Quality Assessment of Endoscopic Forceps Biopsy Samples under Magnifying Narrow Band Imaging for Histological Diagnosis of Cervical Intraepithelial Neoplasia: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
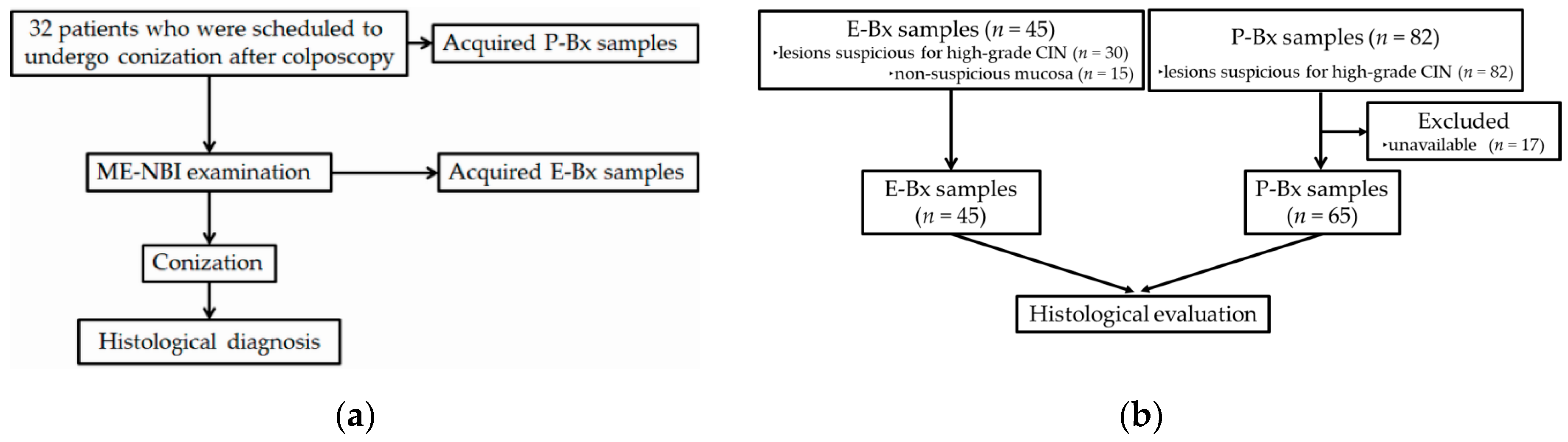
2.1. Study Design, Setting and Participants
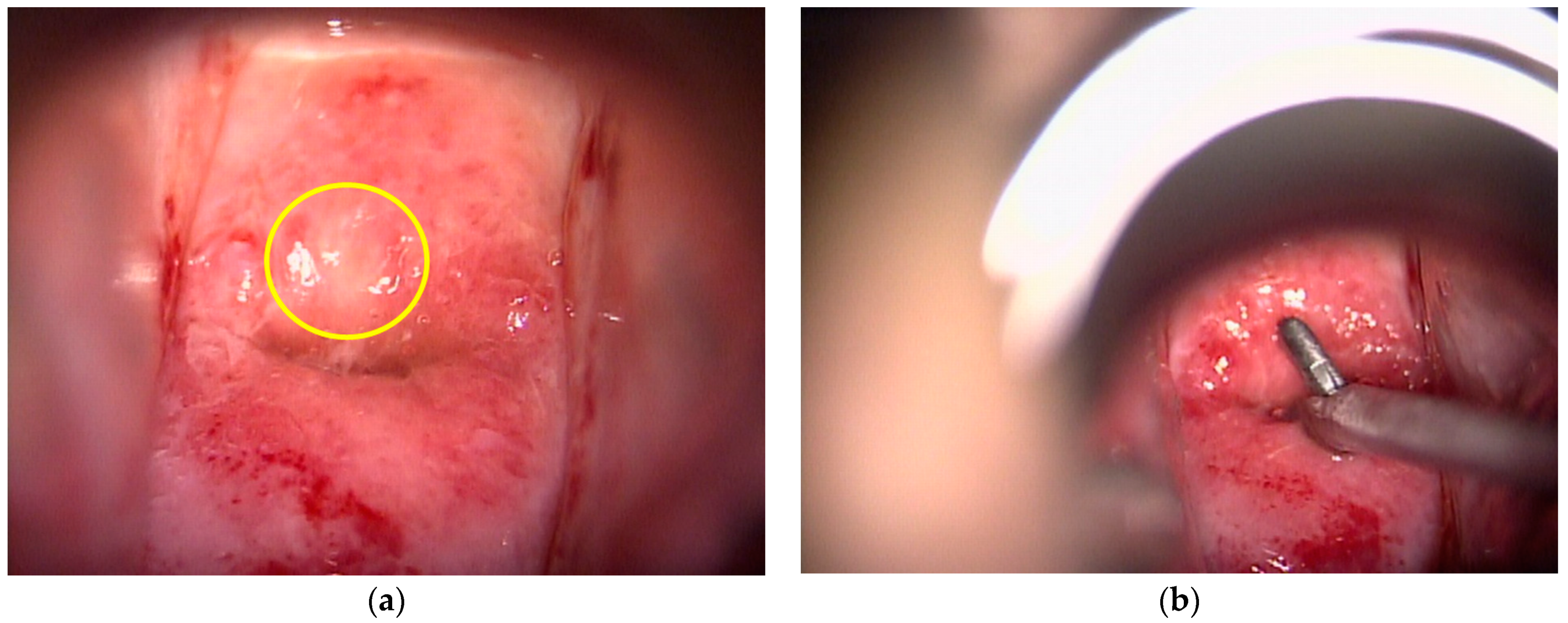
2.2. Colposcopic Procedure
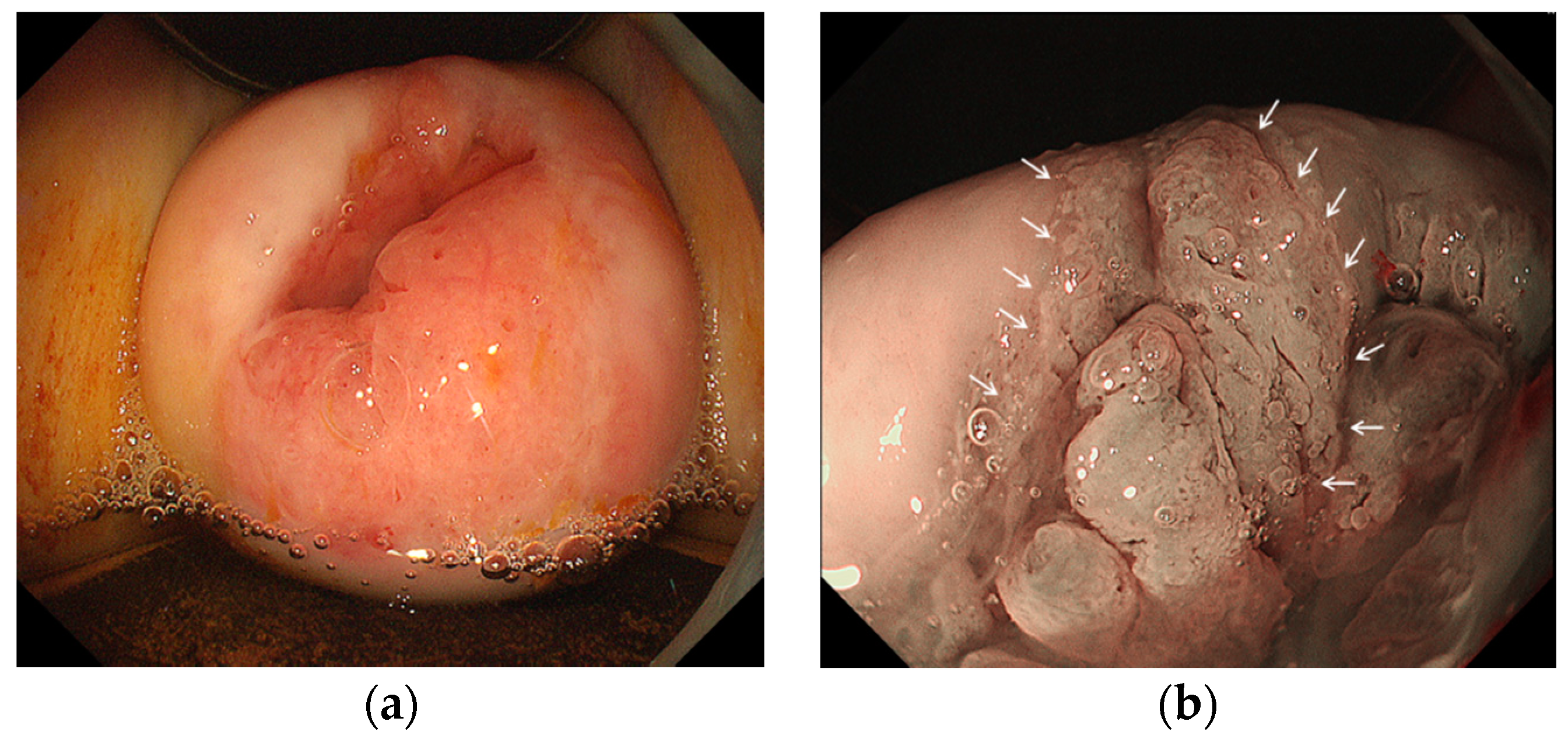
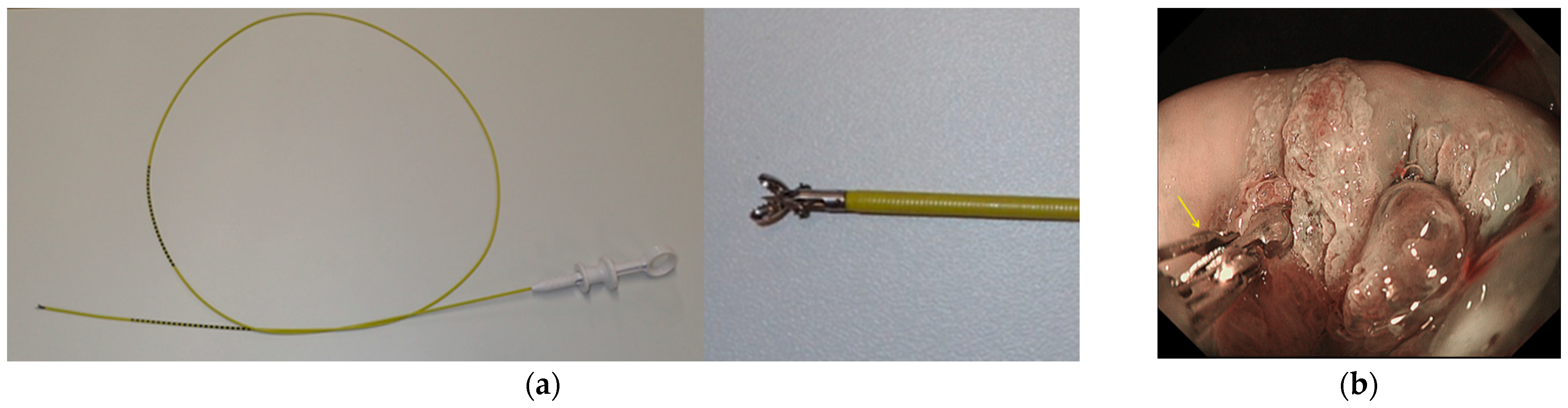
2.3. Endoscopic Procedure
2.4. Evaluation of Biopsy Specimens
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Participants and Descriptive Data
3.2. Outcome Data
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comparetto, C.; Borruto, F. Cervical cancer screening: A never-ending developing program. World J. Clin. Cases 2015, 3, 614–624. [Google Scholar] [CrossRef]
- Guo, P.; Xue, Z.; Mtema, Z.; Yeates, K.; Ginsburg, O.; Demarco, M.; Long, L.R.; Schiffman, M.; Antani, S. Ensemble Deep Learning for Cervix Image Selection toward Improving Reliability in Automated Cervical Precancer Screening. Diagnostics 2020, 10, 451. [Google Scholar] [CrossRef]
- Muto, M.; Yao, K.; Kaise, M.; Kato, M.; Uedo, N.; Yagi, K.; Tajiri, H. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig. Endosc. 2016, 28, 379–393. [Google Scholar] [CrossRef]
- Yao, K.; Doyama, H.; Gotoda, T.; Ishikawa, H.; Nagahama, T.; Yokoi, C.; Oda, I.; Machida, H.; Uchita, K.; Tabuchi, M. Diagnostic performance and limitations of magnifying narrow-band imaging in screening endoscopy of early gastric cancer: A prospective multicenter feasibility study. Gastric Cancer 2014, 17, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Kanenishi, K.; Mori, H.; Kobara, H.; Fujihara, S.; Chiyo, T.; Kobayashi, N.; Matsunaga, T.; Ayaki, M.; Yachida, T.; et al. Flexible magnifying endoscopy with narrow band imaging for the diagnosis of uterine cervical tumors: A cooperative study among gastrointestinal endoscopists and gynecologists to explore a novel micro-vascular classiication system. Oncol. Lett. 2017, 14, 355–362. [Google Scholar] [CrossRef]
- Uchita, K.; Kanenishi, K.; Hirano, K.; Kobara, H.; Nishiyama, N.; Kawada, A.; Fujihara, S.; Ibuki, E.; Haba, R.; Takahashi, Y.; et al. Characteristic findings of high-grade cervical intraepithelial neoplasia or more on magnifying endoscopy with narrow band imaging. Int. J. Clin. Oncol. 2018, 23, 707–714. [Google Scholar] [CrossRef]
- Underwood, M.; Arbyn, M.; Parry-Smith, W.; De Bellis-Ayres, S.; Todd, R.; Redman, C.W.; Moss, E.L. Accuracy of colposcopy-directed punch biopsies: A systematic review and meta-analysis. BJOG 2012, 119, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Coronado, P.J.; Fasero, M. Correlating the accuracy of colposcopy with practitioner experience when diagnosing cervical pathology using the dynamic spectral imaging system. Gynecol. Obstet. Investig. 2014, 78, 224–229. [Google Scholar] [CrossRef]
- Louwers, J.; Zaal, A.; Kocken, M.; Ter Harmsel, W.; Graziosi, G.; Spruijt, J.; Berkhof, J.; Balas, C.; Papagiannakis, E.; Snijders, P.; et al. Dynamic spectral imaging colposcopy: Higher sensitivity for detection of premalignant cervical lesions. BJOG 2011, 118, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, J.; Bentley, J.; Bösze, P.; Girardi, F.; Haefner, H.; Menton, M.; Perrotta, M.; Prendiville, W.; Russell, P.; Sideri, M.; et al. 2011 colposcopic terminology of the International Federation for Cervical Pathology and colposcopy. Obstet. Gynecol. 2012, 120, 166–172. [Google Scholar] [CrossRef]
- Inoue, H.; Kaga, M.; Ikeda, H.; Sato, C.; Sato, H.; Minami, H.; Santi, E.G.; Hayee, B.; Eleftheriadis, N. Magnification endoscopy in esophageal squamous cell carcinoma: A review of the intrapapillary capillary loop classification. Ann. Gastroenterol. 2015, 28, 41–48. [Google Scholar] [PubMed]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; IARC Press: Lyon, France, 2014; pp. 170–206. [Google Scholar]
- Kobara, H.; Mori, H.; Rafiq, K.; Fujihara, S.; Nishiyama, N.; Chiyo, T.; Matsunaga, T.; Ayaki, M.; Yachida, T.; Kato, K.; et al. Analysis of the amount of tissue sample necessary for mitotic count and Ki-67 index in gastrointestinal stromal tumor sampling. Oncol. Rep. 2015, 33, 215–222. [Google Scholar] [CrossRef]
- Wetcho, T.; Rattanaburi, A.; Kanjanapradit, K. Quality of tissue from punch biopsy forceps vs. round loop electrode in colposcopically directed biopsy: A randomized controlled trial. J. Gynecol. Oncol. 2018, 29, e52. [Google Scholar] [CrossRef] [PubMed]
- Bulten, J.; Horvat, R.; Jordan, J.; Herbert, A.; Wiener, H.; Arbyn, M. European guidelines for quality assurance in cervical histopathology. Acta Oncol. 2011, 50, 611–620. [Google Scholar] [CrossRef]
- Pretorius, R.G.; Belinson, J.L.; Burchette, R.J.; Hu, S.; Zhang, X.; Qiao, Y.L. Regardless of skill, performing more biopsies increases the sensitivity of colposcopy. J. Low. Genit. Tract Dis. 2011, 15, 180–188. [Google Scholar] [CrossRef]
- Kikuchi, K.; Uematsu, T.; Akasaka, K. Significance of biopsy under direct view in gastric cancer. Stomach Intest. 1970, 5, 837–842, (In Japanese with English abstract). [Google Scholar]
- Uedo, N.; Yao, K. Endoluminal Diagnosis of Early Gastric Cancer and Its Precursors: Bridging the Gap between Endoscopy and Pathology. Adv. Exp. Med. Biol. 2016, 908, 293–316. [Google Scholar] [PubMed]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; Di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef]
- Waxman, A.G.; Conageski, C.; Silver, M.I.; Tedeschi, C.; Stier, E.A.; Apgar, B.; Huh, W.K.; Wentzensen, N.; Massad, L.S.; Khan, M.J.; et al. ASCCP Colposcopy Standards: How do we perform colposcopy? Implications for establishing standards. J. Low. Genit. Tract Dis. 2017, 21, 235–241. [Google Scholar] [CrossRef]
- Pretorius, R.G.; Belinson, J.L. Colposcopy. Minerva Ginecol. 2012, 64, 173–180. [Google Scholar] [PubMed]
- Baasland, I.; Hagen, B.; Vogt, C.; Valla, M.; Romundstad, P.R. Colposcopy and additive diagnostic value of biopsies from colposcopy-negative areas to detect cervical dysplasia. Acta Obstet. Gynecol. Scand. 2016, 95, 1258–1263. [Google Scholar] [CrossRef]


| Total no. of patient | 32 |
| Mean age (SD) | 43 (10) years |
| No. of P-Bx | 82 |
| Positive (≥CIN2) | 42 |
| Negative (≤CIN1) | 40 |
| Final pathological diagnosis of surgical specimens | |
| ≥CIN3 | 24 |
| CIN2 | 3 |
| CIN1 | 4 |
| No malignancy | 1 |
| E-Bx (n = 45) | P-Bx (n = 65) | p-Value | |
|---|---|---|---|
| Proportion of sufficient samples (95% CI) | 84% (74–96%) | 87% (79–95%) | 0.672 |
| Mean ± SD max. diameter of biopsy samples (mm) | 1.7 ± 0.81 | 5.1 ± 2.2 | <0.001 |
| E-Bx (n = 7) | P-Bx (n = 8) | p-Value | |
|---|---|---|---|
| Lack of subepithelial interstitium, % (n) | 100 (7) | 100 (8) | n/a |
| Lack of entire epithelial layer and subepithelial interstitium, % (n) | 42.9 (3) | 0 (0) | 0.038 1 |
| Sensitivity | 92% |
| Specificity | 81% |
| Positive PV | 89% |
| Negative PV | 87% |
| Accuracy | 88% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchita, K.; Kobara, H.; Yorita, K.; Shigehisa, Y.; Kuroiwa, C.; Nishiyama, N.; Takahashi, Y.; Kai, Y.; Kunikata, J.; Shimokawa, T.; et al. Quality Assessment of Endoscopic Forceps Biopsy Samples under Magnifying Narrow Band Imaging for Histological Diagnosis of Cervical Intraepithelial Neoplasia: A Feasibility Study. Diagnostics 2021, 11, 360. https://doi.org/10.3390/diagnostics11020360
Uchita K, Kobara H, Yorita K, Shigehisa Y, Kuroiwa C, Nishiyama N, Takahashi Y, Kai Y, Kunikata J, Shimokawa T, et al. Quality Assessment of Endoscopic Forceps Biopsy Samples under Magnifying Narrow Band Imaging for Histological Diagnosis of Cervical Intraepithelial Neoplasia: A Feasibility Study. Diagnostics. 2021; 11(2):360. https://doi.org/10.3390/diagnostics11020360
Chicago/Turabian StyleUchita, Kunihisa, Hideki Kobara, Kenji Yorita, Yuriko Shigehisa, Chihiro Kuroiwa, Noriko Nishiyama, Yohei Takahashi, Yuka Kai, Jun Kunikata, Toshio Shimokawa, and et al. 2021. "Quality Assessment of Endoscopic Forceps Biopsy Samples under Magnifying Narrow Band Imaging for Histological Diagnosis of Cervical Intraepithelial Neoplasia: A Feasibility Study" Diagnostics 11, no. 2: 360. https://doi.org/10.3390/diagnostics11020360
APA StyleUchita, K., Kobara, H., Yorita, K., Shigehisa, Y., Kuroiwa, C., Nishiyama, N., Takahashi, Y., Kai, Y., Kunikata, J., Shimokawa, T., Hanaoka, U., Kanenishi, K., Masaki, T., Hirano, K., & Uedo, N. (2021). Quality Assessment of Endoscopic Forceps Biopsy Samples under Magnifying Narrow Band Imaging for Histological Diagnosis of Cervical Intraepithelial Neoplasia: A Feasibility Study. Diagnostics, 11(2), 360. https://doi.org/10.3390/diagnostics11020360







