The Cardiac Effects of Performance-Enhancing Medications: Caffeine vs. Anabolic Androgenic Steroids
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Caffeine as a Performance Enhancing Agent
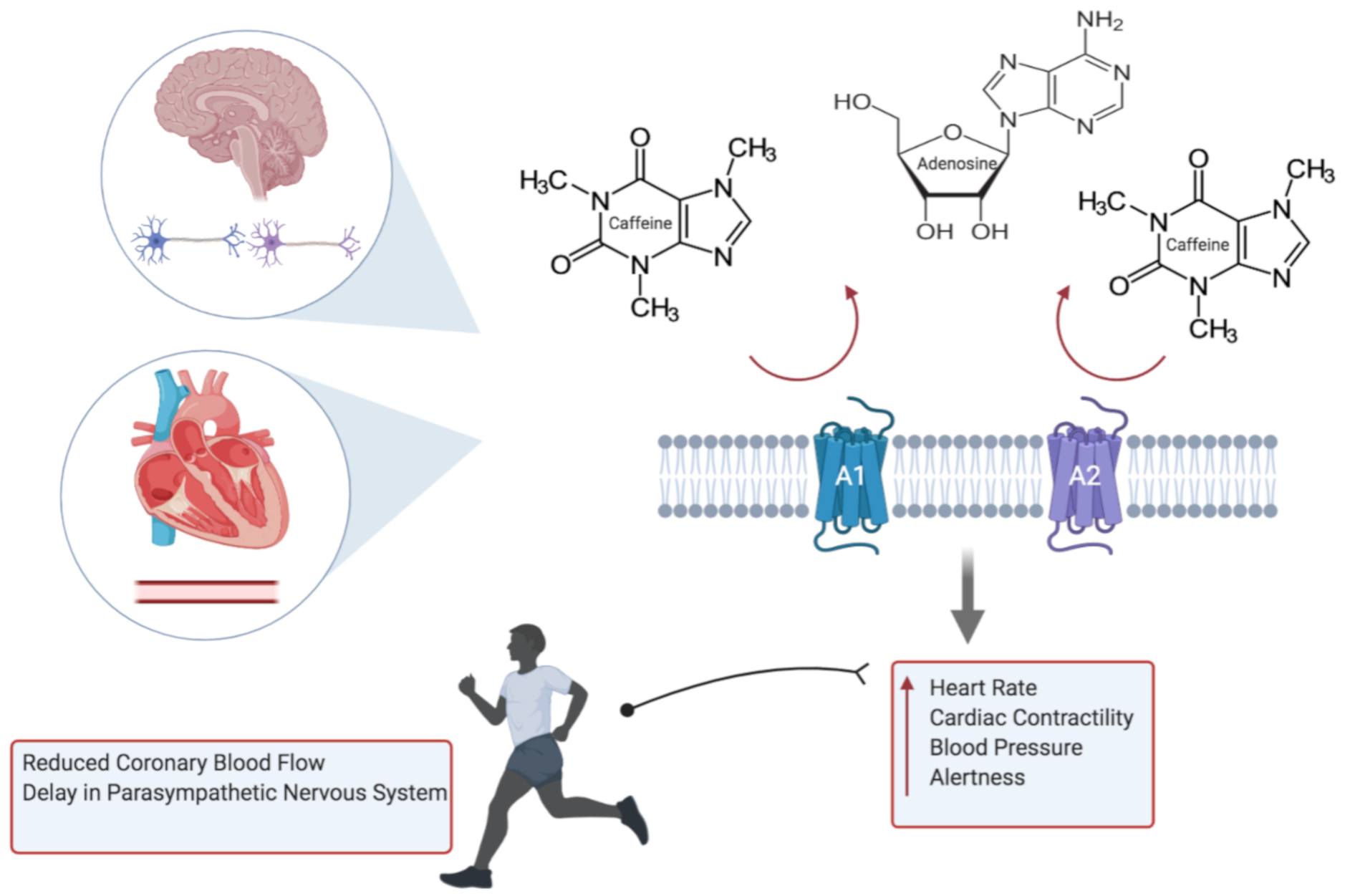
3.1.1. Caffeine Pharmacology and Cardiac Physiology
3.1.2. Caffeine and Risk of Arrhythmia
3.1.3. Caffeine Genetics
3.1.4. Caffeine in Sudden Cardiac Death
3.2. Anabolic Androgenic Steroids as a Performance Enhancing Agent
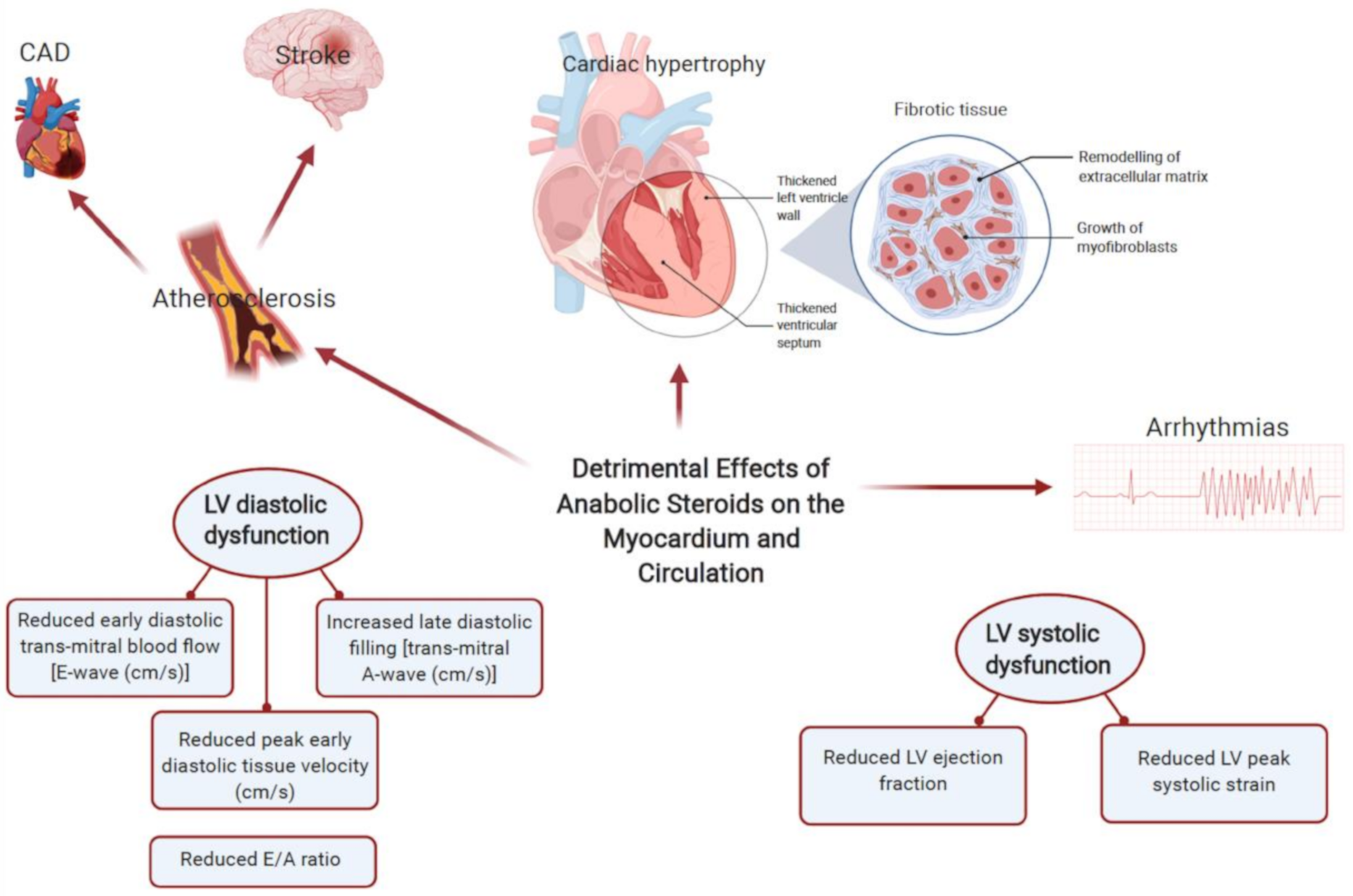
3.2.1. AASs Pharmacology and Cardiac Physiology
3.2.2. AASs and Risk of Arrhythmia
3.2.3. AASs Genetics
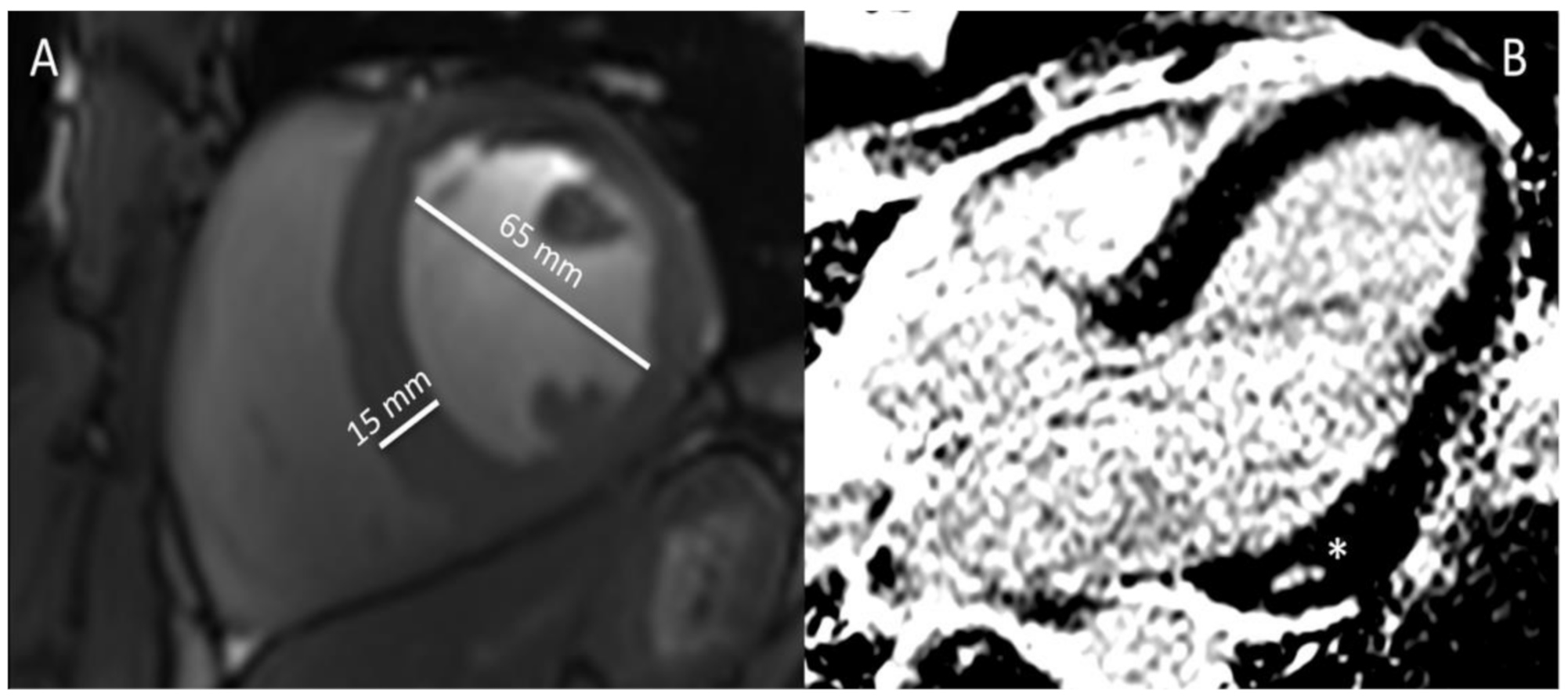
3.2.4. AASs in Sudden Cardiac Death
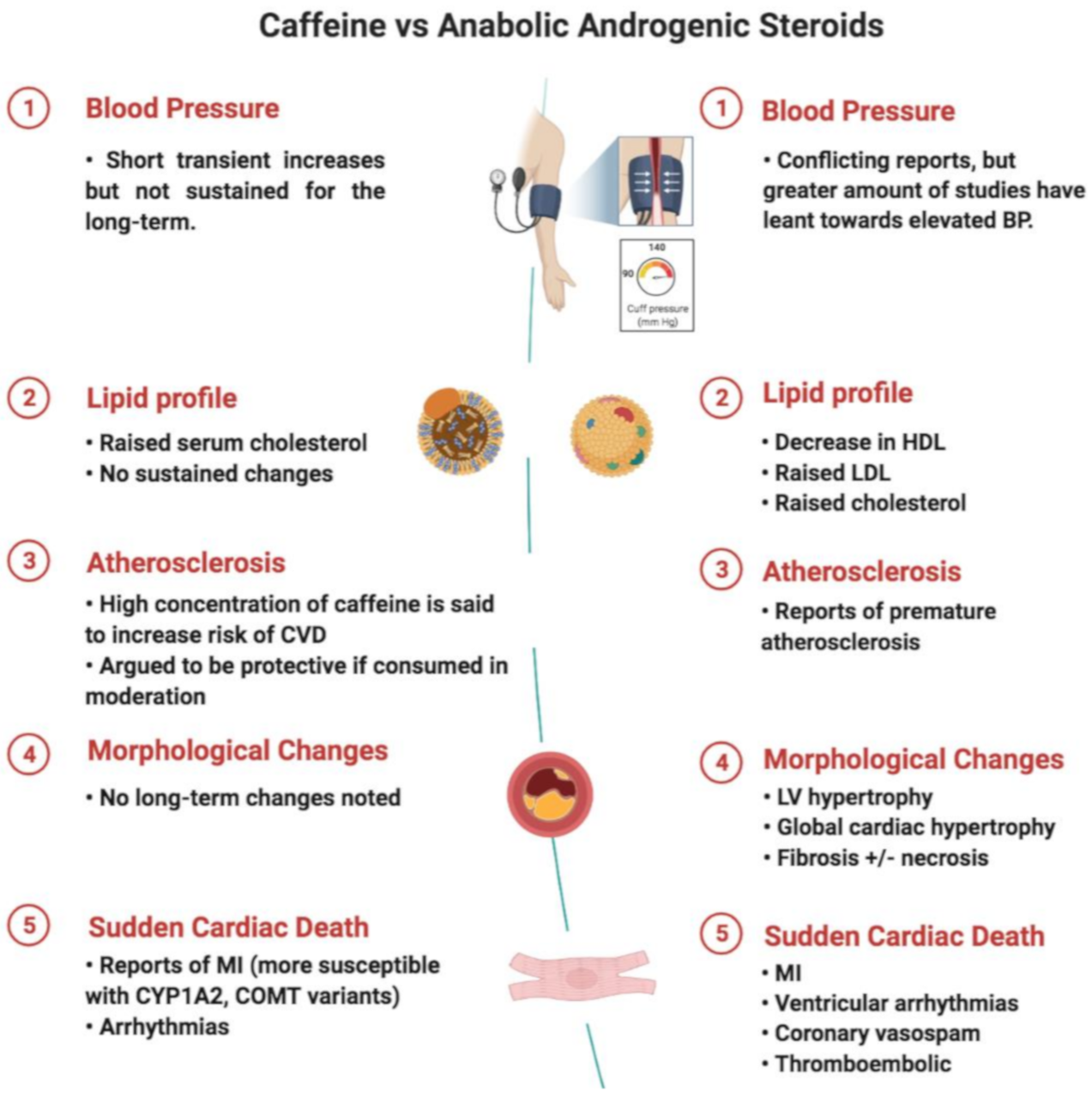
4. Discussion
Clinical Pitfalls and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Pickering, C.; Kiely, J. Are the Current Guidelines on Caffeine Use in Sport Optimal for Everyone? Inter-individual Vari-ation in Caffeine Ergogenicity, and a Move Towards Personalised Sports Nutrition. Sports Med. 2018, 48, 7–16. [Google Scholar] [CrossRef]
- Berglund, B.; Hemmingsson, P. Effects of Caffeine Ingestion on Exercise Performance at Low and High Altitudes in Cross-Country Skiers. Int. J. Sports Med. 1982, 3, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Stadheim, H.K.; Nossum, E.M.; Olsen, R.; Spencer, M.; Jensen, J. Caffeine improves performance in double poling during acute exposure to 2,000-m altitude. J. Appl. Physiol. 2015, 119, 1501–1509. [Google Scholar] [CrossRef]
- Powers, S.K.; Byrd, R.J.; Tulley, R.; Callender, T. Effects of caffeine ingestion on metabolism and performance during graded exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 1983, 50, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Grgic, I.; Pickering, C.; Schoenfeld, B.J.; Bishop, D.J.; Virgile, A.; Pedisic, Z. Infographic. Wake up and smell the coffee: Caffeine supplementation and exercise performance. Br. J. Sports Med. 2020, 54, 304–305. [Google Scholar] [CrossRef]
- Mullen, C.; Whalley, B.J.; Schifano, F.; Baker, J.S. Anabolic androgenic steroid abuse in the United Kingdom: An update. Br. J. Pharmacol. 2020, 177, 2180–2198. [Google Scholar] [CrossRef] [PubMed]
- Sagoe, D.; Molde, H.; Andreassen, C.S.; Torsheim, T.; Pallesen, S. The global epidemiology of anabolic-androgenic steroid use: A meta-analysis and meta-regression analysis. Ann. Epidemiol. 2014, 24, 383–398. [Google Scholar] [CrossRef]
- Reyes-Vallejo, L. Current use and abuse of anabolic steroids. Actas Urológicas Españolas (Engl. Ed.) 2020, 44, 309–313. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Docea, A.O.; Calina, D.; Tsarouhas, K.; Zamfira, L.-M.; Mitrut, R.; Sharifi-Rad, J.; Kovatsi, L.; Siokas, V.; Dardiotis, E.; et al. A Mechanistic and Pathophysiological Approach for Stroke Associated with Drugs of Abuse. J. Clin. Med. 2019, 8, 1295. [Google Scholar] [CrossRef]
- McCullough, D.; Webb, R.; Enright, K.J.; Lane, K.E.; McVeigh, J.; Stewart, C.E.; Davies, I.G. How the love of muscle can break a heart: Impact of anabolic androgenic steroids on skeletal muscle hypertrophy, metabolic and cardiovascular health. Rev. Endocr. Metab. Disord. 2020, 1–17. [Google Scholar] [CrossRef]
- Payne, J.R.; Kotwinski, P.J.; E Montgomery, H. Cardiac effects of anabolic steroids. Heart 2004, 90, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Southward, K.; Rutherfurd-Markwick, K.J.; Ali, A. The Effect of Acute Caffeine Ingestion on Endurance Performance: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.M.; Church, R.J.; Lewander, W. Energy Drinks: The New Eye-Opener For Adolescents. Clin. Pediatr. Emerg. Med. 2008, 9, 35–42. [Google Scholar] [CrossRef]
- Espinola, E.; Dias, R.; Mattei, R.; Carlini, E. Pharmacological activity of Guarana (Paullinia cupana Mart.) in laboratory animals. J. Ethnopharmacol. 1997, 55, 223–229. [Google Scholar] [CrossRef]
- Glatter, K.A.; Myers, R.; Chiamvimonvat, N. Recommendations regarding dietary intake and caffeine and alcohol con-sumption in patients with cardiac arrhythmias: What do you tell your patients to do or not to do? Curr. Treat. Options Cardiovasc. Med. 2012, 14, 529–535. [Google Scholar] [CrossRef]
- Seifert, S.M.; Schaechter, J.L.; Hershorin, E.R.; Lipshultz, S.E. Health Effects of Energy Drinks on Children, Adolescents, and Young Adults. Pediatrics 2011, 127, 511–528. [Google Scholar] [CrossRef]
- Spriet, L.L. Exercise and Sport Performance with Low Doses of Caffeine. Sports Med. 2014, 44, 175–184. [Google Scholar] [CrossRef]
- Del Coso, J.; Muñoz-Fernández, V.E.; Muñoz, G.; Fernández-Elías, V.E.; Ortega, J.F.; Hamouti, N.; Barbero, J.C.; Muñoz-Guerraet, J. Effects of a caffeine containing energy drink on simulated soccer performance. PLoS ONE 2012, 7, e31380. [Google Scholar] [CrossRef]
- Pickering, C.; Grgic, J. Caffeine and Exercise: What Next? Sports Med. 2019, 49, 1007–1030. [Google Scholar] [CrossRef]
- Trapp, G.S.; Allen, K.; O’Sullivan, T.A.; Robinson, M.; Jacoby, P.; Oddy, W.H. ENERGY DRINK CONSUMPTION IS ASSOCIATED WITH ANXIETY IN AUSTRALIAN YOUNG ADULT MALES. Depress. Anxiety 2013, 31, 420–428. [Google Scholar] [CrossRef]
- Graham, T.E.; Rush, J.W.E.; Van Soeren, M.H. Caffeine and Exercise: Metabolism and Performance. Can. J. Appl. Physiol. 1994, 19, 111–138. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, L.A.; Vanderlei, L.C.M.; Gomes, R.L.; Valenti, V.E. Caffeine affects autonomic control of heart rate and blood pressure recovery after aerobic exercise in young adults: A crossover study. Sci. Rep. 2017, 7, 14091. [Google Scholar] [CrossRef]
- Skinner, T.L.; Desbrow, B.; Arapova, J.; Schaumberg, M.A.; Osborne, J.; Grant, G.D.; Anoopkumar-Dukie, S.; Leveritt, M.D. Women Experience the Same Ergogenic Response to Caffeine as Men. Med. Sci. Sports Exerc. 2019, 51, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Haller, C.A.; Jacob, P.; Benowitz, N.L. Pharmacology of ephedra alkaloids and caffeine after single-dose dietary supplement use*. Clin. Pharmacol. Ther. 2002, 71, 421–432. [Google Scholar] [CrossRef]
- Loftfield, E.; Freedman, N.D.; Graubard, B.I.; Guertin, K.A.; Black, A.; Huang, W.-Y.; Shebl, F.M.; Mayne, S.T.; Sinha, R. Association of Coffee Consumption With Overall and Cause-Specific Mortality in a Large US Prospective Cohort Study. Am. J. Epidemiol. 2015, 182, 1010–1022. [Google Scholar] [CrossRef]
- Rosenberg, L.; Palmer, J.R.; Kelly, J.P.; Kaufman, D.W.; Shapiro, S. Coffee drinking and nonfatal myocardial infarction in men under 55 years of age. Am. J. Epidemiol. 1988, 128, 570–578. [Google Scholar] [CrossRef]
- Lacroix, A.Z.; Mead, L.A.; Liang, K.-Y.; Thomas, C.B.; Pearson, T.A. Coffee Consumption and the Incidence of Coronary Heart Disease. New Engl. J. Med. 1986, 315, 977–982. [Google Scholar] [CrossRef]
- Happonen, P.; Voutilainen, S.; Salonen, J.T. Coffee Drinking Is Dose-Dependently Related to the Risk of Acute Coronary Events in Middle-Aged Men. J. Nutr. 2004, 134, 2381–2386. [Google Scholar] [CrossRef]
- Goldfarb, M.; Tellier, C.; Thanassoulis, G. Review of Published Cases of Adverse Cardiovascular Events After Ingestion of Energy Drinks. Am. J. Cardiol. 2014, 113, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Avcı, S.; Sarıkaya, R.; Büyükcam, F. Death of a young man after overuse of energy drink. Am. J. Emerg. Med. 2013, 31, 1624.e3–1624.e4. [Google Scholar] [CrossRef]
- Frost, L.; Vestergaard, P. Caffeine and risk of atrial fibrillation or flutter: The Danish Diet, Cancer, and Health Study. Am. J. Clin. Nutr. 2005, 81, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Pareja-Galeano, H.; Cervellin, G.; Lippi, G.; Earnest, C.P. Energy Drink Overconsumption in Adolescents: Implications for Arrhythmias and Other Cardiovascular Events. Can. J. Cardiol. 2015, 31, 572–575. [Google Scholar] [CrossRef]
- Zuchinali, P.; Zimerman, A.; Giaretta, V.; Salamoni, J.; Fracasso, B.; Pimentel, M.; Souza, G.C.; Chemello, D.; I Zimerman, L.; E Rohde, L. Short-term Effects of High-Dose Caffeine on Cardiac Arrhythmias in Patients With Heart Failure: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 1752–1759. [Google Scholar] [CrossRef]
- Voskoboinik, A.; Kalman, J.M.; Kistler, P.M. Caffeine and Arrhythmias: Time to Grind the Data. JACC Clin. Electrophysiol. 2018, 4, 425–432. [Google Scholar] [CrossRef]
- Souza, D.B.; Del Coso, J.; Casonatto, J.; Polito, M.D. Acute effects of caffeine-containing energy drinks on physical perfor-mance: A systematic review and meta-analysis. Eur. J. Nutr. 2017, 56, 13–27. [Google Scholar] [CrossRef]
- Fletcher, E.A.; Lacey, C.S.; Aaron, M.; Kolasa, M.; Occiano, A.; Shah, S.A. Randomized Controlled Trial of High-Volume Energy Drink Versus Caffeine Consumption on ECG and Hemodynamic Parameters. J. Am. Hear. Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Cornelis, M.C.; Monda, K.L.; Yu, K.; Paynter, N.; Azzato, E.M.; Bennett, S.N.; Berndt, S.I.; Boerwinkle, E.; Chanock, S.; Chatterjee, N.; et al. Genome-Wide Meta-Analysis Identifies Regions on 7p21 (AHR) and 15q24 (CYP1A2) As Determinants of Habitual Caffeine Consumption. PLoS Genet. 2011, 7, e1002033. [Google Scholar] [CrossRef]
- Sulem, P.; Gudbjartsson, D.F.; Geller, F.; Prokopenko, I.; Feenstra, B.; Aben, K.K.H.; Franke, B.; den Heijer, M.; Kovacs, P.; Stumvoll, M.; et al. Sequence variants at CYP1A1- CYP1A2 and AHR associate with coffee consumption. Hum. Mol. Genet. 2011, 20, 2071–2077. [Google Scholar] [CrossRef]
- Denden, S.; Bouden, B.; Khelil, A.H.; Ben Chibani, J.; Hamdaoui, M. Gender and ethnicity modify the association between the CYP1A2 rs762551 polymorphism and habitual coffee intake: Evidence from a meta-analysis. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Happonen, P.; Voutilainen, S.; Tuomainen, T.-P.; Salonen, J.T. Catechol-O-Methyltransferase Gene Polymorphism Modifies the Effect of Coffee Intake on Incidence of Acute Coronary Events. PLoS ONE 2006, 1, e117. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, A.; Frankel, D.S. Arrhythmogenic effects of energy drinks. J. Cardiovasc. Electrophysiol. 2017, 28, 711–717. [Google Scholar] [CrossRef]
- Bird, S.R.; Goebel, C.; Burke, L.M.; Greaves, R.F. Doping in sport and exercise: Anabolic, ergogenic, health and clinical issues. Ann. Clin. Biochem. 2016, 53, 196–221. [Google Scholar] [CrossRef]
- Torrisi, M.; Pennisi, G.; Russo, I.; Amico, F.; Esposito, M.; Liberto, A.; Cocimano, G.; Salerno, M.; Rosi, G.L.; Di Nunno, N.; et al. Sudden Cardiac Death in Anabolic-Androgenic Steroid Users: A Literature Review. Medicina 2020, 56, 587. [Google Scholar] [CrossRef]
- Marsh, J.D.; Lehmann, M.H.; Ritchie, R.H.; Gwathmey, J.K.; Green, G.E.; Schiebinger, R.J. Androgen receptors mediate hyper-trophy in cardiac myocytes. Circulation 1998, 98, 256–261. [Google Scholar] [CrossRef]
- Perry, J.C.; Schuetz, T.M.; Memon, M.D.; Faiz, S.; Cancarevic, I. Anabolic Steroids and Cardiovascular Outcomes: The Controversy. Cureus 2020, 12, e9333. [Google Scholar] [CrossRef]
- Thiblin, I.; Garmo, H.; Garle, M.; Holmberg, L.; Byberg, L.; Michaëlsson, K.; Gedeborg, R. Anabolic steroids and cardiovascular risk: A national population-based cohort study. Drug Alcohol Depend. 2015, 152, 87–92. [Google Scholar] [CrossRef]
- Montisci, M.; El Mazloum, R.; Cecchetto, G.; Terranova, C.; Ferrara, S.D.; Thiene, G.; Basso, C. Anabolic androgenic steroids abuse and cardiac death in athletes: Morphological and toxicological findings in four fatal cases. Forensic Sci. Int. 2012, 217, e13–e18. [Google Scholar] [CrossRef] [PubMed]
- Achar, S.; Rostamian, A.; Narayan, S.M. Cardiac and Metabolic Effects of Anabolic-Androgenic Steroid Abuse on Lipids, Blood Pressure, Left Ventricular Dimensions, and Rhythm. Am. J. Cardiol. 2010, 106, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Sachtleben, T.R.; Berg, K.E.; Elias, B.A.; Cheatham, J.P.; Felix, G.L.; Hofschire, P.J. The effects of anabolic steroids on myocardial structure and cardiovascular fitness. Med. Sci. Sports Exerc. 1993, 25, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Caso, P.; Salerno, G.; Scarafile, R.; De Corato, G.; Mita, C.; Di Salvo, G.; Severino, S.; Cuomo, S.; Liccardo, B.; et al. Left ventricular early myocardial dysfunction after chronic misuse of anabolic androgenic steroids: A Doppler myocardial and strain imaging analysis * COMMENTARY. Br. J. Sports Med. 2007, 41, 149–155. [Google Scholar] [CrossRef]
- Baggish, A.L.; Weiner, R.B.; Kanayama, G.; Hudson, J.I.; Picard, M.H.; Hutter, A.M.; Pope, H.G. Long-Term Anabolic-Androgenic Steroid Use Is Associated With Left Ventricular Dysfunction. Circ. Hear. Fail. 2010, 3, 472–476. [Google Scholar] [CrossRef]
- Riezzo, I.; Di Paolo, M.; Neri, M.; Bello, S.; Cantatore, S.; D’Errico, S.; Dinucci, D.; Parente, R.; Pomara, C.; Rabozzi, R.; et al. Anabolic Steroid - and Exercise - Induced Cardio-Depressant Cytokines and Myocardial β1 Receptor Expression in CD1 Mice. Curr. Pharm. Biotechnol. 2011, 12, 275–284. [Google Scholar] [CrossRef]
- Lieberherr, M.; Grosse, B. Androgens increase intracellular calcium concentration and inositol 1,4,5-trisphosphate and diacylglycerol formation via a pertussis toxin-sensitive G-protein. J. Biol. Chem. 1994, 269, 7217–7223. [Google Scholar] [CrossRef]
- D’Andrea, A.; Radmilovic, J.; Caselli, S.; Carbone, A.; Scarafile, R.; Sperlongano, S.; Tocci, G.; Formisano, T.; Martone, F.; Liccardo, B.; et al. Left atrial myocardial dysfunction after chronic abuse of anabolic andro-genic steroids: A speckle tracking echocardiography analysis. Int. J. Cardiovasc. Imaging. 2018, 34, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Guerra, A.I.; Tapia, J.; Menéndez-Quintanal, L.M.; Lucena, J.S. Sudden cardiac death in anabolic androgenic ster-oids abuse: Case report and literature review. Forensic Sci. Res. 2019, 4, 267–273. [Google Scholar]
- Zhu, D.; Hadoke, P.W.; Wu, J.; Vesey, A.T.; Lerman, D.A.; Dweck, M.R.; Newby, D.E.; Smith, L.B.; MacRae, V.E. Ablation of the an-drogen receptor from vascular smooth muscle cells demonstrates a role for testosterone in vascular calcification. Sci. Rep. 2016, 20, 24807. [Google Scholar] [CrossRef]
- Liu, J.-D.; Wu, Y.-Q. Anabolic-androgenic steroids and cardiovascular risk. Chin. Med J. 2019, 132, 2229–2236. [Google Scholar] [CrossRef] [PubMed]
- Sculthorpe, N.; Grace, F.; Jones, P.; Davies, B. Evidence of altered cardiac electrophysiology following prolonged an-drogenic anabolic steroid use. Cardiovasc. Toxicol. 2010, 10, 239–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alizade, E.; Avcı, A.; Fidan, S.; Tabakçı, M.; Bulut, M.; Zehir, R.; Simsek, Z.; Evlice, M.; Arslantaş, U.; Çakır, H.; et al. The effect of chronic anabolic-androgenic steroid use on TpE interval, Tp-E/Qt ratio, and Tp-E/Qtc ratio in male bodybuilders. Ann. Noninvasive Electrocardiol. 2015, 20, 592–600. [Google Scholar] [CrossRef]
- Shirpoor, A.; Heshmatian, B.; Tofighi, A.; Eliasabad, S.N.; Kheradmand, F.; Zerehpoosh, M. Nandrolone administration with or without strenuous exercise increases cardiac fatal genes overexpression, calcium/calmodulin-dependent protein kinaseiiδ, and monoamine oxidase activities and enhances blood pressure in adult wistar rats. Gene 2019, 697, 131–137. [Google Scholar] [CrossRef]
- Pärssinen, M.; Kujala, U.; Vartiainen, E.; Sarna, S.; Seppälä, T. Increased premature mortality of competitive powerlifters suspected to have used anabolic agents. Int. J. Sports. Med. 2000, 21, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Melchert, R.B.; Welder, A.A. Cardiovascular effects of androgenic-anabolic steroids. Med. Sci. Sports Exerc. 1995, 27, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Rothman, R.D.; Weiner, R.B.; Pope, H.G.; Kanayama, G.; Hutter, A.M.; A Fifer, M.; Dec, G.W.; Baggish, A.L. Anabolic androgenic steroid induced myocardial toxicity: An evolving problem in an ageing population. BMJ Case Rep. 2011, 2011. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Papadakis, M.; Robertus, J.L.; Dhutia, H.; Steriotis, A.K.; Tome, M.; Mellor, G.; Merghani, A.; Malhotra, A.; Behr, E.; et al. Etiology of Sudden Death in Sports: Insights From a United Kingdom Regional Registry. J. Am. Coll. Cardiol. 2016, 67, 2108–2115. [Google Scholar] [CrossRef]
- Sheppard, M.N. Aetiology of sudden cardiac death in sport: A histopathologist’s perspective. Br. J. Sports Med. 2012, 46, i15–i21. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Salerno, M.; Di Mizio, G.; Bertozzi, G.; Messina, G.; Tomaiuolo, B.; Pisanelli, D.; Maglietta, F.; Ricci, P.; Pomara, C. Anabolic Androgenic Steroids: Searching New Molecular Biomarkers. Front. Pharmacol. 2018, 9, 1321. [Google Scholar] [CrossRef] [PubMed]
- Soplinska, A.; Zareba, L.; Wicik, Z.; Eyileten, C.; Jakubik, D.; Siller-Matula, J.M.; De Rosa, S.; Malek, L.A.; Postula, M. MicroRNAs as Biomarkers of Systemic Changes in Response to Endurance Exercise—A Comprehensive Review. Diagnostics 2020, 10, 813. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivalokanathan, S.; Małek, Ł.A.; Malhotra, A. The Cardiac Effects of Performance-Enhancing Medications: Caffeine vs. Anabolic Androgenic Steroids. Diagnostics 2021, 11, 324. https://doi.org/10.3390/diagnostics11020324
Sivalokanathan S, Małek ŁA, Malhotra A. The Cardiac Effects of Performance-Enhancing Medications: Caffeine vs. Anabolic Androgenic Steroids. Diagnostics. 2021; 11(2):324. https://doi.org/10.3390/diagnostics11020324
Chicago/Turabian StyleSivalokanathan, Sanjay, Łukasz A. Małek, and Aneil Malhotra. 2021. "The Cardiac Effects of Performance-Enhancing Medications: Caffeine vs. Anabolic Androgenic Steroids" Diagnostics 11, no. 2: 324. https://doi.org/10.3390/diagnostics11020324
APA StyleSivalokanathan, S., Małek, Ł. A., & Malhotra, A. (2021). The Cardiac Effects of Performance-Enhancing Medications: Caffeine vs. Anabolic Androgenic Steroids. Diagnostics, 11(2), 324. https://doi.org/10.3390/diagnostics11020324






