Wnt/β-Catenin Signaling Regulates CXCR4 Expression and [68Ga] Pentixafor Internalization in Neuroendocrine Tumor Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. RNA Isolation and Quantitative RT-PCR
2.3. Immunohistochemistry
2.4. Western Blot
2.5. CXCR4 Receptor Uptake
2.6. Statistics
3. Results
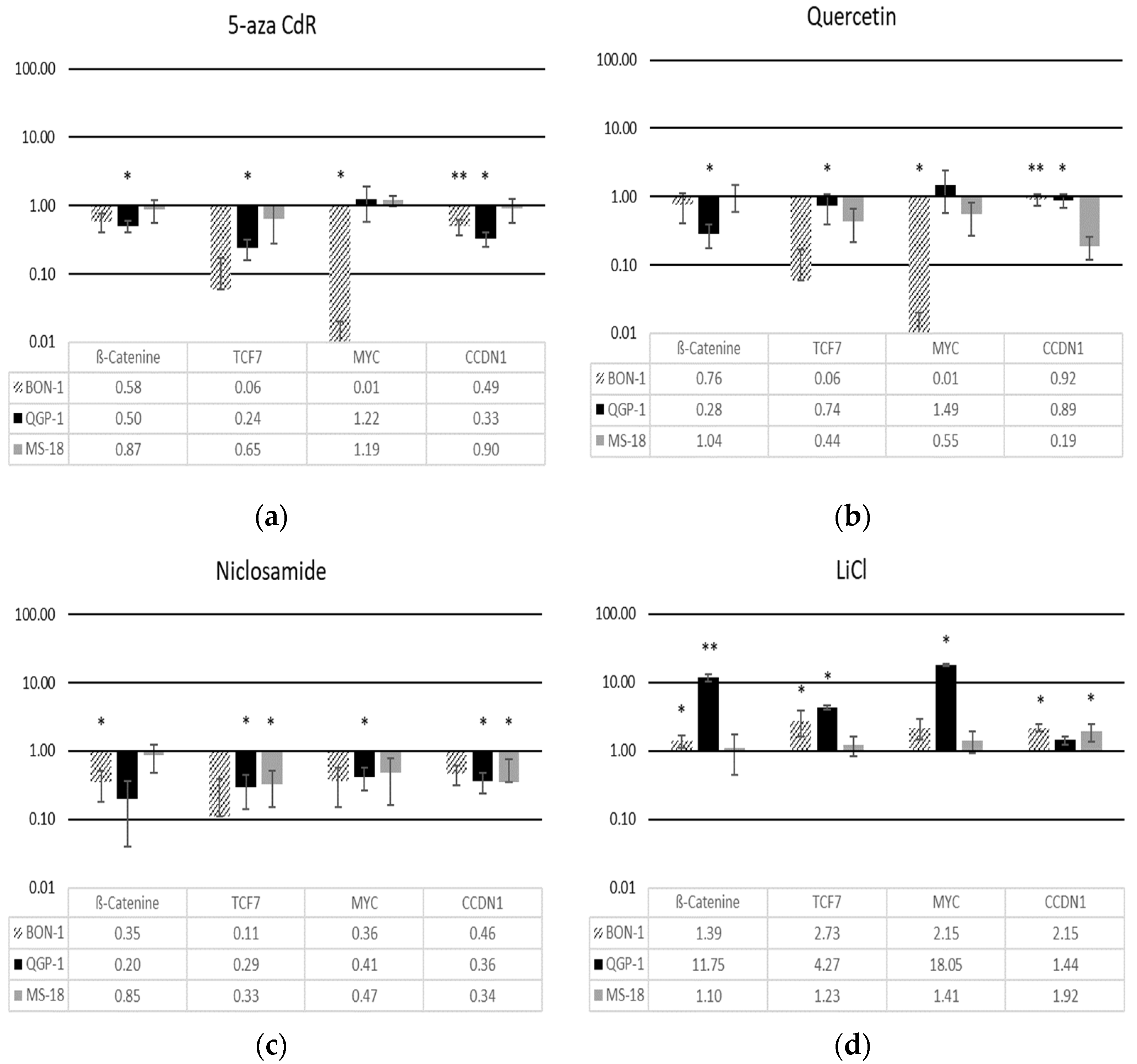
3.1. Modulation of Wnt Pathway Gene Expression
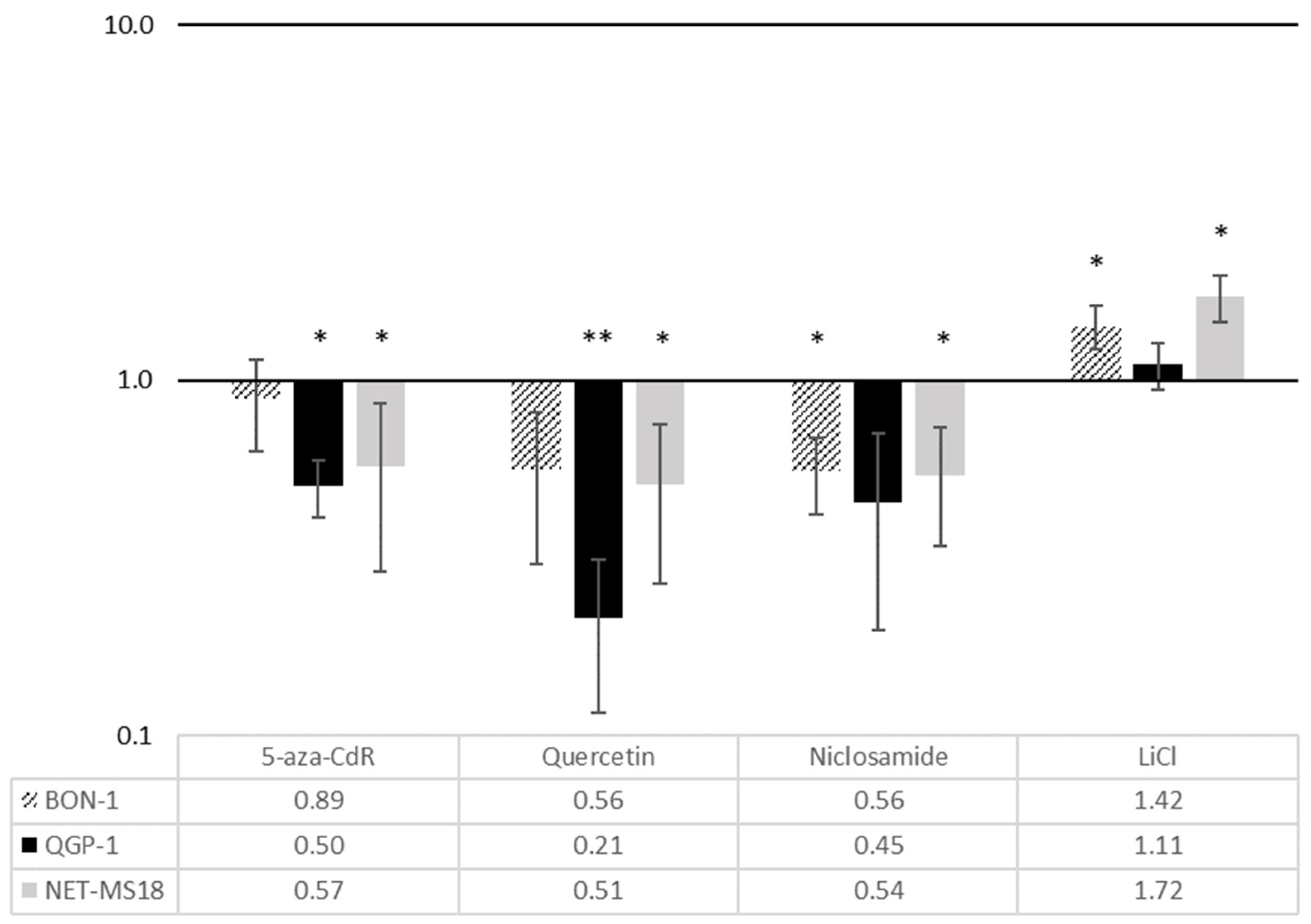
3.2. Effects of Wnt Pathway Modulation on CXCR4 Expression
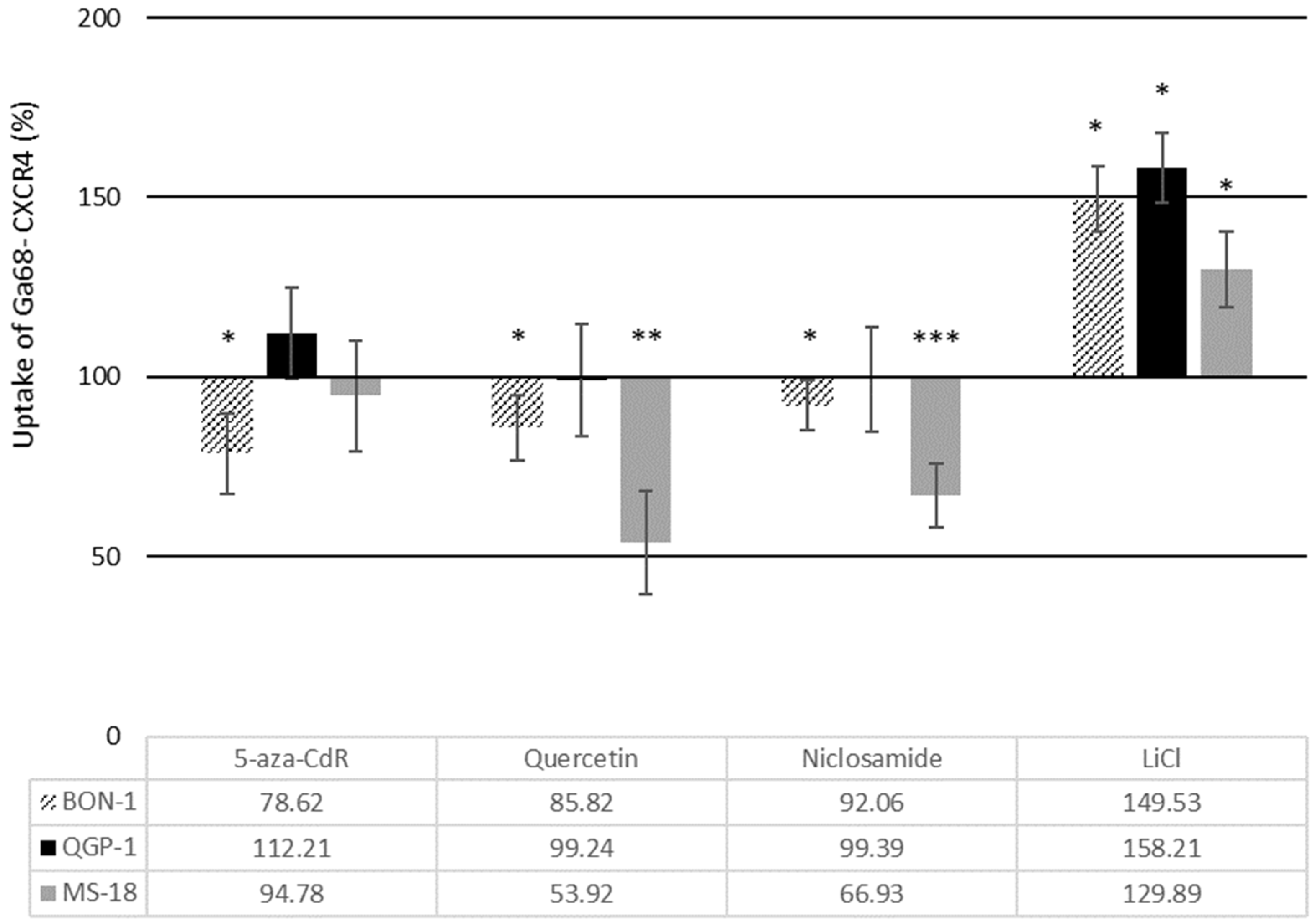
3.3. Effects of Wnt Modulators on [68Ga] Pentixafor Uptake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.Y.; Hong, S.-M. Recent Updates on Neuroendocrine Tumors From the Gastrointestinal and Pancreatobiliary Tracts. Arch. Pathol. Lab. Med. 2016, 140, 437–448. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Träger, T.; Hoffmeister, M.; Sipos, B.; Hommann, M.; Sänger, J.; Schulz, S.; Lupp, A. Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget 2015, 6, 27566–27579. [Google Scholar] [CrossRef] [PubMed]
- Teixidó, J.; Martínez-Moreno, M.; Díaz-Martínez, M.; Sevilla-Movilla, S. The good and bad faces of the CXCR4 chemokine receptor. Int. J. Biochem. Cell Biol. 2018, 95, 121–131. [Google Scholar] [CrossRef]
- Chatterjee, S.; Azad, B.B.; Nimmagadda, S. The intricate role of CXCR4 in cancer. Adv Cancer Res. 2014, 124, 31–82. [Google Scholar] [CrossRef] [PubMed]
- Eckert, F.; Schilbach, K.; Klumpp, L.; Bardoscia, L.; Sezgin, E.C.; Schwab, M.; Zips, D.; Huber, S.M. Potential Role of CXCR4 Targeting in the Context of Radiotherapy and Immunotherapy of Cancer. Front. Immunol. 2018, 9, 3018. [Google Scholar] [CrossRef]
- Rosenberg, E.M., Jr.; Harrison, R.E.S.; Tsou, L.K.; Drucker, N.; Humphries, B.; Rajasekaran, D.; Luker, K.E.; Wu, C.-H.; Song, J.-S.; Wang, C.-J.; et al. Characterization, Dynamics, and Mechanism of CXCR4 Antagonists on a Constitutively Active Mutant. Cell Chem. Biol. 2019, 26, 662–673.e7. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, S.; Liu, M.; Burow, M.E.; Wang, G. Targeting CXCL12/CXCR4 Axis in Tumor Immunotherapy. Curr. Med. Chem. 2019, 26, 3026–3041. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Vogelzang, N.J.; Conkling, P.; Raddad, E.; Polzer, J.; Roberson, S.; Stille, J.R.; Saleh, M.; Thornton, D. A phase I trial of LY2510924, a CXCR4 peptide antagonist, in patients with advanced cancer. Clin. Cancer Res. 2014, 20, 3581–3588. [Google Scholar] [CrossRef] [PubMed]
- Demmer, O.; Dijkgraaf, I.; Schumacher, U.; Marinelli, L.; Cosconati, S.; Gourni, E.; Wester, H.-J.; Kessler, H. Design, synthesis, and functionalization of dimeric peptides targeting chemokine receptor CXCR4. J. Med. Chem. 2011, 54, 7648–7662. [Google Scholar] [CrossRef]
- Gourni, E.; Demmer, O.; Schottelius, M.; D’Alessandria, C.; Schulz, S.; Dijkgraaf, I.; Schumacher, U.; Schwaiger, M.; Kessler, H.; Wester, H.-J. PET of CXCR4 expression by a (68)Ga-labeled highly specific targeted contrast agent. J. Nucl. Med. 2011, 52, 1803–1810. [Google Scholar] [CrossRef]
- Herrmann, K.; Schottelius, M.; Lapa, C.; Osl, T.; Poschenrieder, A.; Hänscheid, H.; Lückerath, K.; Schreder, M.; Bluemel, C.; Knott, M.; et al. First-in-Human Experience of CXCR4-Directed Endoradiotherapy with 177Lu- and 90Y-Labeled Pentixather in Advanced-Stage Multiple Myeloma with Extensive Intra- and Extramedullary Disease. J. Nucl. Med. 2016, 57, 248–251. [Google Scholar] [CrossRef]
- Werner, R.A.; Weich, A.; Higuchi, T.; Schmid, J.S.; Schirbel, A.; Lassmann, M.; Wild, V.; Rudelius, M.; Kudlich, T.; Herrmann, K.; et al. Imaging of Chemokine Receptor 4 Expression in Neuroendocrine Tumors—A Triple Tracer Comparative Approach. Theranostics 2017, 7, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Li, J.; Jang, E.R.; Gulhati, P.; Rychahou, P.G.; Napier, D.L.; Wang, C.; Weiss, H.L.; Lee, E.Y.; Anthony, L.; et al. Deregulation of Wnt/β-catenin signaling through genetic or epigenetic alterations in human neuroendocrine tumors. Carcinogenesis 2013, 34, 953–961. [Google Scholar] [CrossRef]
- Jin, X.-F.; Spoettl, G.; Maurer, J.; Nölting, S.; Auernhammer, C.J. Inhibition of Wnt/beta-Catenin Signaling in Neuroendocrine Tumors in vitro: Antitumoral Effects. Cancers (Basel) 2020, 12, 345. [Google Scholar] [CrossRef]
- Cives, M.; Quaresmini, D.; Rizzo, F.M.; Felici, C.; D’Oronzo, S.; Simone, V.; Silvestris, F. Osteotropism of neuroendocrine tumors: Role of the CXCL12/ CXCR4 pathway in promoting EMT in vitro. Oncotarget 2017, 8, 22534–22549. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, Q. Beta-catenin is a promising key factor in the SDF-1/CXCR4 axis on metastasis of pancreatic cancer. Med Hypotheses 2007, 69, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Choe, Y.; Pleasure, S.J. Wnt signaling regulates intermediate precursor production in the postnatal dentate gyrus by regulating CXCR4 expression. Dev. Neurosci. 2012, 34, 502–514. [Google Scholar] [CrossRef]
- Exner, S.; Prasad, V.; Wiedenmann, B.; Grötzinger, C. Octreotide Does Not Inhibit Proliferation in Five Neuroendocrine Tumor Cell Lines. Front. Endocrinol. 2018, 9, 146. [Google Scholar] [CrossRef]
- Veenstra, M.J.; Van Koetsveld, P.M.; Dogan, F.; Farrell, W.E.; Feelders, R.A.; Lamberts, S.W.; De Herder, W.W.; Vitale, G.; Hofland, L.J. Epidrug-induced upregulation of functional somatostatin type 2 receptors in human pancreatic neuroendocrine tumor cells. Oncotarget 2016, 9, 14791–14802. [Google Scholar] [CrossRef] [PubMed]
- Karahoca, M.; Momparler, R.L. Pharmacokinetic and pharmacodynamic analysis of 5-aza-2’-deoxycytidine (decitabine) in the design of its dose-schedule for cancer therapy. Clin. Epigenet. 2013, 5, 3. [Google Scholar] [CrossRef]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef]
- Burock, S.; Daum, S.; Keilholz, U.; Neumann, K.; Walther, W.; Stein, U. Phase II trial to investigate the safety and efficacy of orally applied niclosamide in patients with metachronous or sychronous metastases of a colorectal cancer progressing after therapy: The NIKOLO trial. BMC Cancer 2018, 18, 1–7. [Google Scholar] [CrossRef]
- Nielsen-Kudsk, F.; Amdisen, A. Analysis of the pharmacokinetics of lithium in man. Eur. J. Clin. Pharmacol. 1979, 16, 271–277. [Google Scholar] [CrossRef]
- Kim, Y.S.; Noh, M.Y.; Kim, J.Y.; Yu, H.-J.; Kim, K.S.; Kim, S.H.; Koh, S.-H. Direct GSK-3beta inhibition enhances mesenchymal stromal cell migration by increasing expression of beta-PIX and CXCR4. Mol. Neurobiol. 2013, 47, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Rogoll, D.; Schauber, J.; Mheta, K.K.; Stich, A.; Scheppach, W. Differential cathelicidin expression in duodenal and gastric biopsies from Tanzanian and German patients. PLoS ONE 2011, 6, e22049. [Google Scholar] [CrossRef]
- Luhrs, H.; Hock, R.; Schauber, J.; Weihrauch, M.; Harrer, M.; Melcher, R.; Scheppach, W.; Bustin, M.; Menzel, T. Modulation of HMG-N2 binding to chromatin by butyrate-induced acetylation in human colon adenocarcinoma cells. Int. J. Cancer 2002, 97, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Cao, Y.; Li, F.; Su, Y.; Li, Y.; Peng, Y.; Cheng, Y.; Zhang, C.; Wang, W.; Ning, G. Targeting β-catenin signaling for therapeutic intervention in MEN1-deficient pancreatic neuroendocrine tumours. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Aristizabal Prada, E.T.; Auernhammer, C.J. Targeted therapy of gastroenteropancreatic neuroendocrine tumours: Preclinical strategies and future targets. Endocr. Connect. 2018, 7, R1–R25. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.; Lines, K.E.; Thakker, R.V. Current and emerging therapies for PNETs in patients with or without MEN1. Nat. Rev. Endocrinol. 2018, 14, 216–227. [Google Scholar] [CrossRef]
- Rennoll, S. Regulation ofMYCgene expression by aberrant Wnt/β-catenin signaling in colorectal cancer. World J. Biol. Chem. 2015, 6, 290–300. [Google Scholar] [CrossRef]
- Shtutman, M.; Zhurinsky, J.; Simcha, I.; Albanese, C.; D’Amico, M.; Pestell, R.; Ben-Ze’Ev, A. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 5522–5527. [Google Scholar] [CrossRef]
- Cadigan, K.M.; Waterman, M.L. TCF/LEFs and Wnt Signaling in the Nucleus. Cold Spring Harb. Perspect. Biol. 2012, 4, a007906. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, T.; Peeters, M.; Dogan, F.; Pauwels, P.; Van Assche, E.; Beyens, M.; Mortier, G.; Vandeweyer, G.; De Herder, W.; Van Camp, G.; et al. Whole-exome characterization of pancreatic neuroendocrine tumor cell lines BON-1 and QGP-1. J. Mol. Endocrinol. 2015, 54, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Luley, K.B.; Biedermann, S.B.; Künstner, A.; Busch, H.; Franzenburg, S.; Schrader, J.; Grabowski, P.; Wellner, U.F.; Keck, T.; Brabant, G.; et al. A Comprehensive Molecular Characterization of the Pancreatic Neuroendocrine Tumor Cell Lines BON-1 and QGP-1. Cancers 2020, 12, 691. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Chang, J.Y.; Hahm, E.R.; Park, S.; Kim, H.-K.; Yang, C.H. Quercetin, a potent inhibitor against beta-catenin/Tcf signaling in SW480 colon cancer cells. Biochem. Biophys. Res. Commun. 2005, 328, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, J.; Lu, J.; Bond, M.C.; Ren, X.-R.; Lyerly, H.K.; Barak, L.S.; Chen, W. The Anti-Helminthic Niclosamide Inhibits Wnt/Frizzled1 Signaling. Biochemistry 2009, 48, 10267–10274. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.S.; Melton, D.A. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 1996, 93, 8455–8459. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.K.; Leng, Y.; Wang, Z.; Leeds, P.; Chuang, D.M. The mood stabilizers valproic acid and lithium enhance mesenchymal stem cell migration via distinct mechanisms. Neuropsychopharmacology 2010, 35, 2225–2237. [Google Scholar] [CrossRef][Green Version]
- Wang, R.; Yang, L.; Li, S.; Ye, D.; Yang, L.; Liu, Q.; Zhao, Z.; Cai, Q.; Tan, J.; Li, X. Quercetin Inhibits Breast Cancer Stem Cells via Downregulation of Aldehyde Dehydrogenase 1A1 (ALDH1A1), Chemokine Receptor Type 4 (CXCR4), Mucin 1 (MUC1), and Epithelial Cell Adhesion Molecule (EpCAM). Med. Sci. Monit. 2018, 24, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Feng, Y.; Wang, J.; Zhang, X.; Shen, J.; Zou, R.; Yuan, Y. Platelet-derived growth factor-BB promotes proliferation and migration of retinal microvascular pericytes by up-regulating the expression of C-X-C chemokine receptor types 4. Exp. Ther. Med. 2019, 18, 4022–4030. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.A.S.; Grochoski, M.; Braun-Prado, K.; Seniski, G.G.; Cavalli, I.J.; Ribeiro, E.M.S.F.; Camargo, A.A.; Costa, F.F.; Klassen, G. Epigenetic changes of CXCR4 and its ligand CXCL12 as prognostic factors for sporadic breast cancer. PLoS ONE 2011, 6, e29461. [Google Scholar] [CrossRef]
- English, E.J.; Mahn, S.A.; Marchese, A. Endocytosis is required for CXC chemokine receptor type 4 (CXCR4)-mediated Akt activation and antiapoptotic signaling. J. Biol. Chem. 2018, 293, 11470–11480. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weich, A.; Rogoll, D.; Gawlas, S.; Mayer, L.; Weich, W.; Pongracz, J.; Kudlich, T.; Meining, A.; Scheurlen, M. Wnt/β-Catenin Signaling Regulates CXCR4 Expression and [68Ga] Pentixafor Internalization in Neuroendocrine Tumor Cells. Diagnostics 2021, 11, 367. https://doi.org/10.3390/diagnostics11020367
Weich A, Rogoll D, Gawlas S, Mayer L, Weich W, Pongracz J, Kudlich T, Meining A, Scheurlen M. Wnt/β-Catenin Signaling Regulates CXCR4 Expression and [68Ga] Pentixafor Internalization in Neuroendocrine Tumor Cells. Diagnostics. 2021; 11(2):367. https://doi.org/10.3390/diagnostics11020367
Chicago/Turabian StyleWeich, Alexander, Dorothee Rogoll, Sophia Gawlas, Lars Mayer, Wolfgang Weich, Judit Pongracz, Theodor Kudlich, Alexander Meining, and Michael Scheurlen. 2021. "Wnt/β-Catenin Signaling Regulates CXCR4 Expression and [68Ga] Pentixafor Internalization in Neuroendocrine Tumor Cells" Diagnostics 11, no. 2: 367. https://doi.org/10.3390/diagnostics11020367
APA StyleWeich, A., Rogoll, D., Gawlas, S., Mayer, L., Weich, W., Pongracz, J., Kudlich, T., Meining, A., & Scheurlen, M. (2021). Wnt/β-Catenin Signaling Regulates CXCR4 Expression and [68Ga] Pentixafor Internalization in Neuroendocrine Tumor Cells. Diagnostics, 11(2), 367. https://doi.org/10.3390/diagnostics11020367






