The Role of Percutaneous Ablation in the Management of Colorectal Cancer Liver Metastatic Disease
Abstract
1. Introduction
2. Ablation Techniques
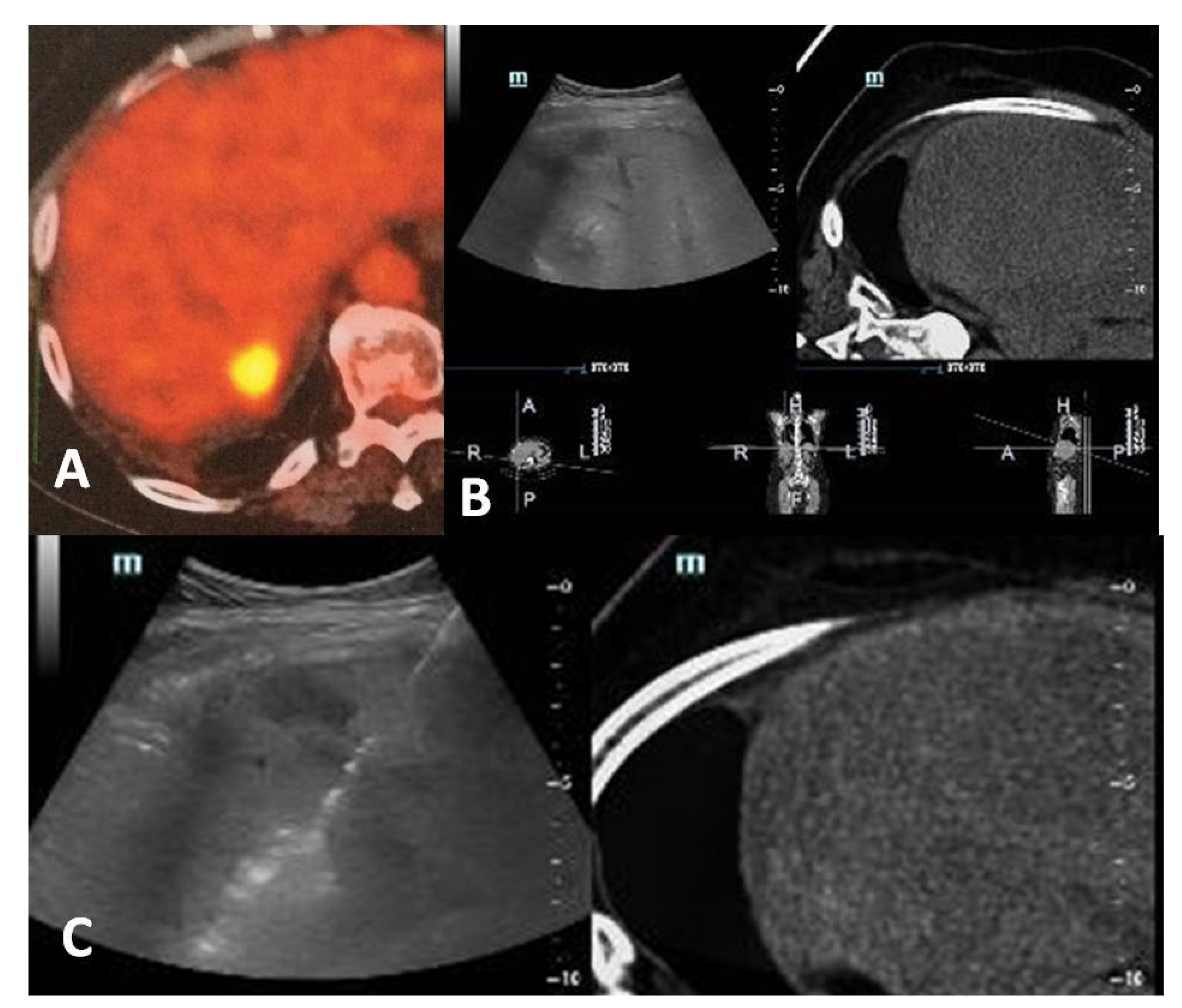
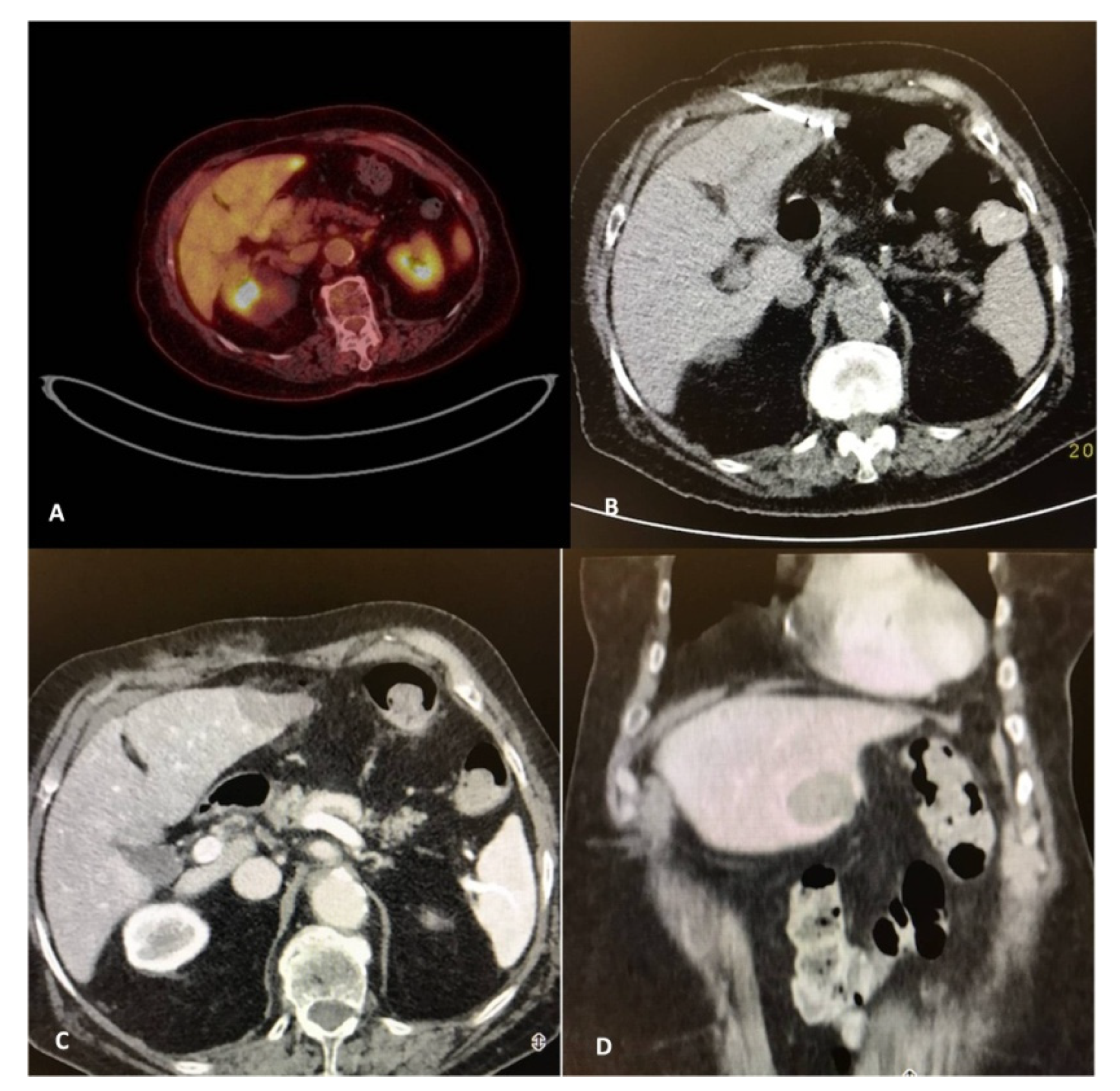
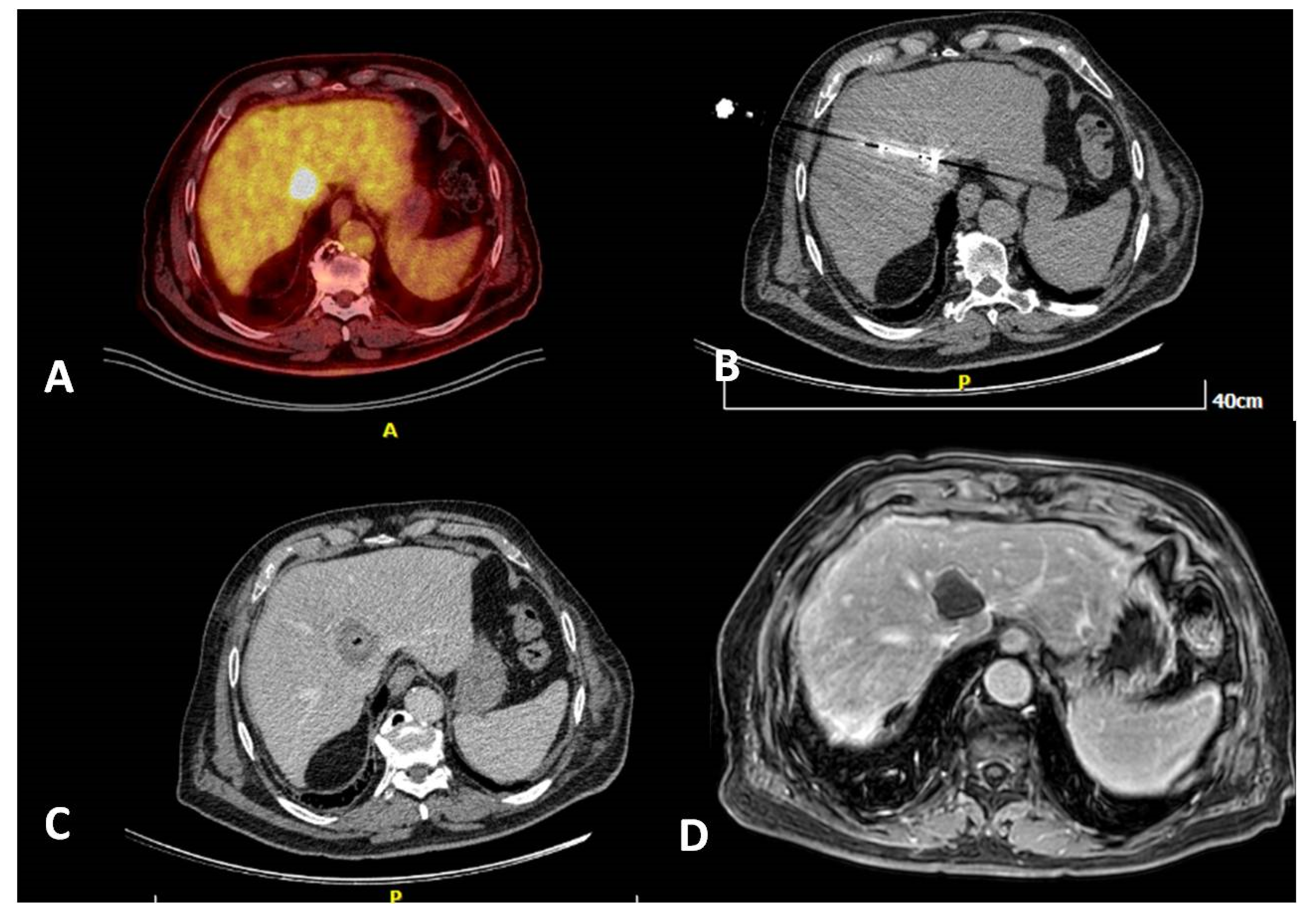
3. Imaging for Diagnosis and Guidance
4. Clinical Applications and Patient Selection
5. Contraindications and Complications
6. Comparing Percutaneous Ablation to Other Therapies
7. Conclusions
Author Contributions
Funding
Institutional review board statement
Informed consent statement
Conflicts of Interest
References
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar]
- Gillams, A.; Goldberg, N.; Ahmed, M.; Bale, R.; Breen, D.; Callstrom, M.; Chen, M.H.; Choi, B.I.; de Baere, T.; Dupuy, D.; et al. Thermal ablation of colorectal liver metastases: A position paper by an international panel of ablation experts, the interventional oncology sans frontières meeting 2013. Eur. Radiol. 2015, 25, 3438–3454. [Google Scholar] [CrossRef] [PubMed]
- Ruers, T.; Van Coevorden, F.; Punt, C.J.A.; Pierie, J.P.E.N.; Rinkes, B.I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.A.; Mauer, M.; et al. Local treatment of unresectable colorectal liver metastases: Results of a randomized phase II Trial. JNCI 2017, 109. [Google Scholar] [CrossRef]
- Meijerink, M.R.; Puijk, R.S.; van Tilborg, A.A.J.M.; Henningsen, K.H.; Fernandez, L.G.; Neyt, M.; Heymans, J.; Frankema, J.S.; de Jong, K.P.; Richel, D.J.; et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: A systematic review and meta-analysis. Cardiovasc. Interv. Radiol. 2018, 41, 1189–1204. [Google Scholar]
- Shady, W.; Petre, E.N.; Gonen, M.; Erinjeri, J.P.; Brown, K.T.; Covey, A.M.; Alago, W.; Durack, J.; Maybody, M.; Brody, L.A.; et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: Factors affecting outcomes—A 10-year experience at a single center. Radiology 2016, 278, 601–611. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Colon Cancer (Version 1.2020). Available online: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 21 January 2020).
- Solbiati, L.; Ahmed, M.; Cova, L.; Ierace, T.; Brioschi, M.; Goldberg, S.N. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: Local response rate and long-term survival with up to 10-year follow-up. Radiology 2012, 265, 958–968. [Google Scholar] [CrossRef]
- Gillams, A.R.; Lees, W.R. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur. Radiol. 2009, 19, 1206–1213. [Google Scholar] [CrossRef]
- Schullian, P.; Johnston, E.W.; Putzer, D.; Laimer, G.; Waroschitz, G.; Braunwarth, E.; Amann, A.; Maglione, M.; Bale, R. Stereotactic radiofrequency ablation (SRFA) for recurrent colorectal liver metastases after hepatic resection. Eur. J. Surg. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kamarinos, V.N.; Kaye, E.A.; Sofocleous, C.T. Image-guided thermal ablation for colorectal liver metastases. Tech. Vasc. Interv. Radiol. 2020, 23, 672. [Google Scholar]
- Camacho, J.C.; Petre, E.N.; Sofocleous, C.T. Thermal ablation of metastatic colon cancer to the liver. Semin. Intervent. Radiol. 2019, 36, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, S.; Puijk, R.S.; van den Bemd, B.; Aldrighetti, L.; Arntz, M.; van den Boezem, P.B.; Bruynzeel, A.M.E.; Burgmans, M.C.; de Cobelli, F.; Coolsen, M.M.E.; et al. Resectability and ablatability criteria for the treatment of liver only colorectal metastases: Multidisciplinary consensus document from the COLLISION trial group. Cancers 2020, 12, 1779. [Google Scholar] [CrossRef]
- Cornelis, F.; Sotirchos, V.; Violari, E.; Sofocleous, C.T.; Schoder, H.; Durack, J.C.; Siegelbaum, R.H.; Maybody, M.; Humm, J.; Solomon, S.B. 18F-FDG PET/CT is an immediate imaging biomarker of treatment success after liver metastasis ablation. J. Nucl. Med. 2016, 57, 1052–1057. [Google Scholar] [CrossRef]
- Mauri, G.; Gennaro, N.; De Beni, S.; Ierace, T.; Goldberg, S.N.; Rodari, M.; Solbiati, L.A. Real-time US-(18)FDG-PET/CT image fusion for guidance of thermal ablation of (18)FDG-PET-positive liver metastases: The added value of contrast enhancement. Cardiovasc. Intervent. Radiol. 2019, 42, 60–68. [Google Scholar] [CrossRef]
- Solbiati, L.; Gennaro, N.; Muglia, R. Augmented reality: From video games to medical clinical practice. Cardiovasc. Intervent. Radiol. 2020, 43, 1427–1429. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Georgiades, C.S. Radiofrequency ablation: Mechanism of action and devices. In Percutaneous tumor ablation. Strategies and Techniques; Hong, K., Georgiades, C.S., Eds.; Thieme: Stuttgart, Germany, 2011; Volume 21, pp. S179–S186. [Google Scholar]
- Wolf, F.; Dupuy, D.E. Microwave ablation: Mechanism of action and devices. In Percutaneous tumor ablation. Strategies and Techniques; Hong, K., Georgiades, C.S., Eds.; Thieme: Stuttgart, Germany, 2011; pp. 27–43. [Google Scholar]
- Lubner, M.G.; Brace, C.L.; Hinshaw, J.L.; Lee, F.T., Jr. Microwave tumor ablation: Mechanism of action, clinical results, and devices. J. Vasc. Interv. Radiol. 2010, 21, S192–S203. [Google Scholar]
- Filippiadis, D.; Mauri, G.; Marra, P.; Charalampopoulos, G.; Gennaro, N.; De Cobelli, F. Percutaneous ablation techniques for renal cell carcinoma: Current status and future trends. Int. J. Hyperth. 2019, 36, 21–30. [Google Scholar] [CrossRef]
- Georgiades, C.S.; Marx, J.K. Cryoablation: Mechanism of action and devices. In Percutaneous tumor ablation. Strategies and Techniques; Hong, K., Georgiades, C.S., Eds.; Thieme: Stuttgart, Germany, 2011; pp. 15–26. [Google Scholar]
- Filippiadis, D.; Efthymiou, E.; Tsochatzis, A.; Kelekis, A.; Prologo, J.D. Percutaneous cryoanalgesia for pain palliation: Current status and future trends. Diagn. Interv. Imaging 2020, 3, S2211–S5684. [Google Scholar] [CrossRef]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; van den Tol, P.M.; Davalos, R.V.; Rubinsky, B.; de Gruijl, T.D.; et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef]
- Ghorra, C.; Pommier, R.; Piveteau, A.; Brandt, R.L.; Vilgrain, V.; Terraz, S.; Ronot, M. The diagnostic performance of a simulated "short" gadoxetic acid-enhanced MRI protocol is similar to that of a conventional protocol for the detection of colorectal liver metastases. Eur. Radiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- McLoney, E.D.; Isaacson, A.J.; Keating, P. The Role of PET Imaging Before, During, and After Percutaneous Hepatic and Pulmonary Tumor Ablation. Semin. Intervent. Radiol. 2014, 31, 187–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Solbiati, L.; Ierace, T.; Tonolini, M.; Cova, L. Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur. J. Radiol. 2004, 51, S19–S23. [Google Scholar] [CrossRef] [PubMed]
- Malone, C.D.; Fetzer, D.T.; Monsky, W.L.; Itani, M.; Mellnick, V.; Velez, P.A.; Middleton, W.D.; Averkiou, M.A.; Ramaswamy, R.S. Contrast-enhanced US for the Interventional Radiologist: Current and Emerging Applications. Radiographics 2020, 40, 562–588. [Google Scholar] [CrossRef]
- Mauri, G.; Porazzi, E.; Cova, L.; Restelli, U.; Tondolo, T.; Bonfanti, M.; Cerri, A.; Ierace, T.; Croce, D.; Solbiati, L. Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: Clinical impact and health technology assessment. Insights Imaging 2014, 5, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ong, Y.T.; Gogna, A.; Venkatanarasimha, N.; Sanamandra, S.K.; Leong, S.; Irani, F.G.; Lo, R.H.G.; Too, C.W. Perfluorobutane contrast-enhanced ultrasonography: A new standard for ultrasonography-guided thermal ablation of sonographically occult liver tumours? Singap. Med. J. 2020, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tsitskari, M.; Filippiadis, D.; Zavridis, P.; Mazioti, A.; Vrachliotis, T.; Alevizos, L.; Brountzos, E.; Kelekis, N. Efficacy and safety of percutaneous computed tomography-guided microwave ablation for colorectal cancer, oligometastatic liver-only disease: A single center’s experience. Ann. Gastroenterol. 2021, 34, 61–67. [Google Scholar] [CrossRef]
- Han, K.; Kim, J.H.; Yang, S.G.; Park, S.H.; Choi, H.K.; Chun, S.Y.; Kim, P.N.; Park, J.; Lee, M. A single-center retrospective analysis of periprocedural variables affecting local tumor progression after radiofrequency ablation of colorectal cancer liver metastases. Radiology 2020. [Google Scholar] [CrossRef]
- Ryan, E.R.; Sofocleous, C.T.; Schöder, H.; Carrasquillo, J.A.; Nehmeh, S.; Larson, S.M.; Thornton, R.; Siegelbaum, R.H.; Erinjeri, J.P.; Solomon, S.B. Split-dose technique for FDG PET/CT-guided percutaneous ablation: A method to facilitate lesion targeting and to provide immediate assessment of treatment effectiveness. Radiology 2013, 268, 288–295. [Google Scholar] [CrossRef]
- Cornelis, F.H.; Petre, E.N.; Vakiani, E.; Klimstra, D.; Durack, J.C.; Gonen, M.; Osborne, J.; Solomon, S.B.; Sofocleous, C.T. Immediate postablation 18F-FDG injection and corresponding SUV are surrogate biomarkers of local tumor progression after thermal ablation of colorectal carcinoma liver metastases. J. Nucl. Med. 2018, 59, 1360–1365. [Google Scholar] [CrossRef]
- Winkelmann, M.T.; Archid, R.; Gohla, G.; Hefferman, G.; Kübler, J.; Weiss, J.; Clasen, S.; Nikolaou, K.; Nadalin, S.; Hoffmann, R. MRI-guided percutaneous thermoablation in combination with hepatic resection as parenchyma-sparing approach in patients with primary and secondary hepatic malignancies: Single center long-term experience. Cancer Imaging 2020, 20, 37. [Google Scholar] [CrossRef]
- Ruers, T.J.M.; Punt, M.; van Coevorden, F.; Ledermann, J.A.; Poston, J.; Bechstein, W.; Lentz, M.; Lutz, M.; Nordlinger, B. 6010 POSTER DISCUSSION Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non- resectable colorectal liver metastases: A randomized EORTC intergroup phase II study (EORTC 40004). Eur. J. Cancer 2011, 47, S394. [Google Scholar] [CrossRef]
- Crocetti, L.; de Baére, T.; Pereira, P.L.; Tarantino, F.P. CIRSE Standards of practice on thermal ablation of liver tumours. Cardiovasc. Intervent. Radiol. 2020, 43, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Siperstein, A.E.; Berber, E.; Ballem, N.; Parikh, R.T. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann. Surg. 2007, 246, 559–565. [Google Scholar] [CrossRef]
- Mulier, S.; Ni, Y.; Jamart, J.; Ruers, T.; Marchal, G.; Michel, L. Local recurrence after hepatic radiofrequency coagulation: Multivariate meta-analysis and review of contributing factors. Ann. Surg. 2005, 242, 158–171. [Google Scholar] [CrossRef]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer. Ann. Surg. 1999, 230, 309. [Google Scholar] [CrossRef] [PubMed]
- Nordlinger, B.; Guiguet, M.; Vaillant, J.C.; Balladur, P.; Boudjema, K.; Bachellier, P.; Jaeck, D. Surgical resection of colorectal carcinoma metastases to the liver: A prognostic scoring system to improve case selection, based on 1568 patients. Cancer 1996, 77, 1254–1262. [Google Scholar] [CrossRef]
- Sofocleous, C.T.; Petre, E.N.; Gonen, M.; Brown, K.T.; Solomon, S.; Covey, A.M.; Alago, W.; Brody, L.A.; Thornton, R.H.; D’Angelica, M.; et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J. Vasc. Interv. Radiol. 2011, 6, 755–761. [Google Scholar] [CrossRef]
- Sotirchos, V.S.; Petrovic, L.M.; Gönen, M.; Klimstra, D.S.; Do, R.K.; Petre, E.N.; Garcia, A.; Barlas, A.; Erinjeri, J.P.; Brown, K.T.; et al. Colorectal cancer liver metastases: Biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology 2016, 280, 949–959. [Google Scholar] [CrossRef]
- Calandri, M.; Yamashita, S.; Gazzera, C.; Fonio, P.; Veltri, A.; Bustreo, S.; Sheth, R.A.; Yevich, S.M.; Vauthey, J.N.; Odisio, B.C. Ablation of colorectal liver metastasis: Interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur. Radiol. 2018, 28, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Shady, W.; Petre, E.N.; Vakiani, E.; Ziv, E.; Gonen, M.; Brown, K.T.; Kemeny, N.E.; Solomon, S.B.; Solit, D.B.; Sofocleous, C.T. Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget 2017, 8, 66117–66127. [Google Scholar] [CrossRef] [PubMed]
- Kurilova, I.; Bendet, A.; Petre, E.N.; Boas, F.E.; Kaye, E.; Gonen, M.; Covey, A.; Brody, L.A.; Brown, K.T.; Kemeny, N.E.; et al. Factors associated with local tumor control and complications after thermal ablation of colorectal cancer liver metastases: A 15-year retrospective cohort study. Clin. Colorectal Cancer 2020, 24. [Google Scholar] [CrossRef] [PubMed]
- Soulen, M.C.; Sofocleous, C.T. Achieving Curative Ablation Outcomes: It is all about the imaging. Radiology. 2020, 10, 3930. [Google Scholar] [CrossRef] [PubMed]
- Kaye, E.A.; Cornelis, F.H.; Petre, E.N.; Tyagi, N.; Shady, W.; Shi, W.; Zhang, Z.; Solomon, S.B.; Sofocleous, C.T.; Durack, J.C. Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur. Radiol. 2018, 29, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Odisio, B.C.; Yamashita, S.; Huang, S.Y.; Harmoush, S.; Kopetz, S.E.; Ahrar, K.; Shin Chun, Y.; Conrad, C.; Aloia, T.A.; Gupta, S.; et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br. J. Surg. 2017, 104, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Shady, W.; Petre, E.N.; Do, K.G.; Gonen, M.; Yarmohammadi, H.; Brown, K.T.; Kemeny, N.E.; D’Angelica, M.; Kingham, P.T.; Solomon, S.B.; et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: Ablation with clear margins (A0) provides the best local tumor control. J. Vasc. Interv. Radiol. 2018, 29, 268–275. [Google Scholar] [CrossRef]
- Wang, X.; Sofocleous, C.T.; Erinjeri, J.P.; Petre, E.N.; Gonen, M.; Do, K.G.; Brown, K.T.; Covey, A.M.; Brody, L.A.; Alago, W.; et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc. Interv. Radiol. 2013, 36, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Tani, S.; Tatli, S.; Hata, N.; Garcia-Rojas, X.; Olubiyi, O.I.; Silverman, S.G.; Tokuda, J. Three-dimensional quantitative assessment of ablation margins based on registration of pre- and post-procedural MRI and distance map. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 1133–1142. [Google Scholar] [CrossRef]
- Sotirchos, V.S.; Fujisawa, S.; Vakiani, E.; Solomon, S.B.; Todorova, M.K.O.; Sofocleous, C.T. Fluorescent tissue assessment of colorectal cancer liver metastases ablation zone: A potential real-time biomarker of complete tumor ablation. Ann. Surg. Oncol. 2019, 26, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Tanis, E.; Spliethoff, J.W.; Evers, D.; Langhout, G.C.; Snaebjornsson, P.; Prevoo, W.; Hendriks, B.H.; Ruers, T.J. Real-time in vivo assessment of radiofrequency ablation of human colorectal liver metastases using diffuse reflectance spectroscopy. Eur. J. Surg. Oncol. 2016, 42, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Laimer, G.; Jaschke, N.; Schullian, P.; Putzer, D.; Eberle, G.; Solbiati, M.; Solbiati, L.; Goldberg, S.N.; Bale, R. Volumetric assessment of the periablational safety margin after thermal ablation of colorectal liver metastases. Eur. Radiol. 2021, 14, 1–11, Online ahead of print. [Google Scholar]
- Amabile, C.; Ahmed, M.; Solbiati, L.; Meloni, M.F.; Solbiati, M.; Cassarino, S.; Tosoratti, N.; Nissenbaum, Y.; Ierace, T.; Goldberg, S.N. Microwave ablation of primary and secondary liver tumours: Ex vivo, in vivo, and clinical characterisation. Int. J. Hyperth. 2017, 33, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.J.; Dupuy, D.E.; Smith, M.W.W. Microwave ablation: Principles and applications. Radiographics 2005, 25, S69–S83. [Google Scholar] [CrossRef]
- Schicho, A.; Niessen, C.; Haimerl, M.; Wiesinger, I.; Stroszczynski, C.; Beyer, L.P.; Wiggermann, P. Long-term survival after percutaneous irreversible electroporation of inoperable colorectal liver metastases. Cancer Manag. Res. 2018, 11, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; Rompianesi, G.; Guzmán, M.I.; Pérez, M.E.; Montalti, R.; Troisi, R.I. Systematic review and meta-analysis of local ablative therapies for resectable colorectal liver metastases. Eur. J. Surg. Oncol. 2020, 46, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.J.; Rahim, S.; Davidson, J.C.; Hanks, S.E.; Tam, A.L.; Walker, T.G.; Wilkins, L.R.; Sarode, R.; Weinberg, I. Society of interventional radiology consensus guidelines for the peri-procedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventionspart II: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J. Vasc. Interv. Radiol. 2019, 30, 1168–1184. [Google Scholar] [PubMed]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse quality assurance document and standards for classification of complications: The CIRSE classification system. Cardiovasc. Intervent. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef]
- Kim, K.R.; Thomas, S. Complications of image-guided thermal ablation of liver and kidney neoplasms. Semin. Interv. Radiol. 2014, 31, 138–148. [Google Scholar]
- Livraghi, T.; Meloni, F.; Solbiati, L.; Zanus, G. Complications of microwave ablation for liver tumors: Results of a multicenter study. Cardiovasc. Interv. Radiol. 2012, 35, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Garnon, J.; Cazzato, R.L.; Caudrelier, J.; Nouri-Neuville, M.; Rao, P.; Boatta, E.; Ramamurthy, N.; Koch, G.; Gangi, A. Adjunctive thermoprotection during percutaneous thermal ablation procedures: Review of current techniques. Cardiovasc. Interv. Radiol. 2018, 42, 344–357. [Google Scholar] [CrossRef]
- Crocetti, L. Radiofrequency Ablation versus Resection for Small Hepatocellular Carcinoma: Are Randomized Controlled Trials Still Needed? Radiology 2018, 287, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Solbiati, L.; Meloni, F.; Ierace, T.; Goldberg, S.N.; Gazelle, G.S. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: The “test-of-time approach”. Cancer 2003, 97, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.L.; Bale, R.; Breen, D.J.; de Baere, T.; Denys, A.; Guiu, B.; Goldberg, N.; Kim, E.; Lewandowski, R.J.; Helmberger, T.; et al. The Management of colorectal cancer liver metastases: The interventional radiology viewpoint. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Gill, B.; Beriwal, S.; Huq, M.S.; Roberts, M.S.; Smith, K.J. Cost-effectiveness analysis of stereotactic body radiation therapy compared with radiofrequency ablation for inoperable colorectal liver metastases. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippiadis, D.K.; Velonakis, G.; Kelekis, A.; Sofocleous, C.T. The Role of Percutaneous Ablation in the Management of Colorectal Cancer Liver Metastatic Disease. Diagnostics 2021, 11, 308. https://doi.org/10.3390/diagnostics11020308
Filippiadis DK, Velonakis G, Kelekis A, Sofocleous CT. The Role of Percutaneous Ablation in the Management of Colorectal Cancer Liver Metastatic Disease. Diagnostics. 2021; 11(2):308. https://doi.org/10.3390/diagnostics11020308
Chicago/Turabian StyleFilippiadis, Dimitrios K., Georgios Velonakis, Alexis Kelekis, and Constantinos T. Sofocleous. 2021. "The Role of Percutaneous Ablation in the Management of Colorectal Cancer Liver Metastatic Disease" Diagnostics 11, no. 2: 308. https://doi.org/10.3390/diagnostics11020308
APA StyleFilippiadis, D. K., Velonakis, G., Kelekis, A., & Sofocleous, C. T. (2021). The Role of Percutaneous Ablation in the Management of Colorectal Cancer Liver Metastatic Disease. Diagnostics, 11(2), 308. https://doi.org/10.3390/diagnostics11020308








