R. Comparison of Image Quality and Radiation Doses between Rapid KV-Switching and Dual-Source DECT Techniques in the Chest. Eur. J. Radiol. 2019, 119, 108639. [Google Scholar] [CrossRef] [PubMed]
- Si-Mohamed, S.; Moreau-Triby, C.; Tylski, P.; Tatard-Leitman, V.; Wdowik, Q.; Boccalini, S.; Dessouky, R.; Douek, P.; Boussel, L. Head-to-Head Comparison of Lung Perfusion with Dual-Energy CT and SPECT-CT. Diagn. Interv. Imaging 2020, 101, 299–310. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jawad, S.; Ulriksen, P.S.; Kalhauge, A.; Hansen, K.L. Acute Pulmonary Embolism Severity Assessment Evaluated with Dual Energy CT Perfusion Compared to Conventional CT Angiographic Measurements. Diagnostics 2021, 11, 495. https://doi.org/10.3390/diagnostics11030495
Jawad S, Ulriksen PS, Kalhauge A, Hansen KL. Acute Pulmonary Embolism Severity Assessment Evaluated with Dual Energy CT Perfusion Compared to Conventional CT Angiographic Measurements. Diagnostics. 2021; 11(3):495. https://doi.org/10.3390/diagnostics11030495
Chicago/Turabian StyleJawad, Samir, Peter Sommer Ulriksen, Anna Kalhauge, and Kristoffer Lindskov Hansen. 2021. "Acute Pulmonary Embolism Severity Assessment Evaluated with Dual Energy CT Perfusion Compared to Conventional CT Angiographic Measurements" Diagnostics 11, no. 3: 495. https://doi.org/10.3390/diagnostics11030495
APA StyleJawad, S., Ulriksen, P. S., Kalhauge, A., & Hansen, K. L. (2021). Acute Pulmonary Embolism Severity Assessment Evaluated with Dual Energy CT Perfusion Compared to Conventional CT Angiographic Measurements. Diagnostics, 11(3), 495. https://doi.org/10.3390/diagnostics11030495





