Internal Jugular Vein Thrombosis: Etiology, Symptomatology, Diagnosis and Current Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources and Searches
2.3. Study Selection
- (1)
- studies reporting patients with an objective diagnosis of IJVthr;
- (2)
- studies correctly addressing the question of the search reported in the abstract;
- (3)
- studies reporting the related clinical outcome.
2.4. Data Extraction and Quality Assessment
3. Results
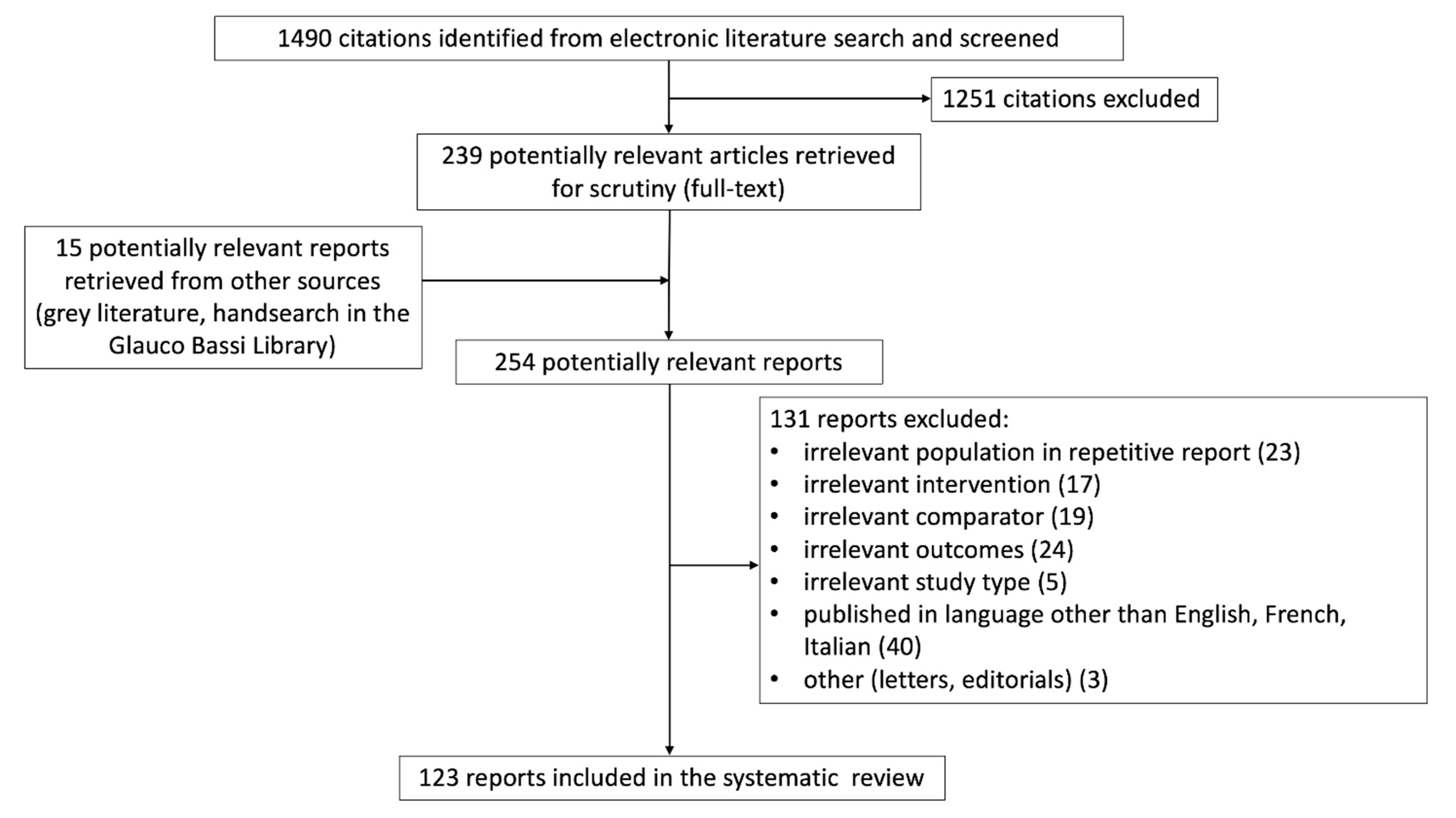
3.1. Study Identification and Selection
3.2. Study Quality
3.3. Clinical Presentation
3.4. Post-Mortem Studies
3.5. Etiology
3.6. Other Less Common Causes
3.7. Pulmonary Embolism and Other Serious Complications
3.8. Combination of Internal Jugular Vein and Cerebral Veins Thrombosis
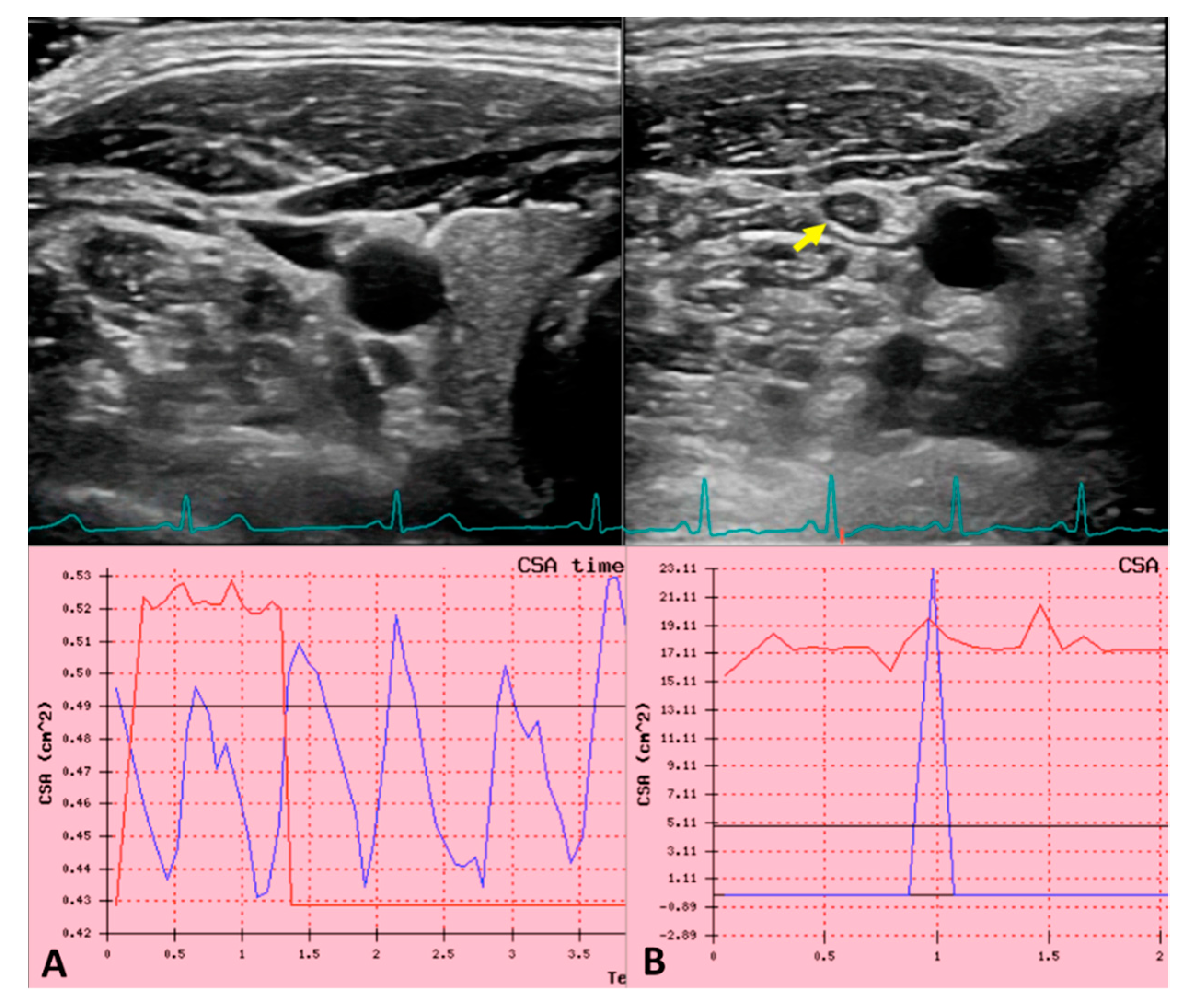
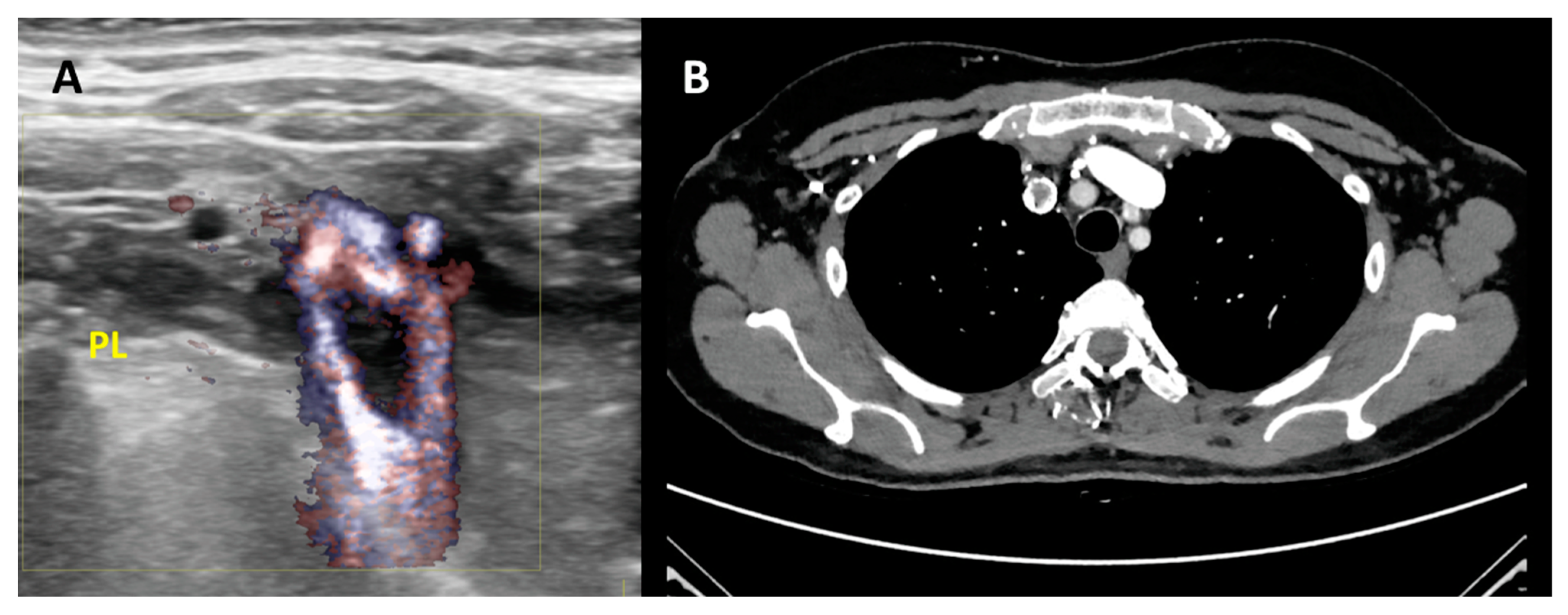
3.9. Ultrasound in IJVthr Screening and Follow up
3.10. Future Perspectives in Non-Invasive IJVthr Diagnostic
3.11. Second Level Diagnosis
3.12. Treatment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, V.; Abbas, A.K.; Fausto, N.; Robbins, S.L.; Cotran, R.S. Robbins and Cotran Pathologic Basis of Disease; Elsevier Saunders: Philadelphia, PA, USA, 1984. [Google Scholar]
- Gbaguidi, X.; Janvresse, A.; Benichou, J.; Cailleux, N.; Lévesque, H.; Marie, I. Internal jugular vein thrombosis: Outcome and risk factors. QJM Int. J. Med. 2010, 104, 209–219. [Google Scholar] [CrossRef]
- Leci-Tahiri, L.; Zherka-Saracini, H.; Tahiri, A.; Koshi, A. Bilateral internal jugular vein thrombosis due to malignant tumor. J. Med. Case Rep. 2018, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Hindi, Z.; Fadhel, E. Idiopathic Bilateral External Jugular Vein Thrombosis. Am. J. Case Rep. 2015, 16, 554–557. [Google Scholar] [PubMed]
- Uzun, K.; Erkoc, R.; Yuca, K.; Etlik, O.; Doğan, E.; Sayarlıoglu, H.; Işlek, A.; Çankaya, H. Internal Jugular Vein thrombosis two Different Etiologies. Electron. J. Gen. Med. 2005, 2, 123–128. [Google Scholar] [CrossRef]
- Bandara, A.R.; Wimalarathna, H.; Kalupahana, R.; Gunathilake, S.S.C. Internal jugular venous thrombosis due to Trousseau’s syndrome as the presenting feature of metastatic prostate carcinoma: A case report. J. Med. Case Rep. 2016, 10, 104. [Google Scholar] [CrossRef]
- Shameem, M.; Akhtar, J.; Bhargava, R.; Ahmed, Z.; Baneen, U.; Khan, N.A. Internal jugular vein thrombosis—A rare presentation of mediastinal lymphoma. Respir. Med. CME 2010, 3, 273–275. [Google Scholar] [CrossRef]
- Williams, D.; Pitre, E.; Ford, C.; Polisena, J.; Weeks, L. Screening for Hepatitis C Virus: A Systematic Review and Meta-Analysis—Project Protocol; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2015; APPENDIX 3, PRISMA FLOW CHART TEMPLATE. Available online: https://www.ncbi.nlm.nih.gov/books/NBK361825/ (accessed on 24 January 2021).
- Bresadola, M. The Bassi Historical International Library of Phlebology at the Ferrara University Hospital. Veins Lymphat. 2014, 3. [Google Scholar] [CrossRef]
- Graves, R.S. Users’ Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice. J. Med. Libr. Assoc. 2002, 90, 483. [Google Scholar]
- Carrington, B.M.; Adams, J.E. Jugular vein thrombosis associated with distant malignancy. Postgrad. Med. J. 1988, 64, 455–458. [Google Scholar] [CrossRef]
- Wada, Y.; Yanagihara, C.; Nishimura, Y. Internal jugular vein thrombosis associated with shiatsu massage of the neck. J. Neurol. Neurosurg. Psychiatry 2005, 76, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Al-Zoubi, N.A. Spontaneous internal jugular vein thrombosis as primary presentation of antiphospholipid syndrome: Case report. Vasc. Health Risk Manag. 2018, 14, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Anthony, R.; Woodhead, C.J. Bilateral internal jugular vein thrombosis in a child with protein S deficiency. J. Laryngol. Otol. 2005, 119, 661–664. [Google Scholar] [CrossRef]
- Hahn, J.; Nordmann-Kleiner, M.; Hoffmann, T.K.; Greve, J. Thrombosis of the internal jugular vein in the ENT-department—Prevalence, causes and therapy: A retrospective analysis. Auris Nasus Larynx 2019, 46, 624–629. [Google Scholar] [CrossRef]
- Graham, R.F.; Wightman, J.M. Neck Pain One Week after Pacemaker Generator Replacement. J. Emerg. Med. 2015, 49, e5–e8. [Google Scholar] [CrossRef]
- Duke, B.J.; Ryu, R.K.; Brega, K.E.; Coldwell, D.M. Traumatic bilateral jugular vein thrombosis: Case report and review of the literature. Neurosurgery 1997, 41, 680–683. [Google Scholar]
- Thapa, S.; Terry, P.B.; Kamdar, B.B. Hemodialysis catheter-associated superior vena cava syndrome and pulmonary embolism: A case report and review of the literature. BMC Res. Notes 2016, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, L.; Zhao, X.; Zhu, M.; Zhang, Y. Cerebral venous thrombosis originating from internal jugular vein outflow impairment: A case report. Medicine 2017, 96, e8975. [Google Scholar] [CrossRef] [PubMed]
- Ilgen, U.; Turan, S.; Emmungil, H. Near-complete recanalization of jugular vein and multiple dural sinus thromboses with warfarin in a case of antiphospholipid syndrome. Reumatologia 2018, 56, 406–408. [Google Scholar] [CrossRef]
- Hacıfazlıoğlu, Ç.; Arslan, E.; Arslan, E.A.; Büyükşerbetci, G. Cerebral venous sinus thrombosis with internal jugular venous thrombosis in a male patient with nephrotic syndrome. Turk. Neurosurg. 2014, 25, 980–983. [Google Scholar] [CrossRef][Green Version]
- Masood, I.; While, A. Bilateral jugular vein thrombosis: A rare cause of papilloedema. Eye 2006, 20, 249–250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Das, S.; Srinivasaraghavan, R.; Krishnamurthy, S.; Mahadevan, S. Internal jugular vein thrombosis complicating disseminated tuberculosis in a 2-year-old child. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qian, W.; Shi, H.; Zhang, W.; Chen, M.; Liang, X. Internal Jugular Vein Thrombosis with Serious Cervical Necrotizing Fasciitis. J. Craniofac. Surg. 2019, 30, e487–e489. [Google Scholar] [CrossRef] [PubMed]
- Toratani, M.; Hayashi, A.; Nishiyama, N.; Nakamura, H.; Chida, R.; Komatsu, T.; Nakahara, S.; Kobayashi, S.; Taguchi, I. Thrombosis in an Internal Jugular Vein and an Upper Limb Deep Vein Treated with Edoxaban. Intern. Med. 2017, 56, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Bilici, M.; Pehlivan, Y.; Kimyon, G.; Kisacik, B. Internal jugular vein thrombosis in Behcet’s disease: A rare complication. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gianesini, S.; Menegatti, E.; Zuolo, M.; Occhionorelli, S.; Ascanelli, S.; Zamboni, P. Spontaneous thrombosis of primary external jugular veins aneurysms. Veins Lymphat. 2013, 2. [Google Scholar] [CrossRef]
- Shakeel, M.; Keh, S.M.; Kynaston, J.; McCluney, N.; Ah See, K.W. Evidence based management of spontaneous internal jugular vein thrombosis: A literature review. J. Otolaryngol. ENT Res. 2015, 2, 00019. [Google Scholar] [CrossRef]
- Hyder, S.M.S.; Iqbal, J.; Lutfi, I.A.; Shazlee, M.K.; Hamid, K.; Rashid, S. Complications Associated with Permanent Internal Jugular Hemodialysis Catheter: A Retrospective Study. Cureus 2019, 11, e4521. [Google Scholar] [CrossRef]
- Mirijello, A.; Impagnatiello, M.; Zaccone, V.; Landolfi, R. ‘Monolateral’ superior vena cava syndrome: Right internal jugular vein occlusion. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef]
- Mantovani, G.; Menegatti, M.; Scerrati, A.; Cavallo, M.A.; De Bonis, P. Controversies and Misconceptions Related to Cerebrospinal Fluid Circulation: A Review of the Literature from the Historical Pioneers’ Theories to Current Models. BioMed Res. Int. 2018, 2018, 2928378. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Chan, C.C.; Fan, C.; Ji, X.; Meng, R. Internal jugular vein stenosis associated with elongated styloid process: Five case reports and literature review. BMC Neurol. 2019, 19, 112. [Google Scholar] [CrossRef]
- Zhou, D.; Meng, R.; Zhang, X.; Guo, L.; Li, S.; Wu, W.; Duan, J.; Song, H.; Ding, Y.; Ji, X. Intracranial hypertension induced by internal jugular vein stenosis can be resolved by stenting. Eur. J. Neurol. 2017, 25, 365-e13. [Google Scholar] [CrossRef]
- De Bonis, P.; Menegatti, E.; Cavallo, M.A.; Sisini, F.; Trapella, G.; Scerrati, A.; Zamboni, P. JEDI (jugular entrapment, dilated ventricles, intracranial hypertension) syndrome: A new clinical entity? A case report. Acta Neurochir. 2019, 161, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, P.; Scerrati, A.; Menegatti, E.; Galeotti, R.; Lapparelli, M.; Traina, L.; Tessari, M.; Ciorba, A.; De Bonis, P.; Pelucchi, S. The eagle jugular syndrome. BMC Neurol. 2019, 19, 333. [Google Scholar] [CrossRef] [PubMed]
- Çakır, Ö.; Ayyıldız, O.; Oruc, A.; Eren, N.; Cakir, O.; Ayyildiz, O. A young adult with coronary artery and jugular vein thrombosis: A case report of combined protein S and protein C deficiency. Heart Vessel. 2002, 17, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Glemarec, J.; Berthelot, J.M.; Chevalet, P.; Guillot, P.; Maugars, Y.; Prost, A. Brachial plexopathy and Horner’s syndrome as the first manifestations of internal jugular vein thrombosis inaugurating polycythemia vera. Rev. Rhum. Engl. Ed. 1998, 65, 358–359. [Google Scholar]
- Sevitt, S.; Gallagher, N. Venous thrombosis and pulmonary embolism. A clinico-pathological study in injured and burned patients. BJS 1961, 48, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, E.; Karwinski, B. Prevalence of pulmonary embolism at necropsy in patients with cancer. J. Clin. Pathol. 1989, 42, 805–809. [Google Scholar] [CrossRef]
- Lambie, J.M.; Mahaffy, R.G.; Barber, D.C.; Karmody, A.M.; Scott, M.M.; Matheson, N.A. Diagnostic Accuracy in Venous Thrombosis. BMJ 1970, 2, 142–143. [Google Scholar] [CrossRef][Green Version]
- Diebold, J.; Löhrs, U. Venous Thrombosis and Pulmonary Embolism. Pathol. Res. Pract. 1991, 187, 260–266. [Google Scholar] [CrossRef]
- Di Nisio, M.; van Es, N.; Büller, H.R. Deep vein thrombosis and pulmonary embolism. Lancet 2016, 388, 3060–3073. [Google Scholar] [CrossRef]
- Giordano, N.J.; Jansson, P.S.; Young, M.N.; Hagan, K.A.; Kabrhel, C. Epidemiology, Pathophysiology, Stratification, and Natural History of Pulmonary Embolism. Tech. Vasc. Interv. Radiol. 2017, 20, 135–140. [Google Scholar] [CrossRef]
- Wilkin, T.D.; Kraus, M.A.; Lane, K.A.; Trerotola, S.O. Internal Jugular Vein Thrombosis Associated with Hemodialysis Catheters. Radiology 2003, 228, 697–700. [Google Scholar] [CrossRef]
- Lordick, F.; Hentrich, M.; Decker, T.; Hennig, M.; Pohlmann, H.; Hartenstein, R.; Peschel, C. Ultrasound screening for internal jugular vein thrombosis aids the detection of central venous catheter-related infections in patients with haemato-oncological diseases: A prospective observational study. Br. J. Haematol. 2003, 120, 1073–1078. [Google Scholar] [CrossRef]
- Boga, C.; Ozdogu, H.; Diri, B.; Oguzkurt, L.; Asma, S.; Yeral, M. Lemierre syndrome variant: Staphylococcus aureus associated with thrombosis of both the right internal jugular vein and the splenic vein after the exploration of a river cave. J. Thromb. Thrombolysis 2007, 23, 151–154. [Google Scholar] [CrossRef]
- Moniot, M.; Montava, M.; Ranque, S.; Scemama, U.; Cassagne, C.; Arthur, V. Malignant Aspergillus flavus Otitis Externa with Jugular Thrombosis. Emerg. Infect. Dis. 2019, 25, 830–832. [Google Scholar] [CrossRef]
- Bostanci, A.; Turhan, M. Internal Jugular Vein Thrombosis following Oropharyngeal Infection. Case Rep. Vasc. Med. 2015, 2015, 538439. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, P.; Menegatti, E.; Cittanti, C.; Sisini, F.; Gianesini, S.; Salvi, F.; Mascoli, F. Fixing the jugular flow reduces ventricle volume and improves brain perfusion. J. Vasc. Surgery Venous Lymphat. Disord. 2016, 4, 434–445. [Google Scholar] [CrossRef]
- Quraishi, H.A.; Wax, M.K.; Granke, K.; Rodman, S.M. Internal Jugular Vein Thrombosis After Functional and Selective Neck Dissection. Arch. Otolaryngol. Head Neck Surg. 1997, 123, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.H.; Mulholland, S.; Yoo, J.H.J.; Gullane, P.J.; Irish, J.C.; Neligan, P.; Keller, A. Internal jugular vein thrombosis following modified neck dissection: Implications for head and neck flap reconstruction. Head Neck 1998, 20, 169–174. [Google Scholar] [CrossRef]
- Chen, K.-H.; Chen, Y.-J.; Liaw, C.-C.; Chang, H.-J.; Yeow, K.-M. Left internal jugular vein thrombosis due to a lung tumor. Chang. Gung Med. J. 2003, 26, 458–462. [Google Scholar]
- Corral, J.; Villanueva, G. A Case Study of Deep Vein Thrombosis of the Right Internal Jugular Vein in a Healthy 21-Year-Old Male. Case Rep. Hematol. 2016, 2016, 7654749. [Google Scholar] [CrossRef] [PubMed]
- Albertyn, L.E.; Alcock, M.K. Diagnosis of internal jugular vein thrombosis. Radiology 1987, 162, 505–508. [Google Scholar] [CrossRef]
- Guijarro, C.; Carril, J.M.F.; Egido, J.; González, J.L. Aseptic thrombosis of the cerebral venous sinuses and polycythemia vera. Neurología 1995, 10, 58–59. [Google Scholar] [PubMed]
- Merhar, G.L.; Colley, D.P.; Clark, R.A.; Herwig, S.R. Computed Tomographic Demonstration of Cervical Abscess and Jugular Vein Thrombosis: A Complication of Intravenous Drug Abuse in the Neck. Arch. Otolaryngol. Head Neck Surg. 1981, 107, 313–315. [Google Scholar] [CrossRef]
- Lin, D.; Reeck, J.B.; Murr, A.H. Internal Jugular Vein Thrombosis and Deep Neck Infection from Intravenous Drug Use: Management Strategy. Laryngoscope 2004, 114, 56–60. [Google Scholar] [CrossRef]
- Mor, Y.S.; Schenker, J.G. Ovarian Hyperstimulation Syndrome and Thrombotic Events. Am. J. Reprod. Immunol. 2014, 72, 541–548. [Google Scholar] [CrossRef]
- Bénifla, J.L.; Conard, J.; Naouri, M.; Darai, E.; Bascou, V.; Neuraz, A.; Deval, B.; Guglielmina, J.N.; Crequat, J.; Madelenat, P. Ovarian hyperstimulation syndrome and thrombosis. Apropos of a case of thrombosis of the internal jugular vein. Review of the literature. J. Gynécologie Obs. Biol. Reprod. 1994, 23, 778–783. [Google Scholar]
- Ellis, M.H.; Ben Nun, I.; Rathaus, V.; Werner, M.; Shenkman, L. Internal jugular vein thrombosis in patients with ovarian hyperstimulation syndrome. Fertil. Steril. 1998, 69, 140–142. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.-W.; Han, M.; Bae, J.W.; Cho, Y.J. Internal jugular vein thrombosis with OHSS. J. Clin. Ultrasound 2017, 45, 450–452. [Google Scholar] [CrossRef]
- Mnejja, M.; Hammami, B.; Bougacha, L.; Jemal, L.; Chakroun, N.; Chakroun, A.; Charfeddine, I.; Ghorbel, A. Internal jugular vein thrombosis by protein C activated resistance. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 269–271. [Google Scholar] [CrossRef][Green Version]
- Pancześnik, K.; Jonczak, L.; Barwijuk, A.J.; Jankowska, S. The internal jugular vein thrombosis during pregnancy with decreased level of protein S. Ginekol. Polska 2005, 76, 564–566. [Google Scholar]
- Lim, B.G.; Kim, Y.M.; Kim, H.; Lim, S.H.; Lee, M.K. Internal jugular vein thrombosis associated with venous hypoplasia and protein S deficiency revealed by ultrasonography. J. Anesthesia 2011, 25, 930–934. [Google Scholar] [CrossRef]
- De Cicco, M.; Matovic, M.; Balestreri, L.; De Angelis, V.; Fracasso, A.; Morassut, S.; Coran, F.; Babare, R.; Buonadonna, A.; Testa, V. Antithrombin III deficiency as a risk factor for catheter-related central vein thrombosis in cancer patients. Thromb. Res. 1995, 78, 127–137. [Google Scholar] [CrossRef]
- Petrov, I.; Grozdinski, L.; Kaninski, G.; Iliev, N.; Iloska, M.; Radev, A. Safety Profile of Endovascular Treatment for Chronic Cerebrospinal Venous Insufficiency in Patients with Multiple Sclerosis. J. Endovasc. Ther. 2011, 18, 314–323. [Google Scholar] [CrossRef]
- Mandato, K.D.; Hegener, P.F.; Siskin, G.P.; Haskal, Z.J.; Englander, M.J.; Garla, S.; Mitchell, N.; Reutzel, L.; Doti, C. Safety of Endovascular Treatment of Chronic Cerebrospinal Venous Insufficiency: A Report of 240 Patients with Multiple Sclerosis. J. Vasc. Interv. Radiol. 2012, 23, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Lupattelli, T.; Onorati, P.; Bellagamba, G.; Toma, G. Successful retrograde recanalization of internal jugular vein passing from omolateral external jugular vein. Veins Lymphat. 2018, 7. [Google Scholar] [CrossRef]
- Siu, S.L.Y.; Yang, J.Y.K.; Hui, J.P.K.; Li, R.C.H.; Cheng, V.Y.W.; Cheung, T.W.Y.; Kwong, A.N.S. Chylothorax secondary to catheter related thrombosis successfully treated with heparin. J. Paediatr. Child Health 2011, 48, E105–E107. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.-X.; Wang, W.; Xu, C.; Shen, S.; Yu, L.; Zhang, G.-Z. Thrombosis of the superior vena cava and auxiliary branches in patients with indwelling catheterization of the internal jugular vein. Chin. Med. J. 2009, 122, 692–696. [Google Scholar]
- Ge, X.; Cavallazzi, R.; Li, C.; Pan, S.M.; Wang, Y.W.; Wang, F.-L. Central venous access sites for the prevention of venous thrombosis, stenosis and infection. Cochrane Database Syst. Rev. 2012, 2012, CD004084. [Google Scholar] [CrossRef] [PubMed]
- Biffi, R.; Pozzi, S.; Bonomo, G.; Della Vigna, P.; Monfardini, L.; Radice, D.; Rotmensz, N.; Zampino, M.G.; Fazio, N.; Orsi, F. Cost Effectiveness of Different Central Venous Approaches for Port Placement and Use in Adult Oncology Patients: Evidence from a Randomized Three-Arm Trial. Ann. Surg. Oncol. 2014, 21, 3725–3731. [Google Scholar] [CrossRef]
- Biffi, R.; Orsi, F.; Pozzi, S.; Pace, U.; Bonomo, G.; Monfardini, L.; Della Vigna, P.; Rotmensz, N.; Radice, D.; Zampino, M.G.; et al. Best choice of central venous insertion site for the prevention of catheter-related complications in adult patients who need cancer therapy: A randomized trial. Ann. Oncol. 2009, 20, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Harter, C.; Salwender, H.J.; Bach, A.; Egerer, G.; Goldschmidt, H.; Ho, A.D. Catheter-related infection and thrombosis of the internal jugular vein in hematologic-oncologic patients undergoing chemotherapy: A prospective comparison of silver-coated and uncoated catheters. Cancer 2002, 94, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Gheith, O.; Al Otaibi, T.; Nampoory, M.R.N.; Attia, H.; Halim, M.; Said, T.; Nair, P.; Balaha, M.; Awadein, W.; Zakariya, Z.; et al. Bilateral chylothorax in a renal transplant recipient: Case report and literature review. Exp. Clin. Transplant. 2014, 12, 148–151. [Google Scholar]
- Marshall-Goebel, K.; Laurie, S.S.; Alferova, I.V.; Arbeille, P.; Auñón-Chancellor, S.M.; Ebert, D.J.; Lee, S.M.C.; Macias, B.R.; Martin, D.S.; Pattarini, J.M.; et al. Assessment of Jugular Venous Blood Flow Stasis and Thrombosis During Spaceflight. JAMA Netw. Open 2019, 2, e1915011. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, P.; Sisini, F.; Menegatti, E.; Taibi, A.; Gadda, G.; Tavoni, V.; Malagoni, A.M.; Tessari, M.; Gianesini, S.; Gambaccini, M. Ultrasound Monitoring of Jugular Venous Pulse during Space Missions: Proof of Concept. Ultrasound Med. Biol. 2018, 44, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Trottier, S.J.; Veremakis, C.; O’Brien, J.; Auer, A.I. Femoral deep vein thrombosis associated with central venous catheterization: Results from a prospective, randomized trial. Crit. Care Med. 1995, 23, 52–59. [Google Scholar] [CrossRef]
- Timsit, J.-F.; Bouadma, L.; Mimoz, O.; Parienti, J.-J.; Garrouste-Orgeas, M.; Alfandari, S.; Plantefeve, G.; Bronchard, R.; Troche, G.; Gauzit, R.; et al. Jugular versus Femoral Short-Term Catheterization and Risk of Infection in Intensive Care Unit Patients. Causal Analysis of Two Randomized Trials. Am. J. Respir. Crit. Care Med. 2013, 188, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Kuban, J.D. Dialysis Catheter Placement in Patients with Exhausted Access. Tech. Vasc. Interv. Radiol. 2017, 20, 65–74. [Google Scholar] [CrossRef]
- Naroienejad, M.; Saedi, D.; Rezvani, A. Prevalence of central vein stenosis following catheterization in patients with end-stage renal disease. Saudi J. Kidney Dis. Transplant. 2010, 21, 975–978. [Google Scholar]
- Hassan, C.; GirishKumar, H.T.; Thatigotla, B.; Asad, M.; Sivakumar, M.; Bhoot, N.; Pokala, N. Value of ultrasound guidance in placement of hemodialysis access catheters in patients with end-stage renal disease. Am. Surg. 2008, 74, 1111–1113. [Google Scholar] [CrossRef]
- Vigo, V.; Lisi, P.; Galgano, G.; Lomonte, C. Lancisi’s sign and central venous catheter tip position: A case report. J. Vasc. Access 2018, 19, 92–93. [Google Scholar] [CrossRef]
- Rajasekhar, A.; Streiff, M.B. How I treat central venous access device–related upper extremity deep vein thrombosis. Blood 2017, 129, 2727–2736. [Google Scholar] [CrossRef]
- Ethiraj, D.; Indiran, V.; Kanakaraj, K.; Madhuraimuthu, P. Trousseau’s sign in the left internal jugular vein in gastric cancer. Indian J. Cancer 2018, 55, 415–416. [Google Scholar] [CrossRef]
- Lupattelli, T.; Bellagamba, G.; Righi, E.; Di Donna, V.; Flaishman, I.; Fazioli, R.; Garaci, F.; Onorati, P. Feasibility and safety of endovascular treatment for chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J. Vasc. Surg. 2013, 58, 1609–1618. [Google Scholar] [CrossRef][Green Version]
- Zamboni, P.; Galeotti, R.; Salvi, F.; Giaquinta, A.; Setacci, C.; Alborino, S.; Guzzardi, G.; Sclafani, S.J.; Maietti, E.; Veroux, P.; et al. Effects of Venous Angioplasty on Cerebral Lesions in Multiple Sclerosis: Expanded Analysis of the Brave Dreams Double-Blind, Sham-Controlled Randomized Trial. J. Endovasc. Ther. 2020, 27, 9–17. [Google Scholar] [CrossRef]
- Zhang, F.-L.; Zhou, H.-W.; Guo, Z.-N.; Yang, Y. Eagle Syndrome as a Cause of Cerebral Venous Sinus Thrombosis. Can. J. Neurol. Sci. 2019, 46, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Scerrati, A.; De Bonis, P.; Zamboni, P. Letter to the Editor Regarding “Styloidectomy and Venous Stenting for Treatment of Styloid-Induced Internal Jugular Vein Stenosis: A Case Report and Literature Review”. World Neurosurg. 2020, 139, 697. [Google Scholar] [CrossRef]
- De Bonis, P.; Musio, A.; Mantovani, G.; Pompucci, A.; Visani, J.; Lofrese, G.; Scerrati, A. Simplified four-step retropharyngeal approach for the upper cervical spine: Technical note. Eur. Spine J. 2020, 29, 2752–2757. [Google Scholar] [CrossRef] [PubMed]
- Scerrati, A.; Lee, J.-S.; Zhang, J.; Ammirati, M. Exposing the Fundus of the Internal Acoustic Meatus without Entering the Labyrinth Using a Retrosigmoid Approach: Is It Possible? World Neurosurg. 2016, 91, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Scerrati, A.; Ercan, S.; Wu, P.; Zhang, J.; Ammirati, M. Intrapetrous Internal Carotid Artery: Evaluation of Exposure, Mobilization and Surgical Maneuvers Feasibility from a Retrosigmoid Approach in a Cadaveric Model. World Neurosurg. 2016, 91, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Scerrati, A.; Lee, J.-S.; Zhang, J.; Ammirati, M. Microsurgical Anatomy of the Internal Acoustic Meatus as Seen Using the Retrosigmoid Approach. Otol. Neurotol. 2016, 37, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Colasanti, R.; Lee, J.; Scerrati, A.; Ercan, S.; Zhang, J.; Ammirati, M. Quantitative evaluation of different far lateral approaches to the cranio-vertebral junction using the microscope and the endoscope: A cadaveric study using a tumor model. Acta Neurochir. 2018, 160, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, M.; Okada, M. Primary venous aneurysms—Case reports. Angiology 1999, 50, 239–244. [Google Scholar] [CrossRef]
- Bateman, R.M.; Sharpe, M.D.; Jagger, J.E.; Ellis, C.G.; Solé-Violán, J.; López-Rodríguez, M.; Herrera-Ramos, E.; Ruíz-Hernández, J.; Borderías, L.; Horcajada, J.; et al. 36th International Symposium on Intensive Care and Emergency Medicine: Brussels, Belgium. 15–18 March 2016. Crit. Care 2016, 20 (Suppl. 2), 94. [Google Scholar] [CrossRef]
- Frizzelli, R.; Tortelli, O.; Di Comite, V.; Ghirardi, R.; Pinzi, C.; Scarduelli, C. Deep venous thrombosis of the neck and pulmonary embolism in patients with a central venous catheter admitted to cardiac rehabilitation after cardiac surgery: A prospective study of 815 patients. Intern. Emerg. Med. 2008, 3, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Saadeh, S.S.; Abdel-Razeq, N.; Khozouz, O.; Abunasser, M.; Taqash, A. Clinical Course and Complications of Catheter and Non-Catheter-Related Upper Extremity Deep Vein Thrombosis in Patients with Cancer. Clin. Appl. Thromb. 2018, 24, 1234–1240. [Google Scholar] [CrossRef]
- Drakos, P.; Ford, B.C.; Labropoulos, N. A systematic review on internal jugular vein thrombosis and pulmonary embolism. J. Vasc. Surgery Venous Lymphat. Disord. 2020, 8, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Griselli, M.; Barrett, A. Congenital extensive central venous thrombosis with chylous ascites and chylothoraces. J. Pediatr. Surg. 2013, 48, e5–e8. [Google Scholar] [CrossRef]
- Osowicki, J.; Kapur, S.; Phuong, L.K.; Dobson, S. The long shadow of Lemierre’s syndrome. J. Infect. 2017, 74, S47–S53. [Google Scholar] [CrossRef]
- Risoud, M.; Mortuaire, G.; Chevalier, D.; Rysman, B. Atypical Lemierre syndrome. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 123–124. [Google Scholar] [CrossRef]
- De Smet, K.; Claus, P.-E.; Alliet, G.; Simpelaere, A.; Desmet, G. Lemierre’s syndrome: A case study with a short review of literature. Acta Clin. Belg. 2018, 74, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Bousser, M.-G.; Ferro, J.M. Cerebral venous thrombosis: An update. Lancet Neurol. 2007, 6, 162–170. [Google Scholar] [CrossRef]
- von Hohenberg, C.C.; Kerl, U.; Sartorius, A.; Schwarz, S. Cerebral Venous Thrombosis Following Strangulation. Prim. Care Companion CNS Disord. 2018, 20. [Google Scholar] [CrossRef]
- Ma, J.-Y.; Zhang, X.; Li, X.-F.; He, L.-J.; Ma, N.; Wei, Y.-Y.; Wu, R.-H.; Wang, F.-Y. Thrombotic storm in a 4-year-old boy with a thrombus in the right atrium. Int. J. Immunopathol. Pharmacol. 2018, 32. [Google Scholar] [CrossRef]
- Hughes, C.; Nichols, T.; Pike, M.; Subbe, C.; Elghenzai, S. Cerebral Venous Sinus Thrombosis as a Presentation of COVID-19. Eur. J. Case Rep. Intern. Med. 2020, 7, 001691. [Google Scholar]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef]
- Kochi, A.N.; Tagliari, A.P.; Forleo, G.B.; Fassini, G.M.; Tondo, C. Cardiac and arrhythmic complications in patients with COVID-19. J. Cardiovasc. Electrophysiol. 2020, 31, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Marietta, M.; Ageno, W.; Artoni, A.; De Candia, E.; Gresele, P.; Marchetti, M.; Marcucci, R.; Tripodi, A. COVID-19 and haemostasis: A position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus. 2020, 18, 167–169. [Google Scholar]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Der Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Li, C.; Chen, Y.; Gerotziafas, G.; Zhang, Z.; Wan, J.; Liu, P.; Elalamy, I.; Wang, C.; On behalf of the Prevention Treatment of VTE Associated with COVID-19 Infection Consensus Statement Group. Pulmonary Embolism Pulmonary Vascular Diseases Group of the Chinese Thoracic SocietyPulmonary Embolism Pulmonary Vascular Disease Working Committee of Prevention and Treatment of Venous Thromboembolism Associated with Coronavirus Disease 2019 Infection: A Consensus Statement before Guidelines. Thromb. Haemost. 2020, 120, 937–948. [Google Scholar] [CrossRef]
- Saposnik, G.; Barinagarrementeria, F.; Brown, R.D., Jr.; Bushnell, C.D.; Cucchiara, B.; Cushman, M.; Deveber, G.; Ferro, J.M.; Tsai, F.Y. Diagnosis and management of cerebral venous thrombosis: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 1158–1192. [Google Scholar] [CrossRef]
- Patronas, N.J.; Duda, E.E.; Mirfakhraee, M.; Wollmann, R.L. Superior sagittal sinus thrombosis diagnosed by computed tomography. Surg. Neurol. 1981, 15, 11–14. [Google Scholar] [CrossRef]
- Zamboni, P.; Malagoni, A.M.; Menegatti, E.; Ragazzi, R.; Tavoni, V.; Tessari, M.; Beggs, C.B. Central venous pressure estimation from ultrasound assessment of the jugular venous pulse. PLoS ONE 2020, 15, e0240057. [Google Scholar] [CrossRef] [PubMed]
- Wachsberg, R.H. B-Flow, a Non-Doppler Technology for Flow Mapping: Early Experience in the Abdomen. Ultrasound Q. 2003, 19, 114–122. [Google Scholar] [CrossRef]
- Yücel, C.; Oktar, S.O.; Erten, Y.; Bursali, A.; Ozdemir, H. B-flow sonographic evaluation of hemodialysis fistulas: A comparison with low- and high-pulse repetition frequency color and power Doppler sonography. J. Ultrasound Med. 2005, 24, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.A.; Jha, P.; Poder, L.; Weinstein, S. Advanced ultrasound applications in the assessment of renal transplants: Contrast-enhanced ultrasound, elastography, and B-flow. Abdom. Radiol. 2018, 43, 2604–2614. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Siddiqui, W.J. Internal Jugular Vein Thrombosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kommareddy, A.; Zaroukian, M.H.; Hassouna, H.I. Upper Extremity Deep Venous Thrombosis. Semin. Thromb. Hemost. 2002, 28, 89–100. [Google Scholar] [CrossRef]
- Prandoni, P. Upper-extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch. Intern. Med. 1997, 157, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Sisini, F.; Tessari, M.; Gadda, G.; Di Domenico, G.; Taibi, A.; Menegatti, E.; Gambaccini, M.; Zamboni, P. An Ultrasonographic Technique to Assess the Jugular Venous Pulse: A Proof of Concept. Ultrasound Med. Biol. 2015, 41, 1334–1341. [Google Scholar] [CrossRef]
- Nakamura, K. Reply: Novel Interest About Cardiac Variation of Internal Jugular Vein for the Evaluation of the Hemodynamics. Ultrasound Med. Biol. 2017, 43, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Constant, J. Using Internal Jugular Pulsations as a Manometer for Right Atrial Pressure Measurements. Cardiology 2000, 93, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, P.; Menegatti, E.; Conforti, P.; Shepherd, S.; Tessari, M.; Beggs, C. Assessment of cerebral venous return by a novel plethysmography method. J. Vasc. Surg. 2012, 56, 677–685.e1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taibi, A.; Gadda, G.; Gambaccini, M.; Menegatti, E.; Sisini, F.; Zamboni, P. Investigation of cerebral venous outflow in microgravity. Physiol. Meas. 2017, 38, 1939–1952. [Google Scholar] [CrossRef]
- García-López, I.; Rodriguez-Villegas, E. Extracting the Jugular Venous Pulse from Anterior Neck Contact Photoplethysmography. Sci. Rep. 2020, 10, 3466. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.L.; Fortuna, R.B.; Jones, B.V.; Gaskill-Shipley, M.F. Imaging of Cerebral Venous Thrombosis: Current Techniques, Spectrum of Findings, and Diagnostic Pitfalls. Radiographics 2006, 26 (Suppl. 1), S19–S41. [Google Scholar] [CrossRef]
- Oppenheim, C.; Domigo, V.; Gauvrit, J.-Y.; Lamy, C.; Mackowiak-Cordoliani, M.-A.; Pruvo, J.-P.; Méder, J.-F. Subarachnoid hemorrhage as the initial presentation of dural sinus thrombosis. Am. J. Neuroradiol. 2005, 26, 614–617. [Google Scholar] [PubMed]
- Ozsvath, R.R.; O Casey, S.; Lustrin, E.S.; Alberico, R.A.; Hassankhani, A.; Patel, M. Cerebral venography: Comparison of CT and MR projection venography. Am. J. Roentgenol. 1997, 169, 1699–1707. [Google Scholar] [CrossRef]
- Liauw, L.; Van Buchem, M.A.; Spilt, A.; De Bruïne, F.T.; Berg, R.V.D.; Hermans, J.; Wasser, M.N.J.M. MR Angiography of the Intracranial Venous System. Radiology 2000, 214, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Debourdeau, P.; Farge, D.; Beckers, M.; Baglin, C.; Bauersachs, R.M.; Brenner, B.; Brilhante, D.; Falanga, A.; Gerotzafias, G.T.; Haim, N.; et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J. Thromb. Haemost. 2013, 11, 71–80. [Google Scholar] [CrossRef]
- Baskin, J.L.; Pui, C.-H.; Reiss, U.; Wilimas, J.A.; Metzger, M.L.; Ribeiro, R.C.; Howard, S.C. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009, 374, 159–169. [Google Scholar] [CrossRef]
- Kreuziger, L.B.; Onwuemene, O.; Kolesar, E.; Crowther, M.A.; Lim, W. Systematic review of anticoagulant treatment of catheter-related thrombosis. Thromb. Res. 2015, 136, 1103–1109. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Einhäupl, K.; Villringer, A.; Mehraein, S.; Garner, C.; Pellkofer, M.; Haberl, R.; Pfister, H.-W.; Schmiedek, P.; Meister, W. Heparin treatment in sinus venous thrombosis. Lancet 1991, 338, 597–600. [Google Scholar] [CrossRef]
- De Bruijn, S.F.T.M.; Stam, J. Randomized, Placebo-Controlled Trial of Anticoagulant Treatment With Low-Molecular-Weight Heparin for Cerebral Sinus Thrombosis. Stroke 1999, 30, 484–488. [Google Scholar] [CrossRef]
- Erkens, P.M.G.; Prins, M.H. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst. Rev. 2010, 2010, CD001100. [Google Scholar] [CrossRef]
- Wasay, M.; Khan, M.; Rajput, H.M.; Farooq, S.; Memon, M.I.; Alrukn, S.A.; Malik, A.; Abd-Allah, F.; Shoaib, R.F.; Shahid, R.; et al. New Oral Anticoagulants versus Warfarin for Cerebral Venous Thrombosis: A Multi-Center, Observational Study. J. Stroke 2019, 21, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Ferro, J.M.; Coutinho, J.M.; Dentali, F.; Kobayashi, A.; Alasheev, A.; Canhão, P.; Karpov, D.; Nagel, S.; Posthuma, L.; Roriz, J.M.; et al. Safety and Efficacy of Dabigatran Etexilate vs Dose-Adjusted Warfarin in Patients with Cerebral Venous Thrombosis: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.I.; Obeid, H.; Matti, L.; Ramakrishna, H.; Shamoun, F.E. Cerebral Venous Thrombosis: Current and Newer Anticoagulant Treatment Options. Neurologist 2015, 20, 80–88. [Google Scholar] [CrossRef] [PubMed]
| Symptoms | Articles |
|---|---|
| Pain | Gbaguidi [2]; Leci-Tahiri [3]; Carrington [11]; Wada [12]; Al-Zoubi [13]; Gunasekaran [14]; Hahn [15]; Shakeel [28]; Graham [16] |
| Asymptomatic | Al-Zoubi [13]; Çakir [36] |
| Difficulty in swallowing, Odinophagia, Disphagia | Carrington [11]; Shakeel [28] |
| Headache | Duke [17]; Wada [12]; Shakeel [28]; Graham [16]; Thapa [18]; Li C [19]; Ilgen [20]; Hacifazlioglu [21]; Masood [22] |
| Numbness/somnolence, alteration of consciousness | Duke [17] |
| Eye discomfort, conjunctival injection | Graham [16] |
| Blurred vision, decrease visual acuity | Duke [17]; Wada [12] |
| Dizziness, Nausea | Thapa [18] |
| Paraesthesia | Wada [12] |
| Signs | |
| Neck swelling and erythema | Gbaguidi [2]; Leci-Tahiri [3]; Uzun [5]; Bandara [6]; Das [23]; Zhang W [24]; Carrington [11]; Toratani [25]; Wada [12]; Al-Zoubi [13]; Hahn [15]; Shakeel [28]; Bilici [26]; Gianesini [27]; Gunasekaran [14]; Duke [17] |
| Palpable cord beneath the sternocleidomastoid muscle | Gbaguidi [2]; Leci-Tahiri [3]; Bandara [6]; Shakeel [28] |
| Superficial varicose collateral veins | Gbaguidi [2]; Mirijello [30] |
| Indurated vein | Gbaguidi [2] |
| Fever | Leci-Tahiri [3]; Shakeel [28]; Thapa [18]; Gunasekaran [14] |
| Oedematous swelling of the face/scalp | Duke [17]; Hyder [29]; Bilici [26]; Thapa [18]; Mirijello [30] |
| Brachial plexopathy | Glemarec [37] |
| Horner Syndrome | Glemarec [37] |
| Papilledema | Duke [17]; Wada [12]; Gunasekaran [14]; Masood [22] |
| Retinal haemorrhage | Duke [17] |
| Intracranial hypertension | Duke [17]; Wada [12]; Shakeel [28]; Li C [19]; Masood [22] |
| VI nerve palsy | Duke [17] |
| Seizures | Wada [12] |
| Agraphia | Wada [12] |
| Progressive Dyspnoea | Bilici [26] |
| Laboratory parameters | |
| Leucocytosis | Leci-Tahiri [3];Uzun [5]; Toratani [25]; Shakeel [28]; Bilici [26]; Gunasekaran [14] |
| D-Dimer raising | Toratani [25]; Shakeel [28]; Li C [32] |
| Etiology | Articles |
|---|---|
| Infection | Leci-Tahiri [3]; Hindi [4]; Uzun [5]; Bandara [6]; Li M [32]; Boga [46]; Das [23]; Lin [57]; Duke [17]; Mnejja [62]; Hahn [15]; Shakeel [28]; Graham [16]; Masood [22] |
| Cervical Neck Fasciitis | Lin [57] |
| Surgery | Gbaguidi [2]; Leci-Tahiri [3]; Uzun [5]; Li M [32]; Das [23]; Zhang W [24]; Zamboni [49]; Quraishi [50]; Duke [17]; Gunasekaran [14]; Shakeel [28]; Petrov [66]; Mandato [67]; Lupattelli [68]; Graham [16] |
| Trauma | Gbaguidi [2]; Leci-Tahiri [3]; Hindi [4]; Uzun [5]; Bandara [6]; Zhang W [24]; Duke [17]; Lin D [57]; Mnejja [62]; Gunasekaran [14]; Shakeel [28] |
| Malignancy | Gbaguidi [2]; Leci-Tahiri [3]; Hindi [4]; Uzun [5]; Bandara [6]; Li M [32]; Das [23]; Zhang W [24]; Duke [17]; Carrington [11]; Toratani [25]; Lin D [57]; Mnejja [62]; Gunasekaran [14]; De Cicco [65]; Harter [63]; Hahn [15]; Shakeel [28]; Siu [69]; Graham [16]; Li [70]; Masood [22] |
| CVC | Gbaguidi [2] Leci-Tahiri [3]; Hindi [4]; Uzun [5]; Bandara [6]; Giordano [43]; Wilkin [44]; Das [23]; Zhang W [24]; Duke [17]; Toratani [25]; Gunasekaran [14]; De Cicco [65]; Ge [71]; Hyder [29]; Biffi [72]; Biffi [73]; Harter [74]; Hahn [15]; Shakeel [28]; Gheith [75]; Siu [69]; Graham [16]; Li H [70]; Thapa [18]; Mirijello [30]; Masood [22] |
| Polycythaemia | Leci-Tahiri [3]; Li M [32]; Das [23], Glemarec [37] |
| Intravenous drug abuse | Leci-Tahiri [3]; Hindi [4]; Bandara [6]; Das [23]; Zhang W [24]; Duke [17]; Merhar [56]; Lin D [57]; Gunasekaran [14]; Shakeel [28]; Graham [16]; Masood [22] |
| Neck massage/Cervical traction | Leci-Tahiri [3]; Li M [32]; Das [23]; Wada [12] |
| Ovarian hyperstimulation syndrome | Gbaguidi [2]; Leci-Tahiri [3]; Hindi [4]; Uzun [5]; Das [23]; Lee SH [61], Hahn [15]; Shakeel [28]; Gunasekaran [14] |
| Hyper-omocysteineimia | Li M [32]; Das [23]; Li H [70] |
| Hypercoagulable state/thrombophilia/coagulation abnormalities | Gbaguidi [2]; Leci-Tahiri [3]; Hindi [4]; Uzun [5]; Bandara [6]; Das [23]; Bostanci [48]; Zhang W [24]; Duke [17]; Al-Zoubi [13]; Mnejja [62]; Çakir [36]; Gunasekaran [14]; Lim [64]; De Cicco [65]; Shakeel [28]; Mirijello [30]; Ilgen [20]; Masood [22] |
| Oral contraceptive, hormonal replacement therapy, pregnancy | Gbaguidi [2]; Hahn [15], Masood [22] |
| Congestive heart failure | Gbaguidi [2]; Uzun [5] |
| Pacemaker | Gbaguidi [2]; Hindi [4]; Graham [16] |
| Immobilization | Gbaguidi [2] |
| Primary (thoracic outlet syndrome, effort, idiopathic) | Gbaguidi [2,49]; Leci-Tahiri [3]; Hindi [4]; Bandara [6]; Toratani [32]; Hahn [15]; Shakeel [28] |
| Substernal Goitre | Shakeel [28] |
| Dehydration status and lack of gravitational gradient | Duke [17]; Marshall-Goebel [76]; Zamboni [77] |
| After balloon venoplasty and/or stenting of the IJV | Mandato [67]; Petrov [66] |
| Beçhet disease | Hahn [15], Bilici [26] |
| Truncular IJV malformation | Lim [64]; Lee [61]; Gianesini [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scerrati, A.; Menegatti, E.; Zamboni, M.; Malagoni, A.M.; Tessari, M.; Galeotti, R.; Zamboni, P. Internal Jugular Vein Thrombosis: Etiology, Symptomatology, Diagnosis and Current Treatment. Diagnostics 2021, 11, 378. https://doi.org/10.3390/diagnostics11020378
Scerrati A, Menegatti E, Zamboni M, Malagoni AM, Tessari M, Galeotti R, Zamboni P. Internal Jugular Vein Thrombosis: Etiology, Symptomatology, Diagnosis and Current Treatment. Diagnostics. 2021; 11(2):378. https://doi.org/10.3390/diagnostics11020378
Chicago/Turabian StyleScerrati, Alba, Erica Menegatti, Matilde Zamboni, Anna Maria Malagoni, Mirko Tessari, Roberto Galeotti, and Paolo Zamboni. 2021. "Internal Jugular Vein Thrombosis: Etiology, Symptomatology, Diagnosis and Current Treatment" Diagnostics 11, no. 2: 378. https://doi.org/10.3390/diagnostics11020378
APA StyleScerrati, A., Menegatti, E., Zamboni, M., Malagoni, A. M., Tessari, M., Galeotti, R., & Zamboni, P. (2021). Internal Jugular Vein Thrombosis: Etiology, Symptomatology, Diagnosis and Current Treatment. Diagnostics, 11(2), 378. https://doi.org/10.3390/diagnostics11020378








