Correlation of an Index-Lesion-Based SPECT Dosimetry Method with Mean Tumor Dose and Clinical Outcome after 177Lu-PSMA-617 Radioligand Therapy
Abstract
1. Introduction
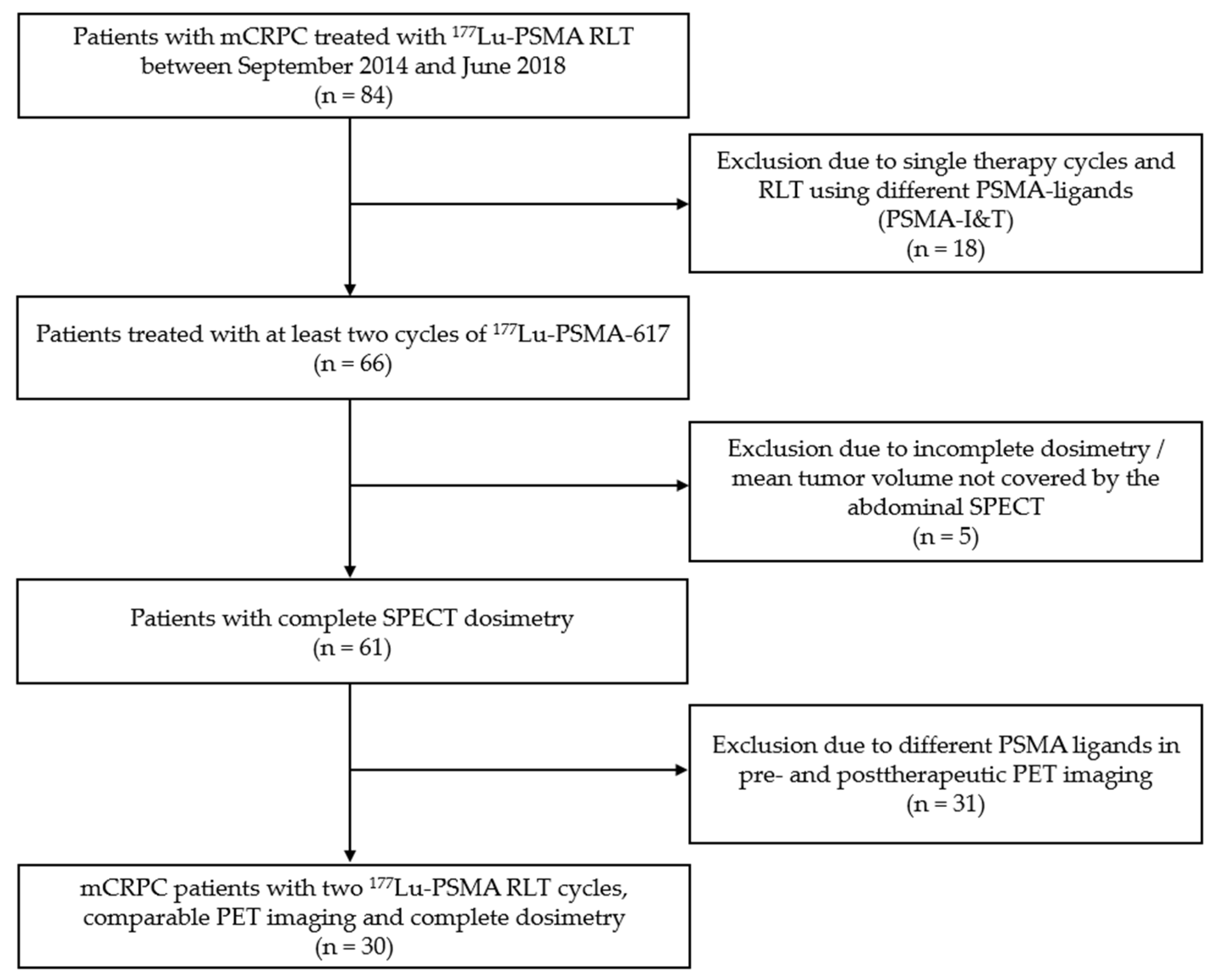
2. Materials and Methods
2.1. Patients
2.2. 177Lu-PSMA-617 Therapy
2.3. PET/CT Imaging
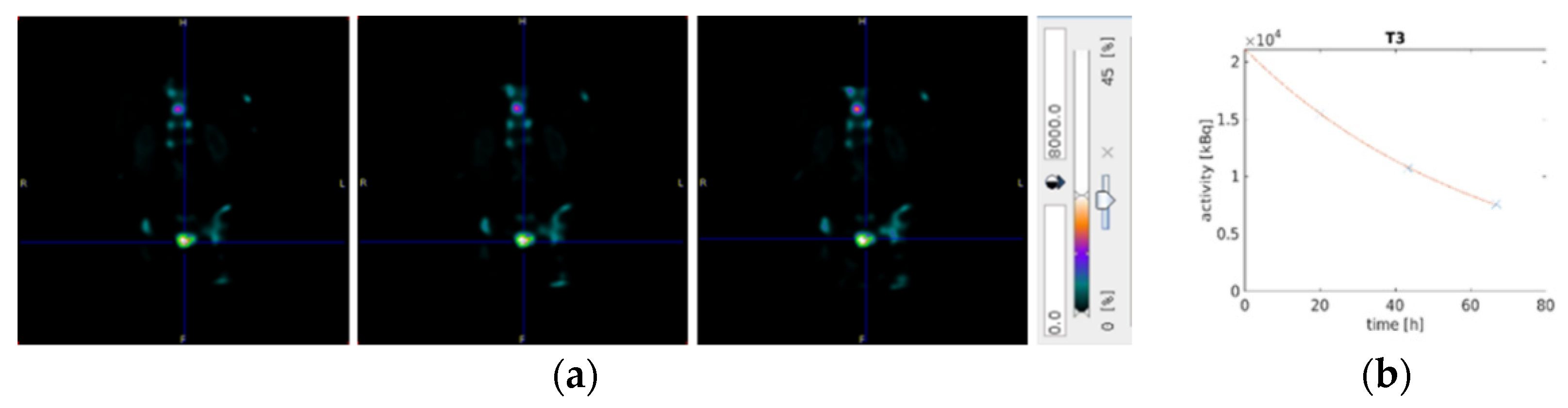
2.4. Dosimetry
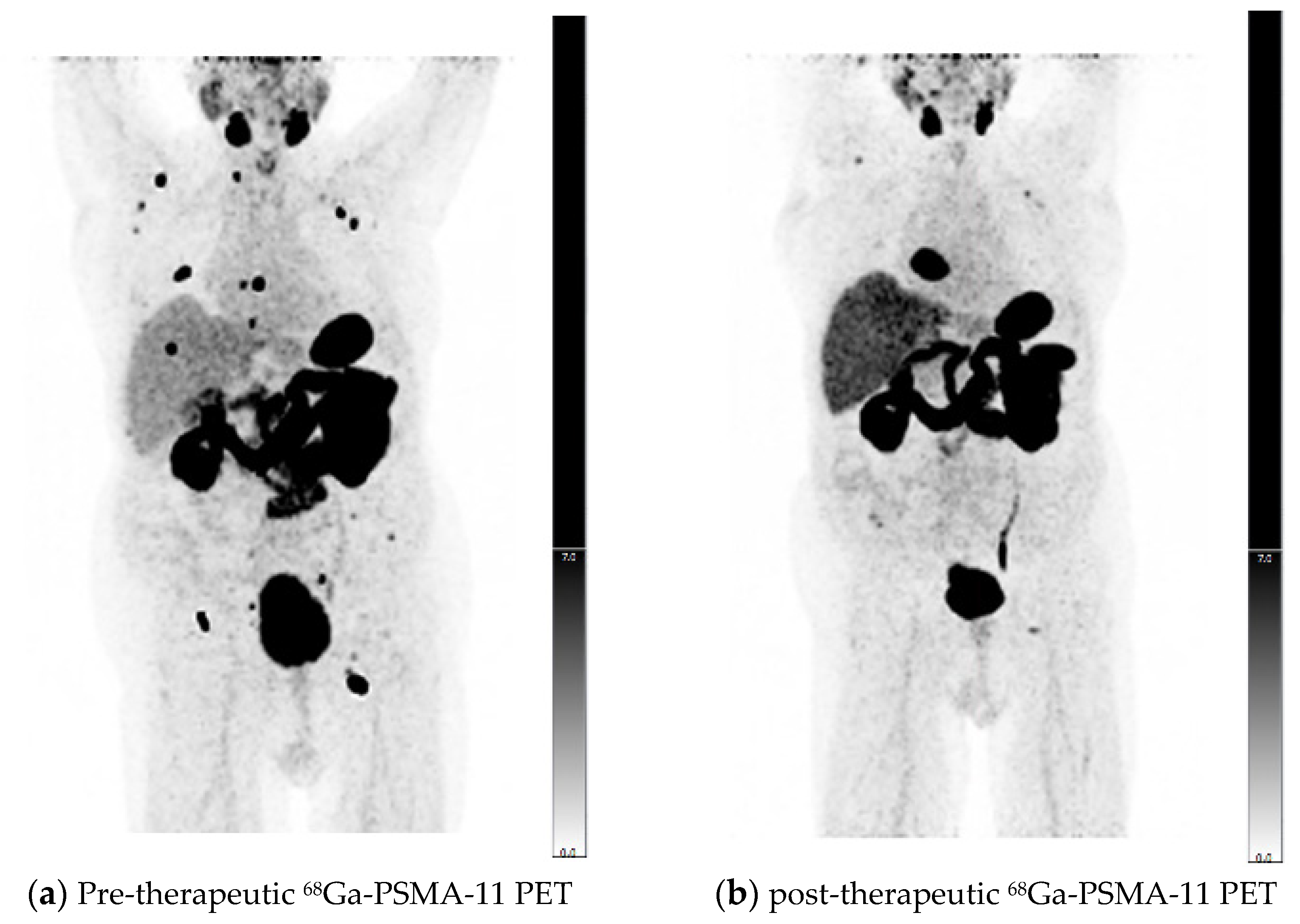
2.5. Response Assessment
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Biochemical Response Assessment
3.3. Therapy Response Assessment Using PERCIST and RECIST 1.1 Criteria
3.4. Comparison of Image-Based and -Biochemical Response Evaluation
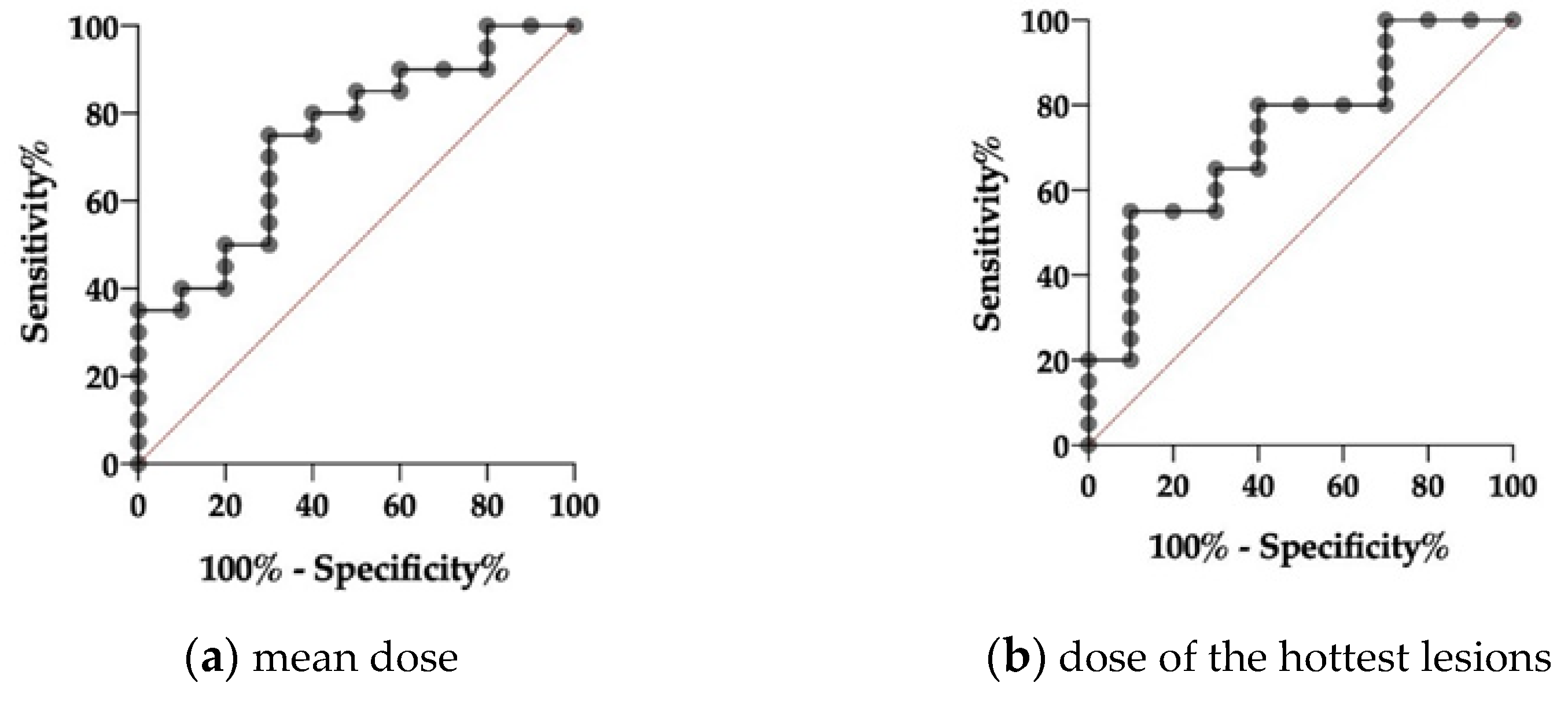
3.5. Dosimetry Results
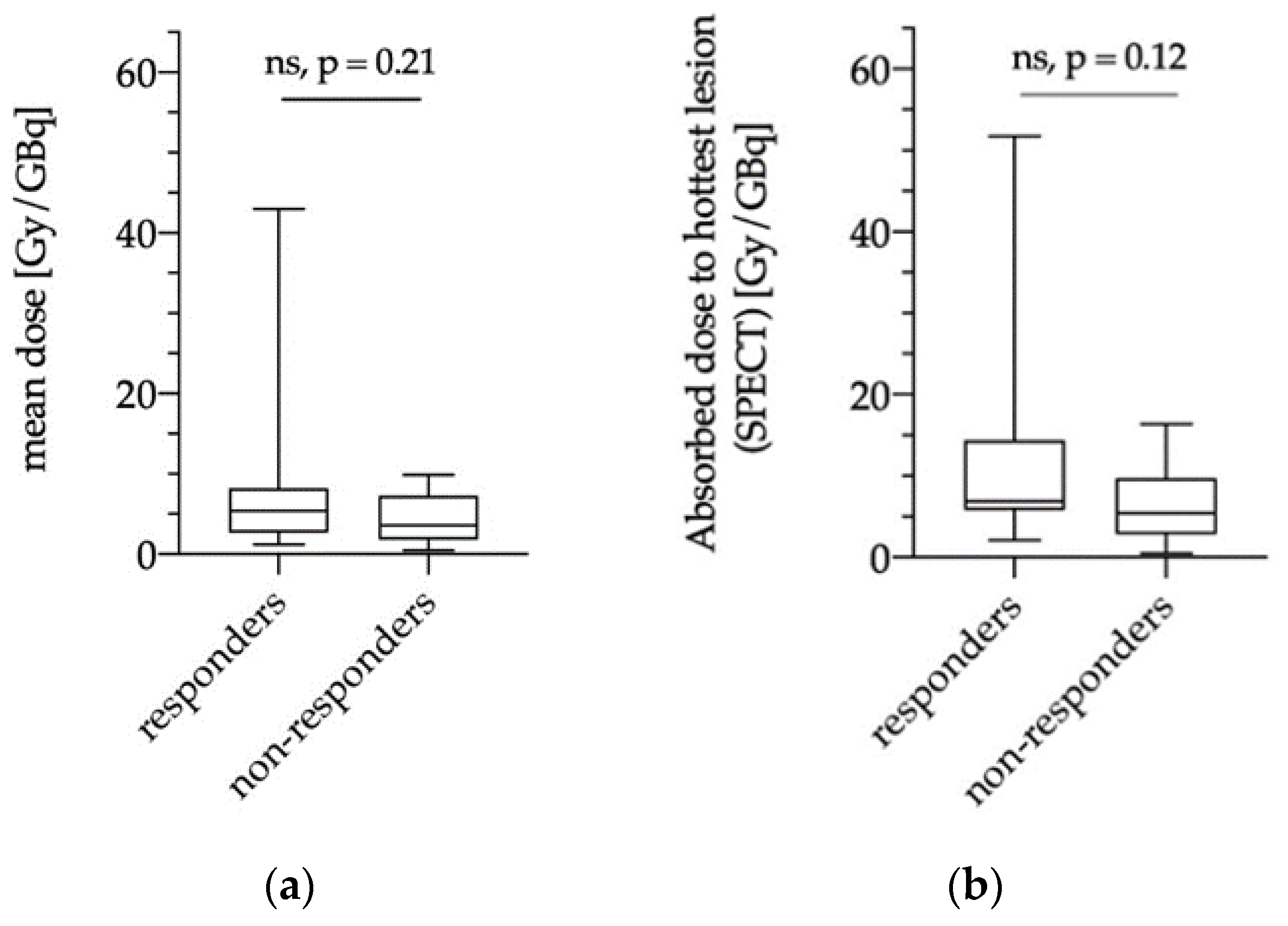
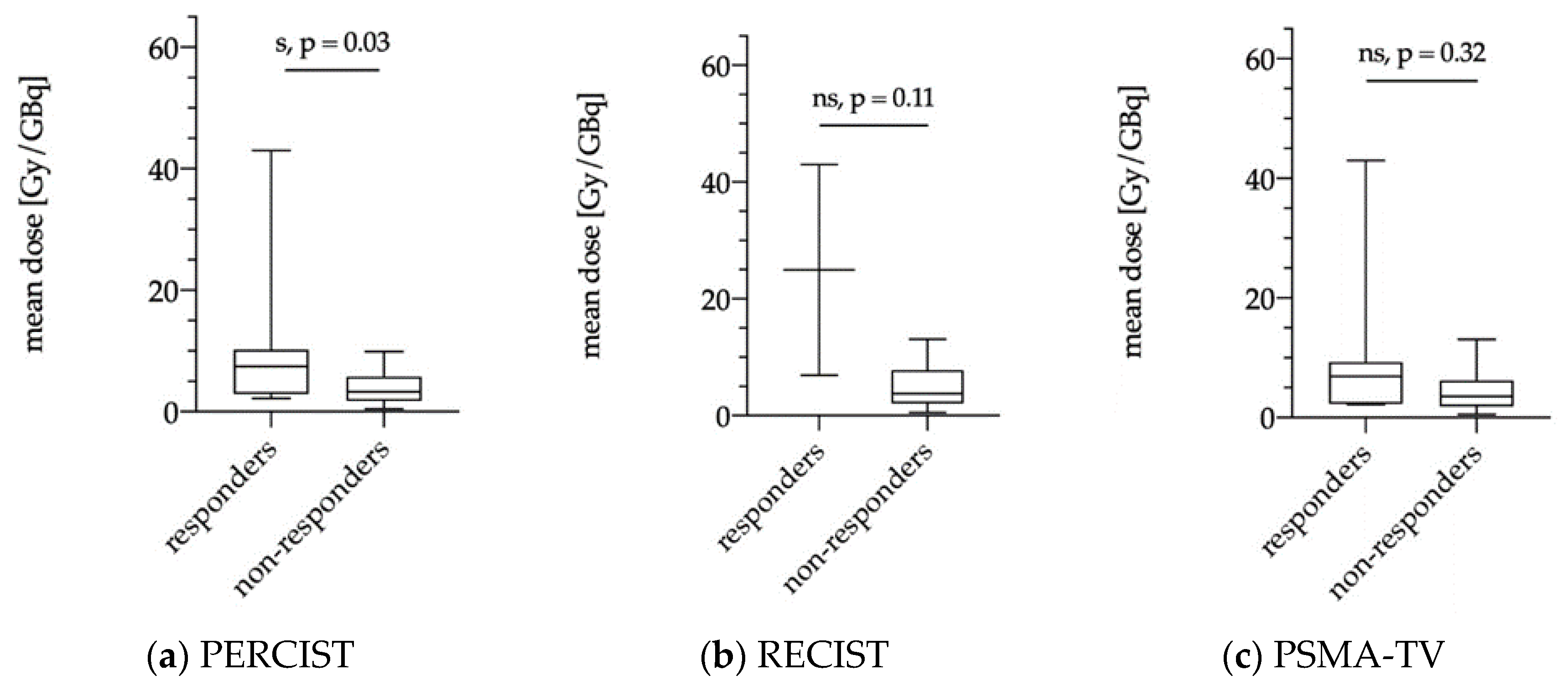
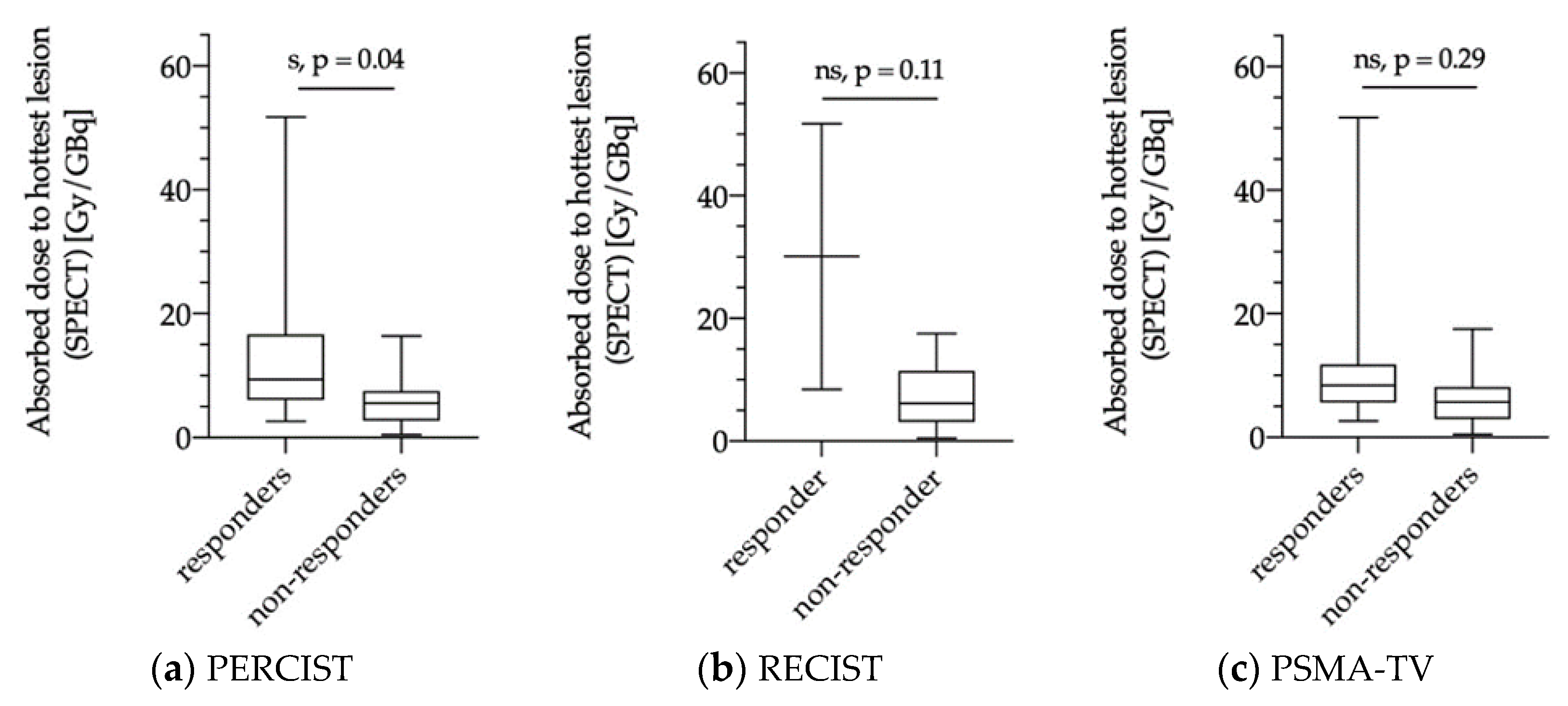
3.5.1. Dose Estimations of Biochemical Responders and Non-Responders
3.5.2. Dose Estimations for Responders and Non-responders as Evaluated via PERCIST, RECIST and PSMA-TV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Dwyer, E.; Bodei, L.; Morris, M.J. The role of theranostics in prostate cancer. Semin. Radiat. Oncol. 2021, 31, 71–82. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Rahbar, K.; Kürpig, S.; Bögemann, M.; Claesener, M.; Eppard, E.; Gärtner, F.; Rogenhofer, S.; Schäfers, M.; Essler, M. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: A two-centre study. EJNMMI Res. 2015, 5, 114. [Google Scholar] [CrossRef]
- Rahbar, K.B.M.; Yordanova, A.; Eveslage, M.; Schäfers, M.; Essler, M.; Ahmadzadehfar, H. Delayed response after repeated 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 243–246. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Tripathi, M.; Damle, N.A.; Sahoo, R.K.; Seth, A.; Bal, C. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: Safety, efficacy, and quality of life assessment. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 81–91. [Google Scholar] [CrossRef]
- Barber, T.W.; Singh, A.; Kulkarni, H.R.; Niepsch, K.; Billah, B.; Baum, R.P. Clinical outcomes of 177Lu-PSMA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J. Nucl. Med. 2019, 60, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: Safety and efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Von Eyben, F.E.; Roviello, G.; Kiljunen, T.; Uprimny, C.; Virgolini, I.; Kairemo, K.; Joensuu, T. Third-line treatment and 177Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 496–508. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Rahbar, K.; Bodei, L.; Morris, M.J. Is the vision of radioligand therapy for prostate cancer becoming a reality? An overview of the phase III VISION trial and its importance for the future of theranostics. J. Nucl. Med. 2019, 60, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Dwivedi, S.N.; Bal, C. Radioligand therapy with 177Lu-PSMA for metastatic castration-resistant prostate cancer: A systematic review and meta-analysis. AJR Am. J. Roentgenol. 2019, 213, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Delker, A.; Fendler, W.P.; Kratochwil, C.; Brunegraf, A.; Gosewisch, A.; Gildehaus, F.J.; Tritschler, S.; Stief, C.G.; Kopka, K.; Haberkorn, U.; et al. Dosimetry for 177Lu-DKFZ-PSMA-617: A new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P.; et al. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: Correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef]
- DGN-Geschäftsstelle. RLT mit Lutetium-177 Markiertem PSMA (Prostata Spezifisches Membranantigen) Antagonist (Lu-177 DKFZ-PSMA-617). Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwj0sNSi5oTvAhUCHqYKHda-CVsQFjAAegQIAxAD&url=https%3A%2F%2Fwww.nuklearmedizin.de%2Fdocs%2FLu-177-PSMA_160224.pdf&usg=AOvVaw0hdulCzjGXOZo3dk3GwgnJ (accessed on 20 December 2020).
- Fendler, W.P.R.; Reinhardt, S.; Ilhan, H.; Delker, A.; Böning, G.; Gildehaus, F.J.; Stief, C.; Bartenstein, P.; Gratzke, C.; Lehner, S.; et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget 2017, 8, 3581–3590. [Google Scholar] [CrossRef] [PubMed]
- Weineisen, M.; Simecek, J.; Margret, S.; Markus, S.; Hans-Jürgen, W. Synthesis and preclinical evaluation of DOTAGAconjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer. EJNMMI Res. 2014, 4, 63. [Google Scholar] [CrossRef]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef]
- Collarino, A.; Pereira Arias-Bouda, L.M.; Valdés Olmos, R.A.; van der Tol, P.; Dibbets-Schneider, P.; de Geus-Oei, L.-F.; van Velden, F.H.P. Experimental validation of absolute SPECT/CT quantification for response monitoring in breast cancer. Med. Phys. 2018, 45, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Violet, J.; Sandhu, S.; Iravani, A.; Ferdinandus, J.; Thang, S.P.; Kong, G.; Kumar, A.R.; Akhurst, T.; Pattison, D.A.; Beaulieu, A.; et al. Long-term follow-up and outcomes of retreatment in an expanded 50-patient single-center phase II prospective trial of. J. Nucl. Med. 2020, 61, 857–865. [Google Scholar] [CrossRef]
- Maffey-Steffan, J.; Scarpa, L.; Svirydenka, A.; Nilica, B.; Nilica, B.; Mair, C.; Buxbaum, S.; Bektic, J.; von Guggenberg, E.; Uprimny, C.; et al. The (68)Ga/(177)Lu-theragnostic concept in PSMA-targeting of metastatic castration-resistant prostate cancer: Impact of post-therapeutic whole-body scintigraphy in the follow-up. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 695–712. [Google Scholar] [CrossRef]
- Grubmüller, B.; Senn, D.; Kramer, G.; Baltzer, P.; D′Andrea, D.; Grubmüller, K.H.; Mitterhauser, M.; Eidherr, H.; Haug, A.R.; Wadsak, W.; et al. Response assessment using (68)Ga-PSMA ligand PET in patients undergoing (177)Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1063–1072. [Google Scholar] [CrossRef]
- Joo Hyun, O.; Martin, A.L.; Richard, L.W. Practical PERCIST: A simplified guide to PET response criteria in solid tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Seitz, A.K.; Rauscher, I.; Haller, B.; Krönke, M.; Luther, S.; Heck, M.M.; Horn, T.; Gschwend, J.E.; Schwaiger, M.; Eiber, M.; et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 602–612. [Google Scholar] [CrossRef]
- Powers, E.; Karachaliou, G.S.; Kao, C.; Harrison, M.R.; Hoimes, C.J.; George, D.J.; Armstrong, A.J.; Zhang, T. Novel therapies are changing treatment paradigms in metastatic prostate cancer. J. Hematol. Oncol. 2020, 13, 144. [Google Scholar] [CrossRef]
- Kluetz, P.G.; Pierce, W.; Maher, V.E.; Zhang, H.; Tang, S.; Song, P.; Liu, Q.; Haber, M.T.; Leutzinger, E.E.; Al-Hakim, A.; et al. Radium Ra 223 dichloride injection: U.S. Food and Drug Administration drug approval summary. Clin. Cancer Res. 2014, 20, 9–14. [Google Scholar] [CrossRef]
- Eberlein, U.; Cremonesi, M.; Lassmann, M. Individualized dosimetry for theranostics: Necessary, nice to have, or counterproductive? J. Nucl. Med. 2017, 58, 97S–103S. [Google Scholar] [CrossRef]
- Pfestroff, A.; Luster, M.; Jilg, C.A.; Olbert, P.J.; Ohlmann, C.H.; Lassmann, M.; Maecke, H.R.; Ezziddin, S.; Bodei, L.; Radionuclide Therapy Committee of the European Association of Nuclear Medicine. Current status and future perspectives of PSMA-targeted therapy in Europe: Opportunity knocks. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1971–1975. [Google Scholar] [CrossRef][Green Version]
- Zacho, H.D.; Nielsen, J.B.; Haberkorn, U.; Stenholt, L.; Petersen, L.J. (68) Ga-PSMA PET/CT for the detection of bone metastases in prostate cancer: A systematic review of the published literature. Clin. Physiol. Funct. Imaging 2017. [Google Scholar] [CrossRef]
- Gupta, M.; Choudhury, P.S.; Rawal, S.; Goel, H.C.; Rao, S.A. Evaluation of RECIST, PERCIST, EORTC, and MDA criteria for assessing treatment response with Ga68-PSMA PET-CT in metastatic prostate cancer patient with biochemical progression: A comparative study. Nucl. Med. Mol. Imaging 2018, 52, 420–429. [Google Scholar] [CrossRef]
- Fanti, S.; Goffin, K.; Hadaschik, B.A.; Herrmann, K.; Maurer, T.; MacLennan, S.; Oprea-Lager, D.E.; Oyen, W.J.; Rouviere, O.; Mottet, N.; et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 469–476. [Google Scholar] [CrossRef]
- Heinzel, A.; Boghos, D.; Mottaghy, F.M.; Gaertner, F.; Essler, M.; von Mallek, D.; Ahmadzadehfar, H. (68)Ga-PSMA PET/CT for monitoring response to (177)Lu-PSMA-617 radioligand therapy in patients with metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1054–1062. [Google Scholar] [CrossRef]
- Sartor, O.; Coleman, R.E.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O′Sullivan, J.M.; Vogelzang, N.J.; Bruland, O.; Kobina, S.; Wilhelm, S.; et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann. Oncol. 2017, 28, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Derlin, T.; Schmuck, S. [(177)Lu]-PSMA-617 radionuclide therapy in patients with metastatic castration-resistant prostate cancer. Lancet Oncol. 2018, 19, e372. [Google Scholar] [CrossRef]
- Rathke, H.; Giesel, F.L.; Flechsig, P.; Kopka, K.; Mier, W.; Hohenfellner, M.; Haberkorn, U.; Kratochwil, C. Repeated (177)Lu-labeled PSMA-617 radioligand therapy using treatment activities of up to 9.3 GBq. J. Nucl. Med. 2018, 59, 459–465. [Google Scholar] [CrossRef]
- Finocchiaro, D.; Gear, J.I.; Fioroni, F.; Flux, G.D.; Murray, I.; Castellani, G.; Versari, A.; Iori, M.; Grassi, E. Uncertainty analysis of tumour absorbed dose calculations in molecular radiotherapy. EJNMMI Phys. 2020, 7, 63. [Google Scholar] [CrossRef]
| Initial Clinical Characteristics | Total Patients = 30 | |
|---|---|---|
| Age (years) | ||
| Median (range) | 71.4 | (52.4–91.2) |
| Previous therapies (n) | ||
| Radical prostatectomy | 23 | (77%) |
| Radiotherapy | 19 | (63%) |
| Androgen deprivation therapy | 27 | (90%) |
| Brachytherapy | 1 | (3%) |
| Abiraterone/enzalutamide | 19 | (63%) |
| Radium-223 | 9 | (30%) |
| Chemotherapy (docetaxel and/or cabazitaxel) | 15 | (50%) |
| Serum PSA baseline (ng/mL) | ||
| Median (range) | 129.0 | (1.4–9237.0) |
| Gleason score (n = 23) | ||
| Median (range) | 9 | (6–10) |
| Metastases localization (n) | ||
| Bone | 27 | (90%) |
| Lymph nodes | 23 | (77%) |
| Liver | 3 | (10%) |
| Lungs | 2 | (7%) |
| Local recurrence | 5 | (17%) |
| Interval baseline PET–1st therapy cycle | ||
| Median (range) (weeks) | 4.4 | (0.6–16.7) |
| Interval 1st–2nd therapy cycle | ||
| Median (range) (weeks) | 8 | (4.3–10.1) |
| Interval 1st therapy cycle–follow-up PET | ||
| Median (range) (weeks) | 17.1 | (13.1–22.2) |
| PSMA-TV | ||||
|---|---|---|---|---|
| PD | SD | PR | ||
| PERCIST | PD | 8 (38%) | 3 (14%) | 1 (5%) |
| SD | 0 (0%) | 0 (0%) | 0 (0%) | |
| PR | 0 (0%) | 3 (14%) | 6 (29%) | |
| RECIST 1.1 | PD | 7 (33%) | 4 (19%) | 1 (5%) |
| SD | 1 (5%) | 2 (10%) | 4 (19%) | |
| PR | 0 (0%) | 0 (0%) | 2 (10%) | |
| PERCIST | RECIST | PSMA-TV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PD | SD | PR | PD | SD | PR | PD | SD | PR | ||
| PD | 8 (27%) | 0 (0%) | 0 (0%) | 8 (27%) | 0 (0%) | 0 (0%) | 4 (19%) | 1 (5%) | 0 (0%) | |
| PSA | SD | 8 (27%) | 0 (0%) | 1 (3%) | 8 (27%) | 1 (3%) | 0 (0%) | 3 (14%) | 2 (10%) | 1 (10%) |
| PR | 4 (13%) | 0 (0%) | 9 (27%) | 4 (13%) | 7 (27%) | 2 (7%) | 1 (5%) | 3 (14%) | 6 (29%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Völter, F.; Mittlmeier, L.; Gosewisch, A.; Brosch-Lenz, J.; Gildehaus, F.J.; Zacherl, M.J.; Beyer, L.; Stief, C.G.; Holzgreve, A.; Rübenthaler, J.; et al. Correlation of an Index-Lesion-Based SPECT Dosimetry Method with Mean Tumor Dose and Clinical Outcome after 177Lu-PSMA-617 Radioligand Therapy. Diagnostics 2021, 11, 428. https://doi.org/10.3390/diagnostics11030428
Völter F, Mittlmeier L, Gosewisch A, Brosch-Lenz J, Gildehaus FJ, Zacherl MJ, Beyer L, Stief CG, Holzgreve A, Rübenthaler J, et al. Correlation of an Index-Lesion-Based SPECT Dosimetry Method with Mean Tumor Dose and Clinical Outcome after 177Lu-PSMA-617 Radioligand Therapy. Diagnostics. 2021; 11(3):428. https://doi.org/10.3390/diagnostics11030428
Chicago/Turabian StyleVölter, Friederike, Lena Mittlmeier, Astrid Gosewisch, Julia Brosch-Lenz, Franz Josef Gildehaus, Mathias Johannes Zacherl, Leonie Beyer, Christian G. Stief, Adrien Holzgreve, Johannes Rübenthaler, and et al. 2021. "Correlation of an Index-Lesion-Based SPECT Dosimetry Method with Mean Tumor Dose and Clinical Outcome after 177Lu-PSMA-617 Radioligand Therapy" Diagnostics 11, no. 3: 428. https://doi.org/10.3390/diagnostics11030428
APA StyleVölter, F., Mittlmeier, L., Gosewisch, A., Brosch-Lenz, J., Gildehaus, F. J., Zacherl, M. J., Beyer, L., Stief, C. G., Holzgreve, A., Rübenthaler, J., Cyran, C. C., Böning, G., Bartenstein, P., Todica, A., & Ilhan, H. (2021). Correlation of an Index-Lesion-Based SPECT Dosimetry Method with Mean Tumor Dose and Clinical Outcome after 177Lu-PSMA-617 Radioligand Therapy. Diagnostics, 11(3), 428. https://doi.org/10.3390/diagnostics11030428








