Effect of the Cut-Off Level for Thyroid-Stimulating Hormone on the Prevalence of Subclinical Hypothyroidism among Infertile Mexican Women
Abstract
1. Introduction
2. Materials and Methods
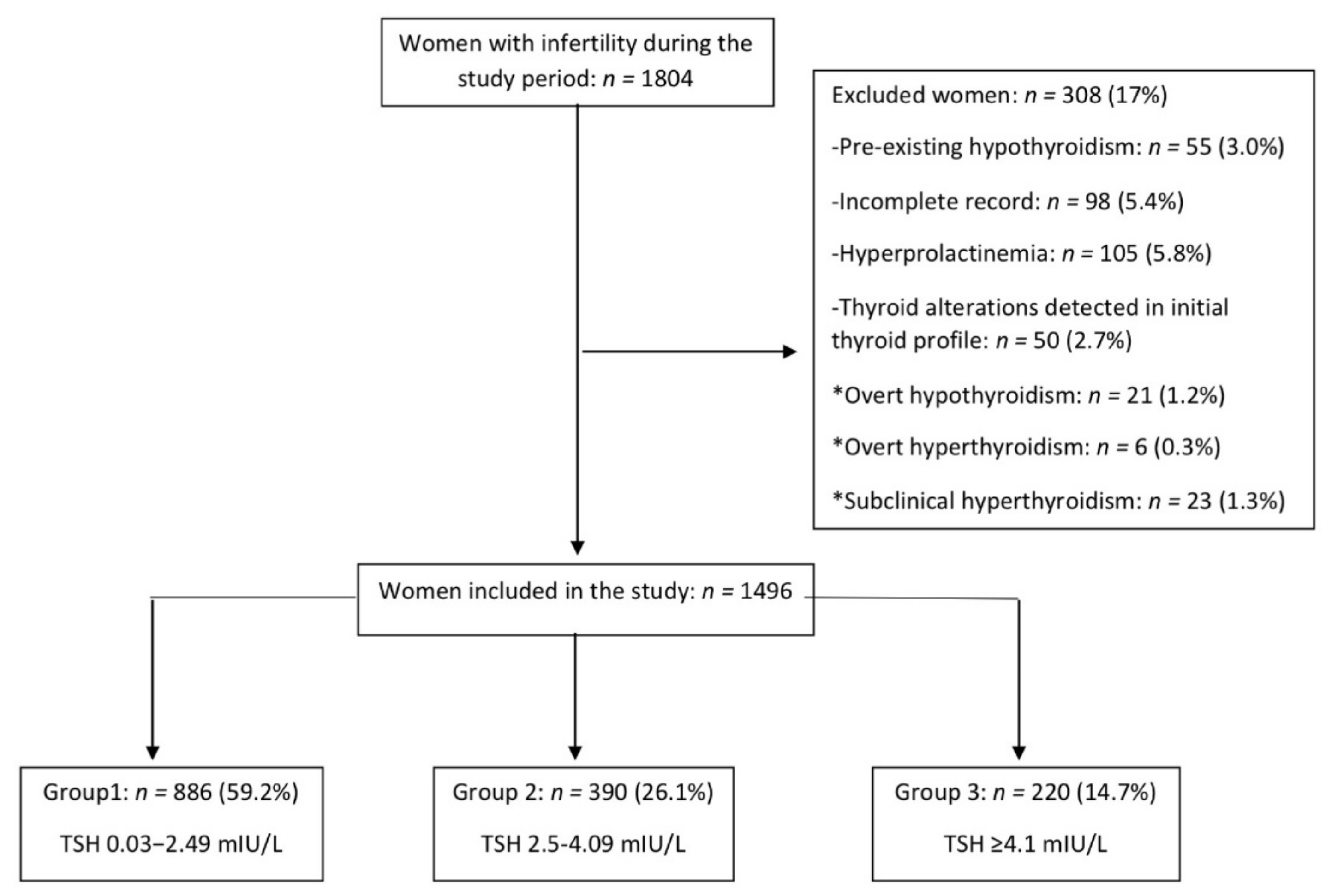
2.1. Study Design and Patients
2.2. Data Collection
2.3. Laboratory Methods
2.4. Study Variables and Endpoints
2.5. Sample Size
2.6. Statistical Analysis
3. Results
3.1. Participants
3.2. SCH Prevalence and Thyroid Profile
3.3. Clinical Features
3.4. Other Biochemical Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef]
- Maraka, S.; Ospina, N.M.; Mastorakos, G.; O’Keeffe, D.T. Subclinical Hypothyroidism in Women Planning Conception and During Pregnancy: Who Should Be Treated and How? J. Endocr. Soc. 2018, 2, 533–546. [Google Scholar] [CrossRef]
- American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: A guideline. Fertil. Steril. 2015, 104, 545–553. [Google Scholar] [CrossRef]
- Stagnaro-Green, A.; Abalovich, M.; Alexander, E.; Azizi, F.; Mestman, J.; Negro, R.; Nixon, A.; Pearce, E.N.; Soldin, O.P.; Sullivan, S.; et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011, 21, 1081–1125. [Google Scholar] [CrossRef]
- Korevaar, T.I.M.; Medici, M.; Visser, T.J.; Peeters, R.P. Thyroid disease in pregnancy: New insights in diagnosis and clinical management. Nat. Rev. Endocrinol. 2017, 13, 610–622. [Google Scholar] [CrossRef]
- Biondi, B.; Cooper, D.S. The clinical significance of subclinical thyroid dysfunction. Endocr. Rev. 2008, 29, 76–131. [Google Scholar] [CrossRef]
- Maheshwari, A.; Bhide, P.; Pundir, I.; Bhattacharya, S. Routine serum thyroid-stimulating hormone testing-optimizing pre-conception health or generating toxic knowledge? Hum. Reprod. 2017, 32, 1779–1785. [Google Scholar] [CrossRef]
- Poppe, K.; Glinoer, D.; Van Steirteghem, A.; Tournaye, H.; Devroey, P.; Schiettecatte, J.; Velkeniers, B. Thyroid dysfunction and autoimmunity in infertile women. Thyroid 2002, 11, 997–1001. [Google Scholar] [CrossRef]
- American College Obstetrics and Gynecologist. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obtet. Gynecol. 2020, 135, e261–e274. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Fertility Problems: Assessment and Treatment. London, 2017. Available online: www.nice.org.uk/guidance/cg156 (accessed on 1 December 2019).
- Vissenberg, R.; Manders, V.D.; Mastenbroek, S.; Fliers, E.; Afink, G.B.; Ris-Stalpers, C.; Goddijn, M.; Bisschop, P.H. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum. Reprod. Update 2015, 21, 378–387. [Google Scholar] [CrossRef]
- Arojoki, M.; Jokimaa, V.; Juuti, A.; Koskinen, P.; Irjala, K.; Anttila, L. Hypothyroidism among infertile women in Finland. Gynecol. Endocrinol. 2000, 14, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Poppe, K.; Glinoer, D. Thyroid autoimmunity and hypothyroidism before and during pregnancy. Hum. Reprod. Update 2003, 9, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Raber, W.; Nowotny, P.; Vytiska-Binstorfer, E.; Vierhapper, H. Thyroxine treatment modified in infertile women according to thyroxine-releasing hormone testing: 5 year follow-up of 283 women referred after exclusion of absolute causes of infertility. Hum. Reprod. 2003, 18, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Shalev, E.; Eliyahu, S.; Ziv, M.; Ben-Ami, M. Routine thyroid function test in infertile women: Are they necessary? Am. J. Obstet. Gynecol. 1994, 171, 1191–1192. [Google Scholar] [CrossRef]
- Reh, A.; Grifo, J.; Danoff, A. What is a normal thyroid-stimulating hormone (TSH) level? Effects of stricter TSH thresholds on pregnancy outcomes after in vitro fertilization. Fertil. Steril. 2010, 94, 2920–2922. [Google Scholar] [CrossRef]
- Méndez-Villa, L.; Elton-Puente, J.E.; Solís-S, J.C.; Sampson-Zaldívar, E.; García-G, C.; Villalobos, P.; Colarossi, A.; García, O.P.; Robles-Osorio, L.; García-Solís, P. Iodine nutrition and thyroid function assessment in childbearing age women from Queretaro, Mexico. Nutr. Hosp. 2014, 29, 204–211. [Google Scholar]
- Flores-Rebollar, A.; Moreno-Castañeda, L.; Vega-Servín, N.S.; López-Carrasco, G.; Ruiz-Juvera, A. Determination of thyrotropin reference values in an adult Mexican population. Endocrinol. Nutr. 2015, 62, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Feldthusen, A.D.; Pedersen, P.L.; Larsen, J.; Kristensen, T.T.; Ellervik, C.; Kvetny, I. Impaired Fertility Associated with Subclinical Hypothyroidism and Thyroid Autoimmunity: The Danish General Suburban Population Study. J. Pregnancy 2015, 2015, 132718. [Google Scholar] [CrossRef] [PubMed]
- Garber, J.R.; Cobin, R.H.; Gharib, H.; Hennessey, J.V.; Klein, I.; Mechanick, J.I.; Pessah-Pollack, R.; Singer, P.A.; Woeber, K.N. American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr. Pr. 2012, 18, 988–1028. [Google Scholar]
- Kim, C.H.; Ahn, J.W.; Kang, S.P.; Kim, S.H.; Chae, H.D.; Kang, B.M. Effect of levothyroxine treatment on in vitro fertilization and pregnancy outcome in infertile women with subclinical hypothyroidism undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil. Steril. 2011, 95, 1650–1654. [Google Scholar] [CrossRef]
- Velkeniers, B.; Van-Meerhaeghe, A.; Poppe, K.; Unuane, D.; Tournaye, H.; Haentjens, P. Levothyroxine treatment and pregnancy outcome in women with subclinical hypothyroidism undergoing assisted reproduction technologies: Systematic review and meta-analysis of RCTs. Hum. Reprod. Update 2013, 19, 251–258. [Google Scholar] [CrossRef]
- Negro, R.; Stagnaro-Green, A. Clinical aspects of hyperthyroidism, hypothyroidism, and thyroid screening in pregnancy. Endocr. Pract. 2014, 20, 597–607. [Google Scholar] [CrossRef]
- Rotterdam ESHRE/ASRM- Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risk related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Abalovich, M.; Mitelberg, L.; Allami, C.; Gutierrez, S.; Alcaraz, G.; Otero, P.; Levalle, O. Subclinical hypothyroidism and thyroid autoimmunity in women with infertility. Gynecol. Endocrinol. 2007, 23, 279–283. [Google Scholar] [CrossRef]
- Grassi, G.; Balsamo, A.; Ansaldi, C.; Balbo, A.; Massobrio, M.; Benedetto, C. Thyroid autoimmunity and infertility. Gynecol. Endocrinol. 2001, 15, 389–396. [Google Scholar] [CrossRef]
- Gerhard, I.; Becker, T.; Eggert-Kruse, W.; Klinga, K.; Runnebaum, B. Thyroid and ovarian function in infertile women. Hum. Reprod. 1991, 6, 338–345. [Google Scholar] [CrossRef]
- Castillo, C.; Lustig, C.; Margozzini, P.; Gomez, A.; Rojas, M.P.; Muzzo, S.; Mosso, L. Thyroid-Stimulating Hormone Reference Ranges in the First Trimester of Pregnancy in an Iodine-Sufficient Country. Endocrinol. Metab. 2018, 33, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Shan, Z.; Teng, X.; Guan, H.; Li, Y.; Teng, D.; Jin, Y.; Yu, X.; Fan, C.; Chong, W.; et al. Efect of iodine intake on thyroid diseases in China. N. Engl. J. Med. 2006, 354, 2783–2793. [Google Scholar] [CrossRef] [PubMed]
- Flores-Rebollar, A.; Pérez-Díaz, I.; Vega-Vega, O.; Rivera-Moscoso, R.; Fagundo-Sierra, R.; Carbajal-Morelos, S.L.; Osorio-Landa, H.K.; López-Carrasco, M.G.; Lira-Reyes, A.; Correa-Rotter, R. Prevalence of thyroid dysfunction in healthy adults according to the estimated iodine intake in 24-h urine samples: The SALMEX cohort. Eur. J. Nutr. 2021, 60, 399–409. [Google Scholar] [CrossRef]
- Benetti-Pinto, C.L.; Berini Piccolo, V.R.; Garmes, H.M.; Teatin Juliato, C.R. Subclinical hypothyroidism in young women with polycystic ovary syndrome: An analysis of clinical, hormonal, and metabolic parameters. Fertil. Steril. 2013, 99, 588–592. [Google Scholar] [CrossRef]
- Van Hulsteijn, L.T.; Pasquali, R.; Casanueva, F.; Haluzik, M.; Ledoux, S.; Monteiro, M.P.; Salvador, I.; Santini, F.; Toplak, H.; Dekkers, O.M. Prevalence of endocrine disorders in obese patients: Systematic review and meta-analysis. Eur. J. Endocrinol. 2020, 182, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, M.; Magri, F.; Chiovato, L. Thyroid and obesity: Not a one-way interaction. J. Clin. Endocrinol. Metab. 2011, 96, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Santini, F.; Marzullo, P.; Rotondi, M.; Ceccarini, G.; Pagano, L.; Ippolito, S.; Chiovato, L.; Biondi, B. Mechanisms in endocrinology: The crosstalk between thyroid gland and adipose tissue: Signal integration in health and disease. Eur. J. Endocrinol. 2014, 171, R137–R152. [Google Scholar] [CrossRef]
- Taylor, P.N.; Richmond, R.; Davies, N.; Sayers, A.; Stevenson, K.; Woltersdorf, W.; Taylor, A.; Groom, A.; Northstone, K.; Ring, S.; et al. Paradoxical Relationship Between Body Mass Index and Thyroid Hormone Levels: A Study Using Mendelian Randomization. J. Clin. Endocrinol. Metab. 2016, 101, 730–738. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fitzgerald, S.P.; Bean, N.G.; Falhammar, H.; Tuke, J. Clinical Parameters Are More Likely to Be Associated with Thyroid Hormone Levels than with Thyrotropin Levels: A Systematic Review and Meta-Analysis. Thyroid 2020, 30, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
| Thyroid Determination | Group 1 (TSH 0.3–2.49 mIU/L) n = 886 | Group 2 (TSH 2.5–4.09 mIU/L) n= 390 | p Group 1 vs. 2 | Group 3 (TSH ≥ 4.1 mIU/L) n = 220 | p Group 1 vs. 3 |
|---|---|---|---|---|---|
| TSH (mUI/L) | 1.55 ± 0.5 | 3.1 ± 0.4 | 0.0001 | 5.6 ± 1.3 * | 0.0001 |
| T3T (ng/dL) | 122 ± 28 | 128 ± 28 | 0.001 | 130 ± 28 | 0.0001 |
| fT4 (ng/dL) | 1.29 ± 0.29 | 1.23 ± 0.24 | 0.0001 | 1.16± 0.21 * | 0.0001 |
| Thyroid antibody determination | n = 115 (13.0%) | n = 252 (64.6%) | n = 170 (77.3%) | ||
| Positive TPO-Abs | n = 9 (7.8%) | n = 33 (13.1%) | 0.19 | n = 33 (19.4%) | 0.01 |
| Positive TG-Abs | n = 4 (3.5%) | n = 14 (5.5%) | 0.55 | n = 11 (6.5%) | 0.4 |
| Thyroid autoimmunity | n = 11 (9.6%) | n = 34 (13.5%) | 0.37 | n = 33 (19.4%) | 0.03 |
| Characteristic | Group 1 TSH 0.3−2.49 mIU/L n = 886 | Group 2 (TSH 2.5−4.09 mIU/L) n = 390 | p Group 1 vs. 2 | Group 3 (TSH ≥ 4.1 mIU/L) n = 220 | p Group 1 vs. 3 |
|---|---|---|---|---|---|
| Age (years) | 29.8 ± 3.9 | 30.1 ± 4.1 | 0.98 | 30.9 ± 4.8 | 0.27 |
| Years of Infertility | 5.1 ± 3 | 5.1 ± 3.1 | 0.98 | 5.1 ± 3 | 0.98 |
| Weight (kg) | 66.2 ±12 | 68.1 ± 12.9 | 0.03 | 70.3 ± 13.7 | 0.0001 |
| Height (m) | 1.55 ± 0.06 | 1.56 ± 0.06 | 0.41 | 1.56 ± 0.06 | 0.14 |
| BMI (kg/m2) | 27.3 ± 4.5 | 27.9 ± 4.8 | 0.11 | 28.7 ± 5.1 | 0.0001 |
| Primary infertility | 668 (75.4) | 300 (76.9) | 0.75 | 168 (76.4) | 0.96 |
| Secondary infertility | 218 (24.6) | 90 (23.1) | 0.75 | 52(23.6) | 0.96 |
| Normal weight (BMI 18.5−24.99 Kg/m2) | 299 (33.7) | 103 (26.4) | 0.01 | 58 (26.4) | 0.04 |
| Overweight (BMI 25−29.99 Kg/m2) | 354 (40) | 184 (47.2) | 0.01 | 77 (35) * | 0.20 |
| Obesity (BMI ≥30 Kg/m2) | 233 (26.3) | 103 (26.4) | 0.97 | 85 (38.6) * | 0.0001 |
| Oligo-anovulation | 438 (49.4) | 202 (51.8) | 0.47 | 111 (50.5) | 0.84 |
| Polycystic ovarian syndrome | 290 (32.7) | 141 (36.2) | 0.26 | 80 (36.4) | 0.34 |
| Characteristics | Group 1 TSH 0.3−2.49 mIU/L n = 886 | Group 2 (TSH 2.5−4.09 mIU/L) n = 390 | p Group 1 vs. 2 | Group 3 (TSH ≥ 4.1 mIU/L) n = 220 | p Group 1 vs. 3 |
|---|---|---|---|---|---|
| Prolactin (ng/mL) | 12.1 (9.2–16) | 12.4 (9.2–17) | 0.55 | 12.7 (9.3–17.7) | 0.24 |
| Progesterone (ng/mL) | 3 (0.65–11.1) | 1.8 (0.52–10.6) | 0.16 | 1.37 (0.56–9.8) | 0.08 |
| Women with glucose and insulin | n= 616 (69.5) | n = 271 (69.5) | n= 155 (70.4) | ||
| Glucose (mg/dL) | 93.1 ± 17 | 93.8 ± 18 | 0.98 | 93.6 ± 11 | 0.96 |
| Insulin (µU/mL) | 8.9 (5.8–12.6) | 10.8 (7.7–16.7) | 0.004 | 12 (7–18.9) | 0.0001 |
| HOMA-IR | 2.1 (1.2–3.4) | 2.4 (1.4–4.0) | 0.008 | 2.7 (1.7–4.3) | 0.0001 |
| Insulin resistance | 246 (40) | 125 (46.1) | 0.09 | 84 (54.2) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arce-Sánchez, L.; Vitale, S.G.; Flores-Robles, C.M.; Godines-Enriquez, M.S.; Noventa, M.; Urquia-Figueroa, C.M.; Martínez-Cruz, N.; Estrada-Gutierrez, G.; Espino y Sosa, S.; Romo-Yañez, J.; et al. Effect of the Cut-Off Level for Thyroid-Stimulating Hormone on the Prevalence of Subclinical Hypothyroidism among Infertile Mexican Women. Diagnostics 2021, 11, 417. https://doi.org/10.3390/diagnostics11030417
Arce-Sánchez L, Vitale SG, Flores-Robles CM, Godines-Enriquez MS, Noventa M, Urquia-Figueroa CM, Martínez-Cruz N, Estrada-Gutierrez G, Espino y Sosa S, Romo-Yañez J, et al. Effect of the Cut-Off Level for Thyroid-Stimulating Hormone on the Prevalence of Subclinical Hypothyroidism among Infertile Mexican Women. Diagnostics. 2021; 11(3):417. https://doi.org/10.3390/diagnostics11030417
Chicago/Turabian StyleArce-Sánchez, Lidia, Salvatore Giovanni Vitale, Claudia Montserrat Flores-Robles, Myrna Souraye Godines-Enriquez, Marco Noventa, Carmen Marcela Urquia-Figueroa, Nayeli Martínez-Cruz, Guadalupe Estrada-Gutierrez, Salvador Espino y Sosa, José Romo-Yañez, and et al. 2021. "Effect of the Cut-Off Level for Thyroid-Stimulating Hormone on the Prevalence of Subclinical Hypothyroidism among Infertile Mexican Women" Diagnostics 11, no. 3: 417. https://doi.org/10.3390/diagnostics11030417
APA StyleArce-Sánchez, L., Vitale, S. G., Flores-Robles, C. M., Godines-Enriquez, M. S., Noventa, M., Urquia-Figueroa, C. M., Martínez-Cruz, N., Estrada-Gutierrez, G., Espino y Sosa, S., Romo-Yañez, J., Montoya-Estrada, A., & Reyes-Muñoz, E. (2021). Effect of the Cut-Off Level for Thyroid-Stimulating Hormone on the Prevalence of Subclinical Hypothyroidism among Infertile Mexican Women. Diagnostics, 11(3), 417. https://doi.org/10.3390/diagnostics11030417









