IDH1 Non-Canonical Mutations and Survival in Patients with Glioma
Abstract
1. Introduction
2. Materials and Methods
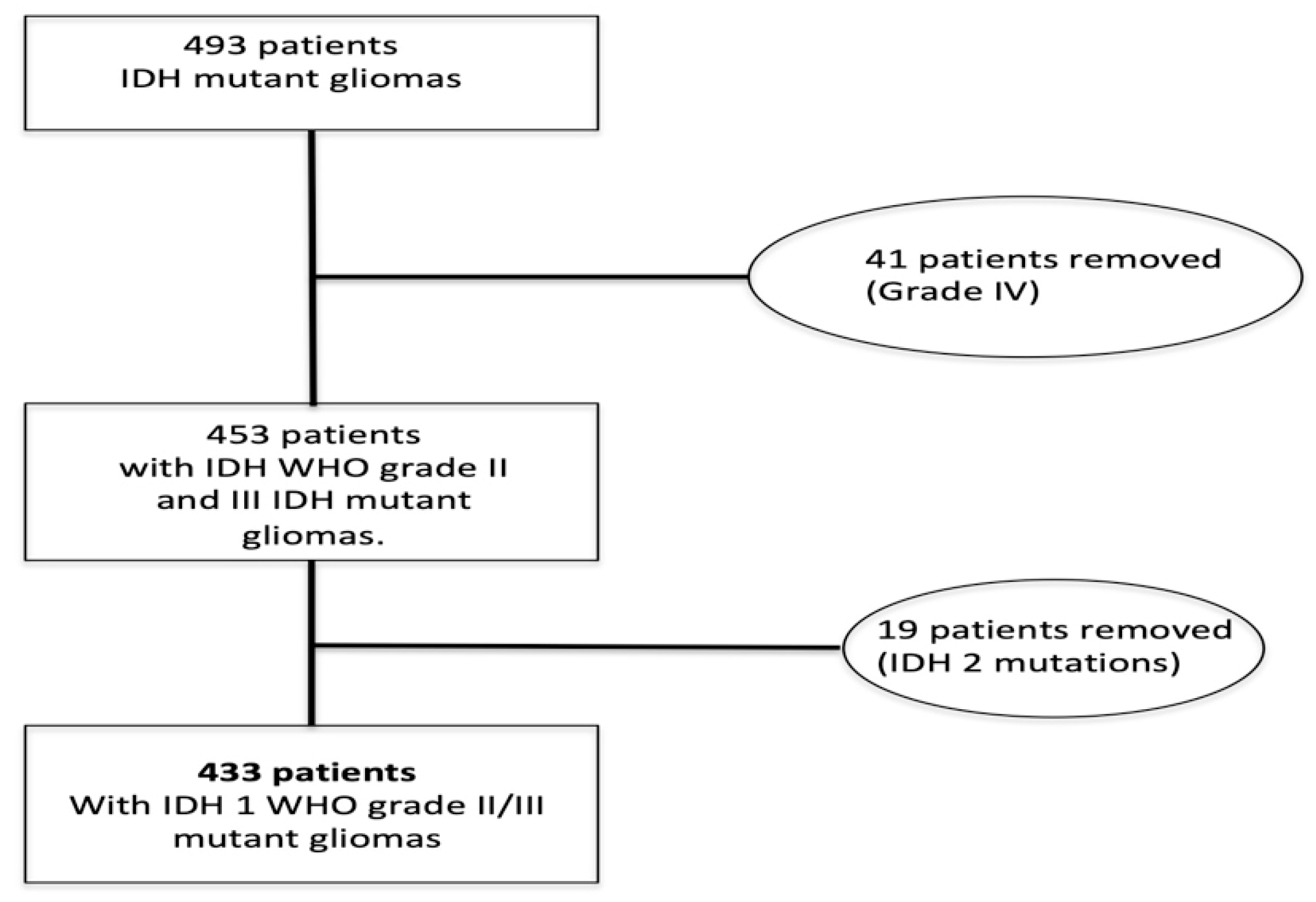
2.1. Patient Selection
2.2. IDH1 Analysis
2.3. Statistical Analysis
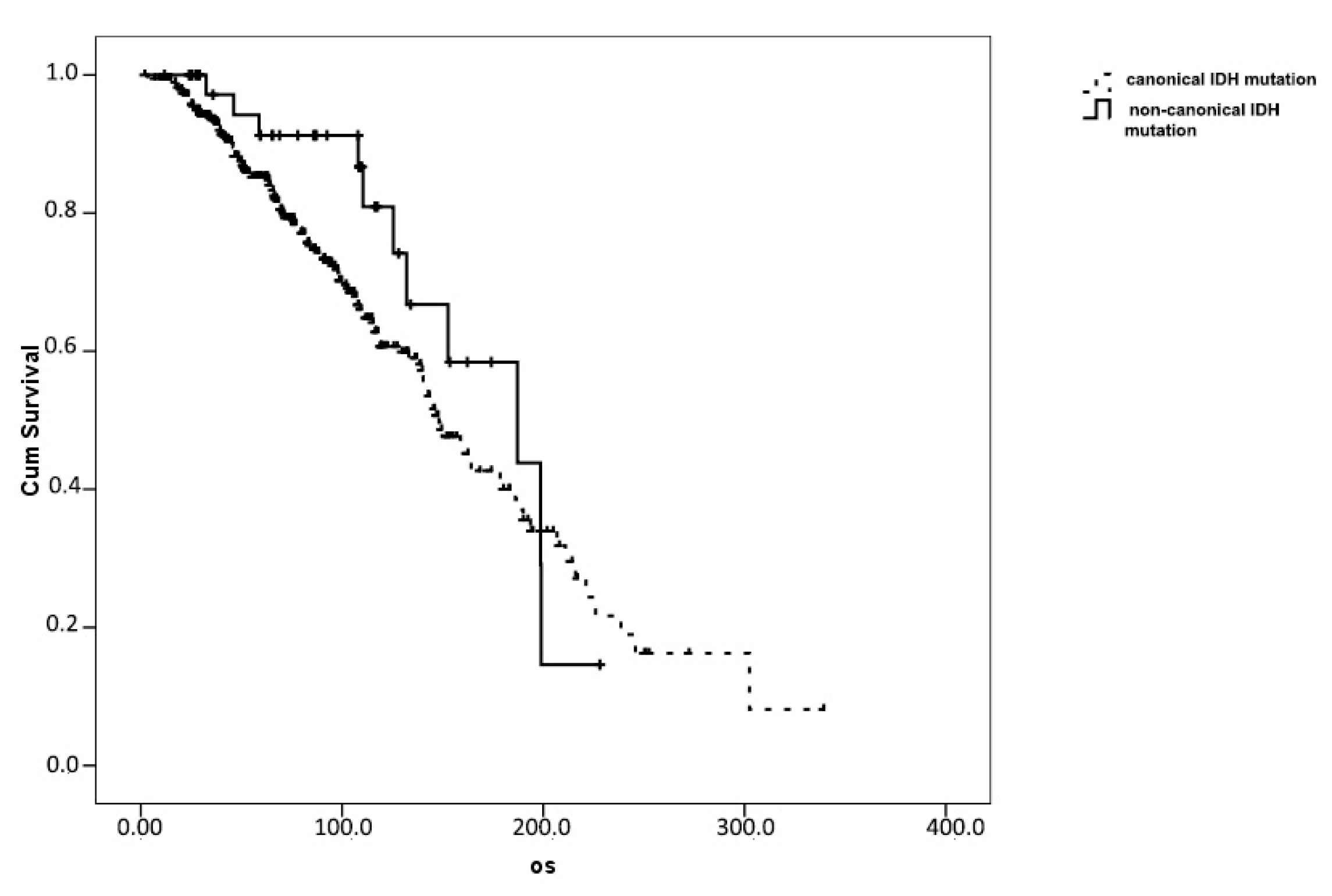
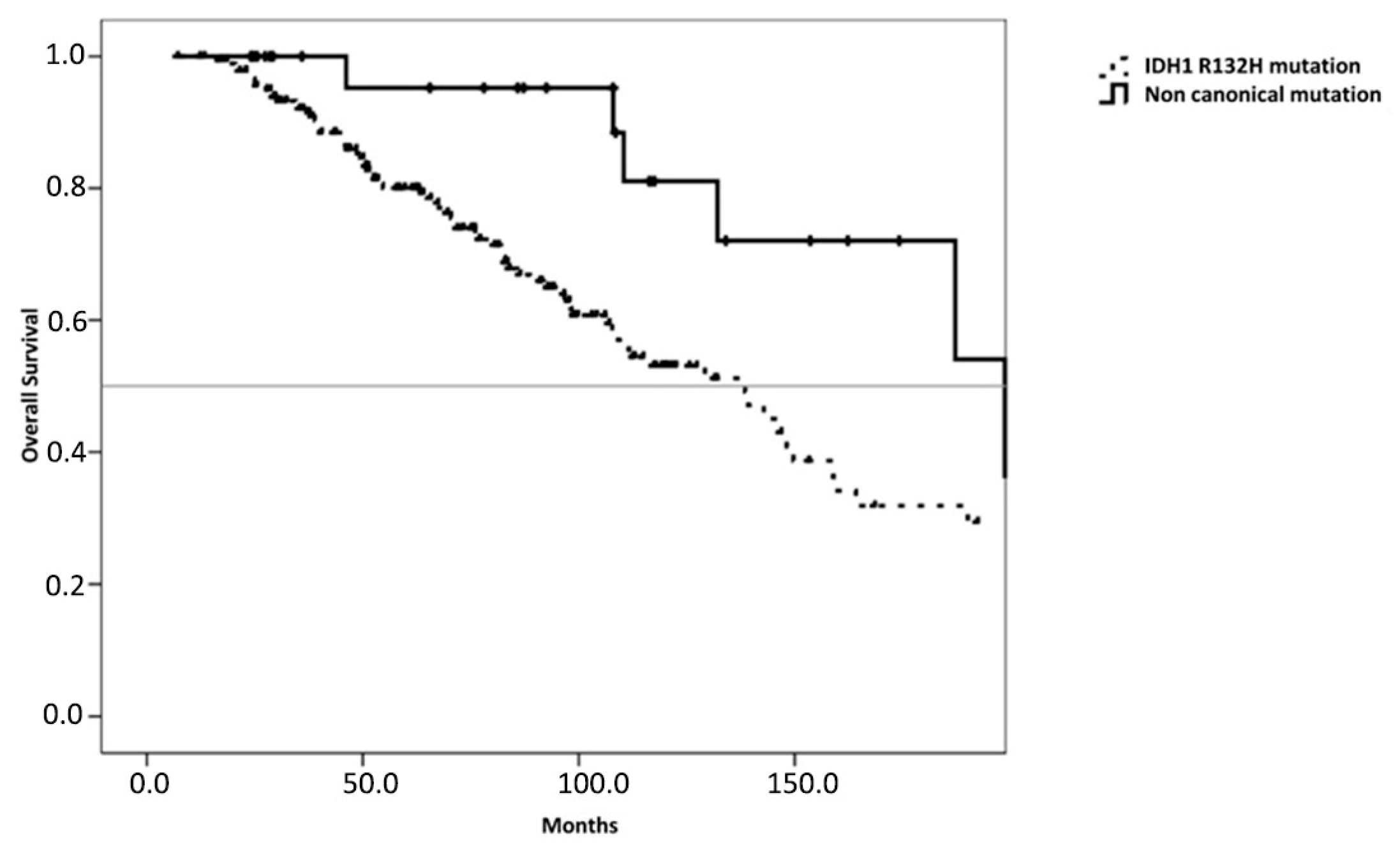
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1andIDH2Mutations in Gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef]
- Sanson, M.; Marie, Y.; Paris, S.; Idbaih, A.; Laffaire, J.; Ducray, F.; El Hallani, S.; Boisselier, B.; Mokhtari, K.; Hoang-Xuan, K.; et al. Isocitrate Dehydrogenase 1 Codon 132 Mutation Is an Important Prognostic Biomarker in Gliomas. J. Clin. Oncol. 2009, 27, 4150–4154. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMTGene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Stupp, R.; Reifenberger, G.; Brandes, A.A.; Bent, M.J.V.D.; Wick, W.; Hegi, M.E. MGMT promoter methylation in malignant gliomas: Ready for personalized medicine? Nat. Rev. Neurol. 2009, 6, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, P.; Bent, M.V.D.; Perry, A. Oligodendroglioma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015, 129, 809–827. [Google Scholar] [CrossRef]
- Van den Bent, M.J.; Dubbink, H.J.; Sanson, M.; van der Lee-Haarloo, C.R.; Hegi, M.; Jeuken, J.W.; Ibdaih, A.; Brandes, A.A.; Taphoorn, M.J.B.; Kros, J.M.; et al. MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: A report from EORTC Brain Tumor Group Study 26951. J. Clin. Oncol. 2009, 27, 5881–5886. [Google Scholar] [CrossRef]
- Brandes, A.A.; Tosoni, A.; Cavallo, G.; Reni, M.; Franceschi, E.; Bonaldi, L.; Bertorelle, R.; Gardiman, M.; Ghimenton, C.; Iuzzolino, P.; et al. Correlations Between O6-Methylguanine DNA Methyltransferase Promoter Methylation Status, 1p and 19q Deletions, and Response to Temozolomide in Anaplastic and Recurrent Oligodendroglioma: A Prospective GICNO Study. J. Clin. Oncol. 2006, 24, 4746–4753. [Google Scholar] [CrossRef]
- Mair, M.J.; Geurts, M.; Bent, M.J.V.D.; Berghoff, A.S. A basic review on systemic treatment options in WHO grade II-III gliomas. Cancer Treat. Rev. 2021, 92, 102124. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Bent, M.V.D.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2020, 1–17. [Google Scholar] [CrossRef]
- Visani, M.; Acquaviva, G.; Marucci, G.; Paccapelo, A.; Mura, A.; Franceschi, E.; Grifoni, D.; Pession, A.; Tallini, G.; De Biase, D.; et al. Non-canonical IDH1 and IDH2 mutations: A clonal and relevant event in an Italian cohort of gliomas classified according to the 2016 World Health Organization (WHO) criteria. J. Neuro Oncol. 2017, 135, 245–254. [Google Scholar] [CrossRef]
- Zacher, A.; Kaulich, K.; Stepanow, S.; Wolter, M.; Köhrer, K.; Felsberg, J.; Malzkorn, B.; Reifenberger, G. Molecular Diagnostics of Gliomas Using Next Generation Sequencing of a Glioma-Tailored Gene Panel. Brain Pathol. 2017, 27, 146–159. [Google Scholar] [CrossRef]
- Chen, N.; Yu, T.; Gong, J.; Nie, L.; Chen, X.; Zhang, M.; Xu, M.; Tan, J.; Su, Z.; Zhong, J.; et al. IDH1/2 gene hotspot mutations in central nervous system tumours: Analysis of 922 Chinese patients. Pathology 2016, 48, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Camelo-Piragua, S.; Jansen, M.; Ganguly, A.; Kim, J.C.; Cosper, A.K.; Dias-Santagata, D.; Nutt, C.L.; Iafrate, A.J.; Louis, D.N. A Sensitive and Specific Diagnostic Panel to Distinguish Diffuse Astrocytoma From Astrocytosis: Chromosome 7 Gain With Mutant Isocitrate Dehydrogenase 1 and p53. J. Neuropathol. Exp. Neurol. 2011, 70, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Tang, K.; Liang, T.-Y.; Zhang, W.-Z.; Li, J.-Y.; Wang, W.; Hu, H.-M.; Li, M.-Y.; Wang, H.-Q.; He, X.-Z.; et al. The comparison of clinical and biological characteristics between IDH1 and IDH2 mutations in gliomas. J. Exp. Clin. Cancer Res. 2016, 35, 1–9. [Google Scholar] [CrossRef]
- Shen, X.; Voets, N.L.; Larkin, S.J.; De Pennington, N.; Plaha, P.; Stacey, R.; Mccullagh, J.S.O.; Schofield, C.J.; Clare, S.; Jezzard, P.; et al. A Noninvasive Comparison Study between Human Gliomas with IDH1 and IDH2 Mutations by MR Spectroscopy. Metabolites 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- De Biase, D.; Visani, M.; Baccarini, P.; Polifemo, A.M.; Maimone, A.; Fornelli, A.; Giuliani, A.; Zanini, N.; Fabbri, C.; Pession, A.; et al. Next Generation Sequencing Improves the Accuracy of KRAS Mutation Analysis in Endoscopic Ultrasound Fine Needle Aspiration Pancreatic Lesions. PLoS ONE 2014, 9, e87651. [Google Scholar] [CrossRef]
- De Biase, D.; Visani, M.; Malapelle, U.; Simonato, F.; Cesari, V.; Bellevicine, C.; Pession, A.; Troncone, G.; Fassina, A.; Tallini, G. Next-Generation Sequencing of Lung Cancer EGFR Exons 18-21 Allows Effective Molecular Diagnosis of Small Routine Samples (Cytology and Biopsy). PLoS ONE 2013, 8, e83607. [Google Scholar] [CrossRef]
- Balss, J.; Meyer, J.; Mueller, W.; Korshunov, A.; Hartmann, C.; von Deimling, A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008, 116, 597–602. [Google Scholar] [CrossRef]
- Gravendeel, L.A.; Kloosterhof, N.K.; Bralten, L.B.; van Marion, R.; Dubbink, H.J.; Dinjens, W.; Bleeker, F.E.; Hoogenraad, C.C.; Michiels, E.; French, P.J.; et al. Segre- gation of non-p.R132H mutations in IDH1 in distinct molecular subtypes of glioma. Hum. Mutat. 2010, 31, E1186–E1199. [Google Scholar] [CrossRef]
- Hartmann, C.; Meyer, J.; Balss, J.; Capper, D.; Mueller, W.; Christians, A.; Felsberg, J.; Wolter, M.; Mawrin, C.; von Deimling, A.; et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: A study of 1010 diffuse gliomas. Acta Neuropathol. 2009, 118, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Metellus, P.; Coulibaly, B.; Colin, C.; de Paula, A.M.; Vasiljevic, A.; Taieb, D.; Barlier, A.; Boisselier, B.; Mokhtari, K.; Figarella-Branger, D.; et al. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010, 120, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, L.; Network, P.; Bronnimann, C.; Loiseau, H.; Frénel, J.S.; Siegfried, A.; Seizeur, R.; Gauchotte, G.; Cappellen, D.; Carpentier, C.; et al. Characteristics of IDH-mutant gliomas with non-canonical IDH mutation. J. Neuro Oncol. 2021, 151, 279–286. [Google Scholar] [CrossRef]
- Wijnenga, M.M.J.; French, P.J.; Dubbink, H.J.; Dinjens, W.N.M.; Atmodimedjo, P.N.; Kros, J.M.; Smits, M.; Gahrmann, R.; Rutten, G.-J.; Verheul, J.B.; et al. The impact of surgery in molecularly defined low-grade glioma: An integrated clinical, radiological, and molecular analysis. Neuro Oncol. 2017, 20, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Jakola, A.S.; Skjulsvik, A.J.; Myrmel, K.S.; Sjåvik, K.; Unsgård, G.; Torp, S.H.; Aaberg, K.; Berg, T.; Dai, H.Y.; Johnsen, K.; et al. Surgical resection versus watchful waiting in low-grade gliomas. Ann. Oncol. 2017, 28, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef]
- Pusch, S.; Schweizer, L.; Beck, A.-C.; Lehmler, J.-M.; Weissert, S.; Balss, J.; Miller, A.K.; Von Deimling, A. D-2-Hydroxyglutarate producing neo-enzymatic activity inversely correlates with frequency of the type of isocitrate dehydrogenase 1 mutations found in glioma. Acta Neuropathol. Commun. 2014, 2, 19. [Google Scholar] [CrossRef]
- Matteo, D.A.; Grunseth, A.J.; Gonzalez, E.R.; Anselmo, S.L.; Kennedy, M.A.; Moman, P.; Scott, D.A.; Hoang, A.; Sohl, C.D. Molecular mechanisms of isocitrate dehydrogenase 1 (IDH1) mutations identified in tumors: The role of size and hydrophobicity at residue 132 on catalytic efficiency. J. Biol. Chem. 2017, 292, 7971–7983. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH mutation in glioma: Molecular mechanisms and potential therapeutic targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef]
- Franceschi, E.; Tosoni, A.; Bartolini, S.; Minichillo, S.; Mura, A.; Asioli, S.; Bartolini, D.; Gardiman, M.; Gessi, M.; Ghimenton, C.; et al. Histopathological grading affects survival in patients with IDH-mutant grade II and grade III diffuse gliomas. Eur. J. Cancer 2020, 137, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, M.; Rice, T.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Hansen, H.; Sicotte, H.; Kollmeyer, T.M.; McCoy, L.S.; Sarkar, G.; et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: Additional prognostic roles of ATRX and TERT. Acta Neuropathol. 2017, 133, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Reuss, D.E.; Sahm, F.; Schrimpf, D.; Wiestler, B.; Capper, D.; Koelsche, C.; Schweizer, L.; Korshunov, A.; Jones, D.T.W.; Hovestadt, V.; et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015, 129, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Weber, R.G.; Willscher, E.; Riehmer, V.; Hentschel, B.; Kreuz, M.; Felsberg, J.; Beyer, U.; Löffler-Wirth, H.; Kaulich, K.; et al. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015, 129, 679–693. [Google Scholar] [CrossRef]
| IDH1 Mutation | Patients (Percentage) |
|---|---|
| R132H | 390 (90.1%) |
| R132C | 19 (4.4%) |
| R132G | 12 (2.8%) |
| R132S | 9 (2.1%) |
| R132L | 3 (0.7%) |
| Variables | Overall Population | IDH1 Canonical | IDH1 Non-Canonical | p-Value |
|---|---|---|---|---|
| (n = 433) | (n = 390) | (n = 43) | ||
| Median age years (range) | 38 (18–76) | 39 (18–76) | 29 (19–47) | <0.001 * |
| Gender | 0.675 | |||
| Male | 253 (58.4%) | 229 (58.7%) | 24 (55.8%) | |
| Female | 180 (41.6%) | 161 (41.3%) | 19 (44.2%) | |
| Grade | 0.675 | |||
| 2 | 269 (62.1%) | 239 (61.3%) | 30 (69.8%) | |
| 3 | 164 (37.9%) | 151 (38.7%) | 13 (30.2%) | |
| 1p19q Codeletion | 0.017 * | |||
| Present | 167 (38.6%) | 157 (40.2%) | 10 (25.6%) | |
| Absent | 216 (49.9%) | 187 (48.0%) | 29 (67.4%) | |
| Miss | 50 (11.5%) | 46 (11.8%) | 4 (7.0%) | |
| Surgical Approach | 0.039 * | |||
| Biopsy | 36 (8.2%) | 35 (9.0%) | 1 (2.2%) | |
| Partial Resection | 254 (58.7%) | 233 (59.7%) | 21 (48.7%) | |
| Total resection | 127 (29.3%) | 108 (27.7%) | 19 (44.7%) | |
| Miss | 16 (3.8%) | 14 (3.6%) | 2 (4.4%) | |
| Post surgical treatment | 0.332 | |||
| Follow up | 165 (38.1%) | 145 (37.2%) | 20 (46.6%) | |
| Radiotherapy | 67 (15.5%) | 61 (15.6%) | 6 (14.1%) | |
| Chemotherapy | 40 (9.2%) | 39 (10.0%) | 1 (2.2%) | |
| Radiotherapy and Chemotherapy | 149 (34.4%) | 134 (34.3%) | 15 (34.9%) | |
| Miss | 12 (2.8%) | 11 (2.9%) | 1 (2.2) |
| Variables | Overall Population | IDH1 Canonical | IDH1 Non-Canonical | p-Value |
|---|---|---|---|---|
| (n = 433) | (n = 390) | (n = 43) | ||
| Median age years (range) | 38 (18–76) | 39 (18–76) | 29 (19–47) | <0.001 * |
| Gender | 0.675 | |||
| Male | 253 (58.4%) | 229 (58.7%) | 24 (55.8%) | |
| Female | 180 (41.6%) | 161 (41.3%) | 19 (44.2%) | |
| Grade | 0.675 | |||
| 2 | 269 (62.1%) | 239 (61.3%) | 30 (69.8%) | |
| 3 | 164 (37.9%) | 151 (38.7%) | 13 (30.2%) | |
| 1p19q Codeletion | 0.017 * | |||
| Present | 167 (38.6%) | 157 (40.2%) | 10 (25.6%) | |
| Absent | 216 (49.9%) | 187 (48.0%) | 29 (67.4%) | |
| Miss | 50 (11.5%) | 46 (11.8%) | 4 (7.0%) | |
| Surgical Approach | 0.039 * | |||
| Biopsy | 36 (8.2%) | 35 (9.0%) | 1 (2.2%) | |
| Partial Resection | 254 (58.7%) | 233 (59.7%) | 21 (48.7%) | |
| Total resection | 127 (29.3%) | 108 (27.7%) | 19 (44.7%) | |
| Miss | 16 (3.8%) | 14 (3.6%) | 2 (4.4%) | |
| Post surgical treatment | 0.332 | |||
| Follow up | 165 (38.1%) | 145 (37.2%) | 20 (46.6%) | |
| Radiotherapy | 67 (15.5%) | 61 (15.6%) | 6 (14.1%) | |
| Chemotherapy | 40 (9.2%) | 39 (10.0%) | 1 (2.2%) | |
| Radiotherapy and Chemotherapy | 149 (34.4%) | 134 (34.3%) | 15 (34.9%) | |
| Miss | 12 (2.8%) | 11 (2.9%) | 1 (2.2) |
| Variables | Overall Population | IDH1 Canonical | IDH1 Non-Canonical | p-Value |
|---|---|---|---|---|
| (n = 433) | (n = 390) | (n = 43) | ||
| Median age years (range) | 38 (18–76) | 39 (18–76) | 29 (19–47) | <0.001 * |
| Gender | 0.675 | |||
| Male | 253 (58.4%) | 229 (58.7%) | 24 (55.8%) | |
| Female | 180 (41.6%) | 161 (41.3%) | 19 (44.2%) | |
| Grade | 0.675 | |||
| 2 | 269 (62.1%) | 239 (61.3%) | 30 (69.8%) | |
| 3 | 164 (37.9%) | 151 (38.7%) | 13 (30.2%) | |
| 1p19q Codeletion | 0.017 * | |||
| Present | 167 (38.6%) | 157 (40.2%) | 10 (25.6%) | |
| Absent | 216 (49.9%) | 187 (48.0%) | 29 (67.4%) | |
| Miss | 50 (11.5%) | 46 (11.8%) | 4 (7.0%) | |
| Surgical Approach | 0.039 * | |||
| Biopsy | 36 (8.2%) | 35 (9.0%) | 1 (2.2%) | |
| Partial Resection | 254 (58.7%) | 233 (59.7%) | 21 (48.7%) | |
| Total resection | 127 (29.3%) | 108 (27.7%) | 19 (44.7%) | |
| Miss | 16 (3.8%) | 14 (3.6%) | 2 (4.4%) | |
| Post surgical treatment | 0.332 | |||
| Follow up | 165 (38.1%) | 145 (37.2%) | 20 (46.6%) | |
| Radiotherapy | 67 (15.5%) | 61 (15.6%) | 6 (14.1%) | |
| Chemotherapy | 40 (9.2%) | 39 (10.0%) | 1 (2.2%) | |
| Radiotherapy and Chemotherapy | 149 (34.4%) | 134 (34.3%) | 15 (34.9%) | |
| Miss | 12 (2.8%) | 11 (2.9%) | 1 (2.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceschi, E.; De Biase, D.; Di Nunno, V.; Pession, A.; Tosoni, A.; Gatto, L.; Tallini, G.; Visani, M.; Lodi, R.; Bartolini, S.; et al. IDH1 Non-Canonical Mutations and Survival in Patients with Glioma. Diagnostics 2021, 11, 342. https://doi.org/10.3390/diagnostics11020342
Franceschi E, De Biase D, Di Nunno V, Pession A, Tosoni A, Gatto L, Tallini G, Visani M, Lodi R, Bartolini S, et al. IDH1 Non-Canonical Mutations and Survival in Patients with Glioma. Diagnostics. 2021; 11(2):342. https://doi.org/10.3390/diagnostics11020342
Chicago/Turabian StyleFranceschi, Enrico, Dario De Biase, Vincenzo Di Nunno, Annalisa Pession, Alicia Tosoni, Lidia Gatto, Giovanni Tallini, Michela Visani, Raffaele Lodi, Stefania Bartolini, and et al. 2021. "IDH1 Non-Canonical Mutations and Survival in Patients with Glioma" Diagnostics 11, no. 2: 342. https://doi.org/10.3390/diagnostics11020342
APA StyleFranceschi, E., De Biase, D., Di Nunno, V., Pession, A., Tosoni, A., Gatto, L., Tallini, G., Visani, M., Lodi, R., Bartolini, S., & Brandes, A. A. (2021). IDH1 Non-Canonical Mutations and Survival in Patients with Glioma. Diagnostics, 11(2), 342. https://doi.org/10.3390/diagnostics11020342








