Rapid Diagnosis of Pseudomonas aeruginosa in Wounds with Point-Of-Care Fluorescence Imaing
Abstract
1. Introduction
Evidence for Cyan Fluorescence Detected from Pseudomonas aeruginosa
2. Materials and Methods
2.1. Patient Population
2.2. Fluorescence Imaging
2.3. Sampling and Bacterial Quantification
2.4. Sample Size Calculation and Data Analysis
3. Results
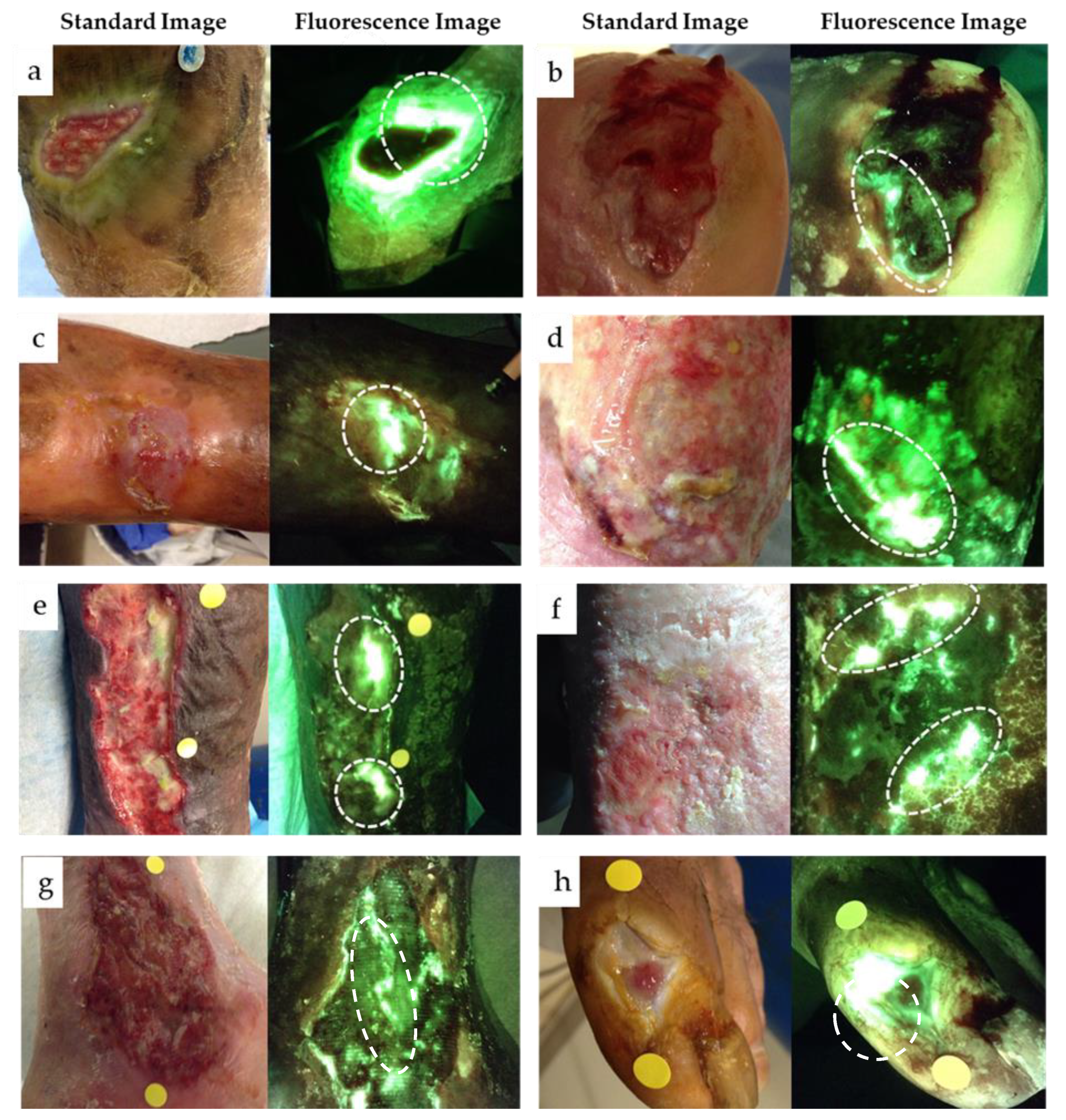
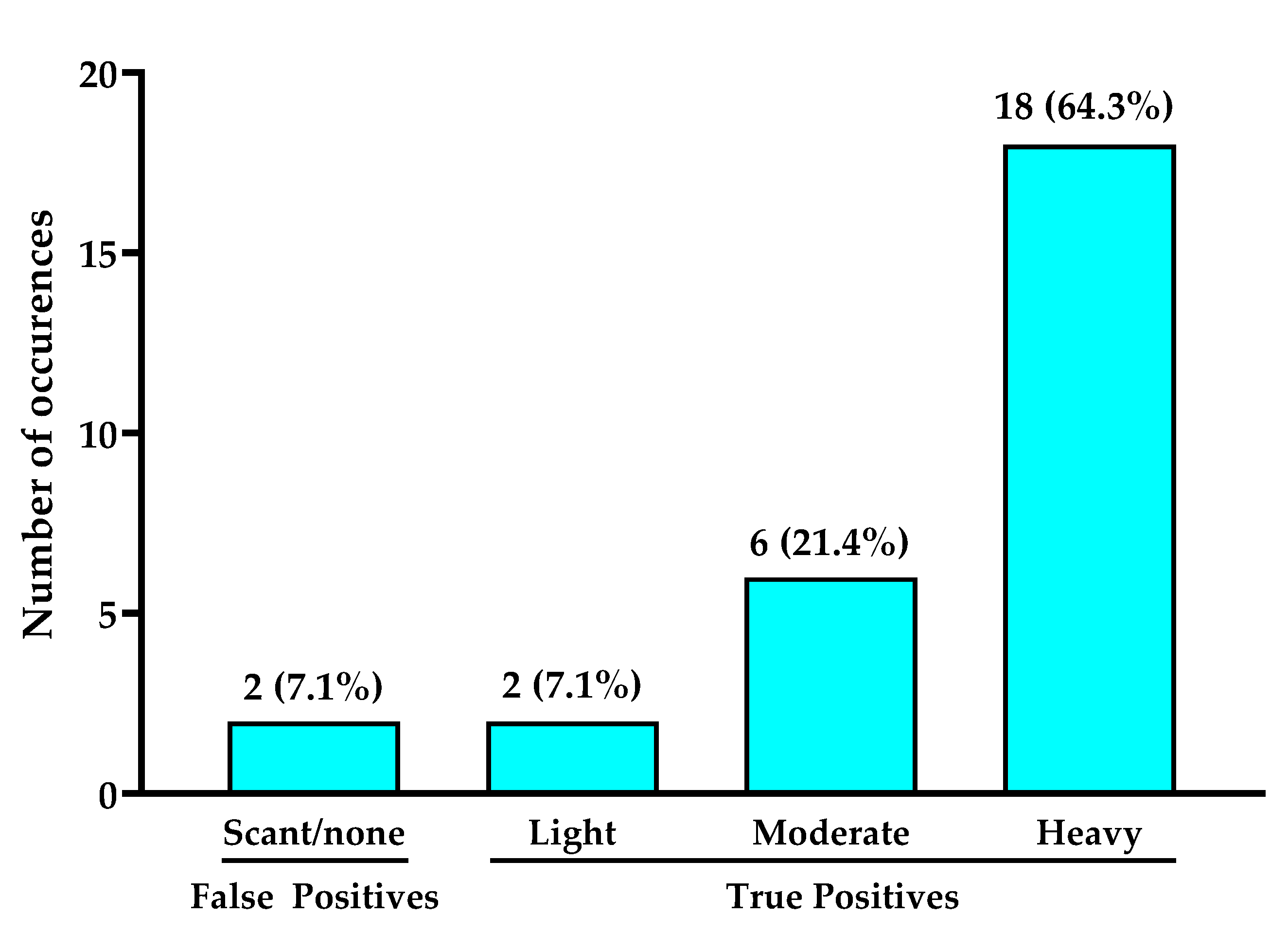
3.1. Positive Predictive Value and Bacterial Loads of Cyan Fluorescence
3.2. Other Microbiological Findings
3.3. Assessment of “Classic” Pseudomonas spp. Symptoms
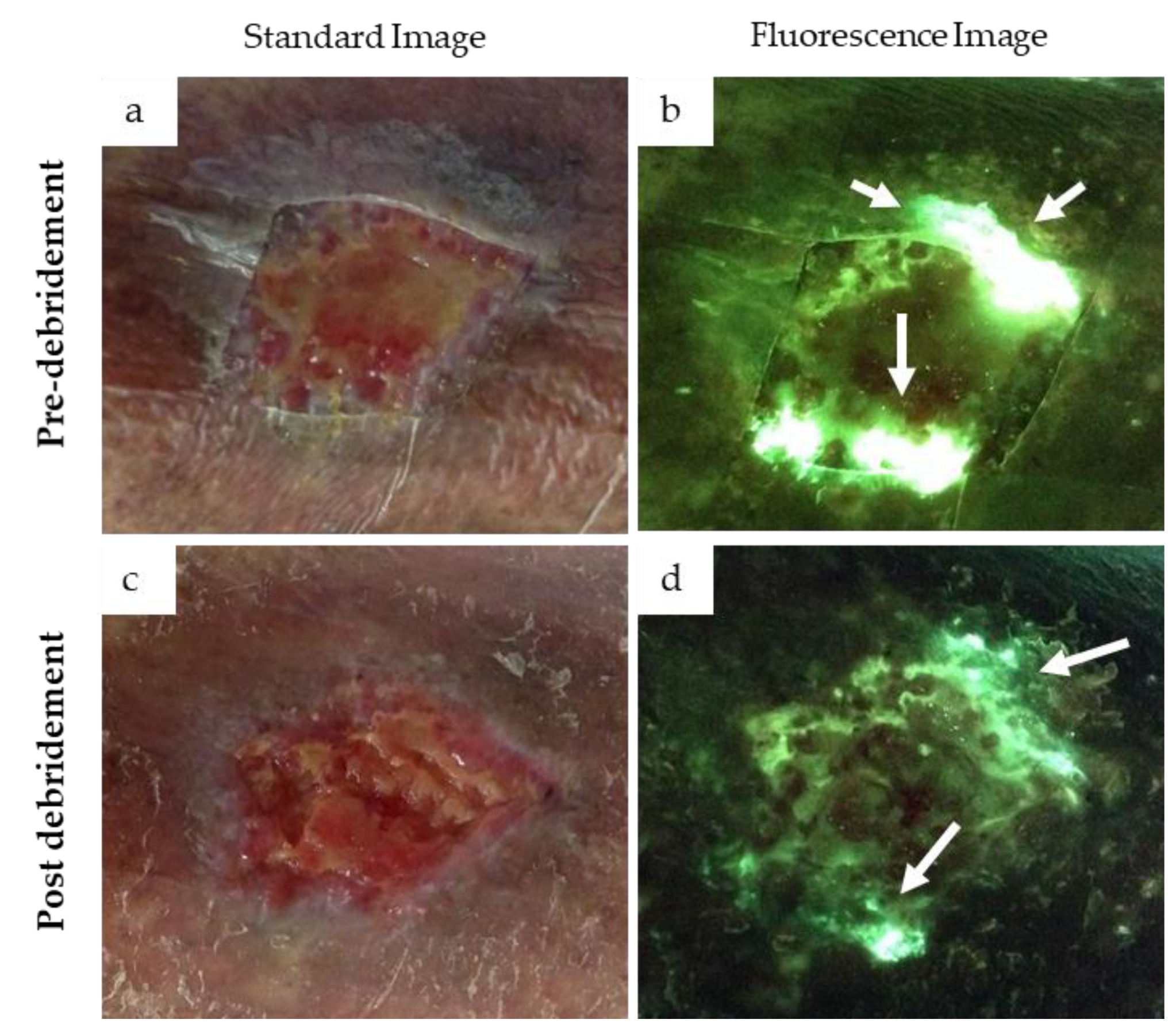
3.4. Cyan Signal Monitored after Mechanical and Antimicrobial Treatments
4. Discussion
Limitations
5. Clinical Implications and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caldwell, M.D. Bacteria and Antibiotics in Wound Healing. Surg. Clin. N. Am. 2020, 100, 757–776. [Google Scholar] [CrossRef]
- Xu, L.; McLennan, S.V.; Lo, L.; Natfaji, A.; Bolton, T.; Liu, Y.; Twigg, S.M.; Yue, D.K. Bacterial load predicts healing rate in neuropathic diabetic foot ulcers. Diabetes Care 2007, 30, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hsia, H.C. The Impact of Microbial Communities on Wound Healing: A Review. Ann. Plast. Surg. 2018, 81, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Hogsberg, T.; Bjarnsholt, T.; Thomsen, J.S.; Kirketerp-Moller, K. Success rate of split-thickness skin grafting of chronic venous leg ulcers depends on the presence of Pseudomonas aeruginosa: A retrospective study. PLoS ONE 2011, 6, e20492. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the chronic wound microbiota of 2963 patients by 16S rDNA pyrosequencing. Wound Repair. Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial Contribution in Chronicity of Wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef]
- Kirketerp-Møller, K.; Jensen, P.Ø.; Fazli, M.; Madsen, K.G.; Pedersen, J.; Moser, C.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Distribution, organization, and ecology of bacteria in chronic wounds. J. Clin. Microbiol. 2008, 46, 2717–2722. [Google Scholar] [CrossRef]
- Bodey, G.P.; Bolivar, R.; Fainstein, V.; Jadeja, L. Infections caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 1983, 5, 279–313. [Google Scholar] [CrossRef]
- Gjødsbøl, K.; Christensen, J.J.; Karlsmark, T.; Jørgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn Wound Infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Prevaldi, C.; Paolillo, C.; Locatelli, C.; Ricci, G.; Catena, F.; Ansaloni, L.; Cervellin, G. Management of traumatic wounds in the Emergency Department: Position paper from the Academy of Emergency Medicine and Care (AcEMC) and the World Society of Emergency Surgery (WSES). World J. Emerg. Surg. 2016, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Jockenhofer, F.; Gollnick, H.; Herberger, K.; Isbary, G.; Renner, R.; Stucker, M.; Valesky, E.; Wollina, U.; Weichenthal, M.; Karrer, S.; et al. Bacteriological pathogen spectrum of chronic leg ulcers: Results of a multicenter trial in dermatologic wound care centers differentiated by regions. J. Dtsch. Dermatol. Ges. 2013, 11, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.M.; Westh, H.; Danielsen, L.; Rosdahl, V.T. Bacterial colonization and healing of venous leg ulcers. Apmis 1996, 104, 895–899. [Google Scholar] [CrossRef]
- Halbert, A.R.; Stacey, M.C.; Rohr, J.B.; Jopp-McKay, A. The effect of bacterial colonization on venous ulcer healing. Australas. J. Dermatol. 1992, 33, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Fazli, M.; Bjarnsholt, T.; Kirketerp-Møller, K.; Jørgensen, B.; Andersen, A.S.; Krogfelt, K.A.; Givskov, M.; Tolker-Nielsen, T. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J. Clin. Microbiol. 2009, 47, 4084–4089. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.H.; Everett, J.; Trivedi, U.; Rumbaugh, K.P.; Whiteley, M. Requirements for Pseudomonas aeruginosa Acute Burn and Chronic Surgical Wound Infection. PLoS Genet. 2014, 10, e1004518. [Google Scholar] [CrossRef]
- McManus, A.T.; Mason, A.D., Jr.; McManus, W.F.; Pruitt, B.A., Jr. Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur. J. Clin. Microbiol. 1985, 4, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.R.; Fleuchot, B.; Lauciello, L.; Jafari, P.; Applegate, L.A.; Raffoul, W.; Que, Y.-A.; Perron, K. Effect of Human Burn Wound Exudate on Pseudomonas aeruginosa Virulence. mSphere 2016, 1, e00111–e00115. [Google Scholar] [CrossRef]
- Schaber, J.A.; Triffo, W.J.; Suh, S.J.; Oliver, J.W.; Hastert, M.C.; Griswold, J.A.; Auer, M.; Hamood, A.N.; Rumbaugh, K.P. Pseudomonas aeruginosa Forms Biofilms in Acute Infection Independent of Cell-to-Cell Signaling. Infect. Immun. 2007, 75, 3715–3721. [Google Scholar] [CrossRef] [PubMed]
- Kamali, E.; Jamali, A.; Ardebili, A.; Ezadi, F.; Mohebbi, A. Evaluation of antimicrobial resistance, biofilm forming potential, and the presence of biofilm-related genes among clinical isolates of Pseudomonas aeruginosa. BMC Res. Notes 2020, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Drenkard, E.; Ausubel, F.M. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 2002, 416, 740–743. [Google Scholar] [CrossRef]
- Aloush, V.; Navon-Venezia, S.; Seigman-Igra, Y.; Cabili, S.; Carmeli, Y. Multidrug-Resistant Pseudomonas aeruginosa: Risk Factors and Clinical Impact. Antimicrob. Agents Chemother. 2006, 50, 43–48. [Google Scholar] [CrossRef]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Mutluoglu, M.; Uzun, G. Pseudomonas infection in a postoperative foot wound. Can. Med. Assoc. J. 2011, 183, E499. [Google Scholar] [CrossRef][Green Version]
- Barankin, B.; Levy, J. Answer: Can you identify this condition? Can. Fam. Physician 2012, 58, 1104. [Google Scholar]
- Lau, G.W.; Hassett, D.J.; Ran, H.; Kong, F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004, 10, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Le, L.; Baer, M.; Briggs, P.; Bullock, N.; Cole, W.; DiMarco, D.; Hamil, R.; Harrell, K.; Kasper, M.; Li, W.; et al. Diagnostic Accuracy of Point-of-Care Fluorescence Imaging for the Detection of Bacterial Burden in Wounds: Results from the 350-Patient Fluorescence Imaging Assessment and Guidance Trial. Adv. Wound Care 2020. [Google Scholar] [CrossRef] [PubMed]
- Janniger, C.K.; Schwartz, R.A.; Szepietowski, J.C.; Reich, A. Intertrigo and common secondary skin infections. Am. Fam. Physician 2005, 72, 833–838. [Google Scholar] [PubMed]
- Polk, H.C., Jr.; Ward, C.G.; Clarkson, J.G.; Taplin, D. Early detection of Pseudomonas burn infection. Clinical experience with Wood’s light fluorescence. Arch. Surg. 1969, 98, 292–295. [Google Scholar] [CrossRef]
- Ponka, D.; Baddar, F. Wood lamp examination. Can. Fam. Physician 2012, 58, 976. [Google Scholar]
- Meyer, J.M.; Abdallah, M. The Fluorescent Pigment of Pseudomonas fluorescens: Biosynthesis, Purification and Physicochemical Properties. Microbiology 1978, 107, 9. [Google Scholar] [CrossRef]
- Schalk, I.J.; Guillon, L. Pyoverdine biosynthesis and secretion in Pseudomonas aeruginosa: Implications for metal homeostasis. Environ. Microbiol. 2013, 15, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.; McKay, G.A. Iron acquisition and its control in Pseudomonas aeruginosa: Many roads lead to Rome. Front. Biosci. 2003, 8, d661–d686. [Google Scholar] [CrossRef] [PubMed]
- Lamont, I.L.; Beare, P.A.; Ochsner, U.; Vasil, A.I.; Vasil, M.L. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2002, 99, 7072–7077. [Google Scholar] [CrossRef]
- Goldberg, J.B. Why is Pseudomonas aeruginosa a pathogen? F1000 Biol. Rep. 2010, 2, 29. [Google Scholar] [CrossRef]
- Meyer, J.M.; Neely, A.; Stintzi, A.; Georges, C.; Holder, I.A. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 1996, 64, 518–523. [Google Scholar] [CrossRef]
- Takase, H.; Nitanai, H.; Hoshino, K.; Otani, T. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect. Immun. 2000, 68, 1834–1839. [Google Scholar] [CrossRef]
- Minandri, F.; Imperi, F.; Frangipani, E.; Bonchi, C.; Visaggio, D.; Facchini, M.; Pasquali, P.; Bragonzi, A.; Visca, P. Role of Iron Uptake Systems in Pseudomonas aeruginosa Virulence and Airway Infection. Infect. Immun. 2016, 84, 2324–2335. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.S.; West, S.A.; Buckling, A. Cooperation and competition in pathogenic bacteria. Nature 2004, 430, 1024–1027. [Google Scholar] [CrossRef]
- Kang, D.; Revtovich, A.V.; Chen, Q.; Shah, K.N.; Cannon, C.L.; Kirienko, N.V. Pyoverdine-Dependent Virulence of Pseudomonas aeruginosa Isolates From Cystic Fibrosis Patients. Front. Microbiol. 2019, 10, 2048. [Google Scholar] [CrossRef]
- Little, W.; Lopez, A.; Gomez, A.; Keim, K.C.; Rennie, M.Y.; Smith, A.C. An investigation of Pseudomonas aeruginosa cyan fluorescence with the MolecuLight i:X bacterial fluorescence imaging device. In Proceedings of the SAWC Spring 2018, San Antonio, TX, USA, 25–29 April 2018. [Google Scholar]
- Lopez, A.J.; Reynolds, L.; Diaz, R.C.; George, I.K.; Little, W.; Fleming, D.; D’souza, A.; Rennie, M.Y.; Rumbaugh, K.P.; Smith, A.C. Detection of bacterial fluorescence from in vivo wound biofilms using a point-of-care fluorescence imaging device. Int. Wound J. 2021, 1–13. [Google Scholar] [CrossRef]
- Warncke, P.; Fink, S.; Wiegand, C.; Hipler, U.C.; Fischer, D. A shell-less hen’s egg test as infection model to determine the biocompatibility and antimicrobial efficacy of drugs and drug formulations against Pseudomonas aeruginosa. Int. J. Pharm. 2020, 585, 119557. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Woo, K. A Prospective Multisite Observational Study Incorporating Bacterial Fluorescence Information Into the UPPER/LOWER Wound Infection Checklists. Wounds 2020, 32, 299–308. [Google Scholar]
- Raizman, R.; Dunham, D.; Lindvere-Teene, L.; Jones, L.M.; Tapang, K.; Linden, R.; Rennie, M.Y. Use of a bacterial fluorescence imaging device: Wound measurement, bacterial detection and targeted debridement. J. Wound Care 2019, 28, 824–834. [Google Scholar] [CrossRef]
- Serena, T.E.; Harrell, K.; Serena, L.; Yaakov, R.A. Real-time bacterial fluorescence imaging accurately identifies wounds with moderate-to-heavy bacterial burden. J. Wound Care 2019, 28, 346–357. [Google Scholar] [CrossRef]
- Pijpe, A.; Ozdemir, Y.; Sinnige, J.C.; Kwa, K.A.A.; Middelkoop, E.; Meij-de Vries, A. Detection of bacteria in burn wounds with a novel handheld autofluorescence wound imaging device: A pilot study. J. Wound Care 2019, 28, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Farhan, N.; Jeffery, S. Utility of MolecuLight i:X for managing bacterial burden in paediatric burns. J. Burn Care Res. 2019. [Google Scholar] [CrossRef]
- Hurley, C.M.; McClusky, P.; Sugrue, R.M.; Clover, J.A.; Kelly, J.E. Efficacy of a bacterial fluorescence imaging device in an outpatient wound care clinic: A pilot study. J. Wound Care 2019, 28, 438–443. [Google Scholar] [CrossRef]
- Blackshaw, E.L.; Jeffery, S.L.A. Efficacy of an imaging device at identifying the presence of bacteria in wounds at a plastic surgery outpatients clinic. J. Wound Care 2018, 27, 20–26. [Google Scholar] [CrossRef]
- Kleintjes, W.G.; Kotzee, E.P. MolecuLight i:X: A New Tool for Wound Infection Diagnosis. S. Afr. J. Plast. Reconstr. Aesthet. Surg. Burns. 2019, 2, 68–71. [Google Scholar] [CrossRef]
- Rennie, M.Y.; Dunham, D.; Lindvere-Teene, L.; Raizman, R.; Hill, R.; Linden, R. Understanding Real-Time Fluorescence Signals from Bacteria and Wound Tissues Observed with the MolecuLight i:X(TM). Diagnostics 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.Y.; Lindvere-Teene, L.; Tapang, K.; Linden, R. Point-of-care fluorescence imaging predicts the presence of pathogenic bacteria in wounds: A clinical study. J. Wound Care 2017, 26, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Robinowitz, N.; Chaulk, P.; Johnson, K. Comparison of chronic wound culture techniques: Swab versus curetted tissue for microbial recovery. Br. J. Community Nurs. 2014, 19 (Suppl. 9), S22–S26. [Google Scholar] [CrossRef]
- Dowd, S.E.; Sun, Y.; Secor, P.R.; Rhoads, D.D.; Wolcott, B.M.; James, G.A.; Wolcott, R.D. Survey of bacterial diversity in chronic wounds using Pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol. 2008, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Gill, S.S.; Wu, W.; Kalkar, S.R.; Rochon, P.A. Does this patient have an infection of a chronic wound? JAMA 2012, 307, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Raizman, R. Fluorescence imaging guided dressing change frequency during negative pressure wound therapy: A case series. J. Wound Care 2019, 28, S28–S37. [Google Scholar] [CrossRef]
- Kang, D.; Kirienko, N.V. High-Throughput Genetic Screen Reveals that Early Attachment and Biofilm Formation Are Necessary for Full Pyoverdine Production by Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 1707. [Google Scholar] [CrossRef] [PubMed]
- Visaggio, D.; Pasqua, M.; Bonchi, C.; Kaever, V.; Visca, P.; Imperi, F. Cell aggregation promotes pyoverdine-dependent iron uptake and virulence in Pseudomonas aeruginosa. Front. Microbiol. 2015, 6, 902. [Google Scholar] [CrossRef]
- Gilchrist, B.; Reed, C. The bacteriology of chronic venous ulcers treated with occlusive hydrocolloid dressings. Br. J. Dermatol. 1989, 121, 337–344. [Google Scholar] [CrossRef]
- Gardiner, M.; Vicaretti, M.; Sparks, J.; Bansal, S.; Bush, S.; Liu, M.; Darling, A.; Harry, E.; Burke, C.M. A longitudinal study of the diabetic skin and wound microbiome. PeerJ 2017, 5, e3543. [Google Scholar] [CrossRef]
- Price, L.B.; Liu, C.M.; Melendez, J.H.; Frankel, Y.M.; Engelthaler, D.; Aziz, M.; Bowers, J.; Rattray, R.; Ravel, J.; Kingsley, C.; et al. Community analysis of chronic wound bacteria using 16S rRNA gene-based pyrosequencing: Impact of diabetes and antibiotics on chronic wound microbiota. PLoS ONE 2009, 4, e6462. [Google Scholar] [CrossRef]
- Hungerer, C.; Troup, B.; Romling, U.; Jahn, D. Regulation of the hemA gene during 5-aminolevulinic acid formation in Pseudomonas aeruginosa. J. Bacteriol. 1995, 177, 1435–1443. [Google Scholar] [CrossRef]
- Jones, L.M.; Dunham, D.; Rennie, M.Y.; Kirman, J.; Lopez, A.J.; Keim, K.C.; Little, W.; Gomez, A.; Bourke, J.; Ng, H.; et al. In vitro detection of porphyrin-producing wound bacteria with real-time fluorescence imaging. Future Microbiol. 2020, 15, 319–332. [Google Scholar] [CrossRef]
- Meyer, J.-M. Pyoverdines: Pigments, siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch. Microbiol. 2000, 174, 135–142. [Google Scholar] [CrossRef]
- Ravel, J.; Cornelis, P. Genomics of pyoverdine-mediated iron uptake in pseudomonads. Trends Microbiol. 2003, 11, 195–200. [Google Scholar] [CrossRef]
- Cole, W.; Coe, S. Use of a bacterial fluorescence imaging system to target wound debridement and accelerate healing: A pilot study. J. Wound Care 2020, 29, S44–S52. [Google Scholar] [CrossRef]
- Rennie, M.Y.; D’souza, A.; Serena, T. Quantitative vs Semi-quantitative Measurements of Bacterial Load in Wounds: Assessment of 1053 Data Points from a 350-Patient Trial. In Proceedings of the Symposium on Advanced Wound Care 2020, Virtual, Las Vegas, NV, USA, 18–20 September 2020. [Google Scholar]
- Roy, S.; Elgharably, H.; Sinha, M.; Ganesh, K.; Chaney, S.; Mann, E.; Miller, C.; Khanna, S.; Bergdall, V.K.; Powell, H.M.; et al. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J. Pathol. 2014, 233, 331–343. [Google Scholar] [CrossRef]
- Buivydas, A.; Pasanen, T.; Senčilo, A.; Daugelavičius, R.; Vaara, M.; Bamford, D.H. Clinical isolates of Pseudomonas aeruginosa from superficial skin infections have different physiological patterns. FEMS Microbiol. Lett. 2013, 343, 183–189. [Google Scholar] [CrossRef]
- De Oliveira, F.P.; Pires, B.; De Cassia Ferreira de Almeida Silva, K.; de Carvalho, B.T.F.; Teixeira, L.A.; de Paula, G.R.; de Oliveira, B. Prevalence, Antimicrobial Susceptibility, and Clonal Diversity of Pseudomonas aeruginosa in Chronic Wounds. J. Wound Ostomy Cont. Nurs. 2017, 44, 528–535. [Google Scholar] [CrossRef]
- Thaden, J.T.; Park, L.P.; Maskarinec, S.A.; Ruffin, F.; Fowler, V.G., Jr.; van Duin, D. Results from a 13-Year Prospective Cohort Study Show Increased Mortality Associated with Bloodstream Infections Caused by Pseudomonas aeruginosa Compared to Other Bacteria. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- International Wound Infection Institute (IWII). Wound infection in clinical practice. Wounds Int. 2016. [Google Scholar]
- Hersh, A.L.; Chambers, H.F.; Maselli, J.H.; Gonzales, R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch. Intern. Med. 2008, 168, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef] [PubMed]
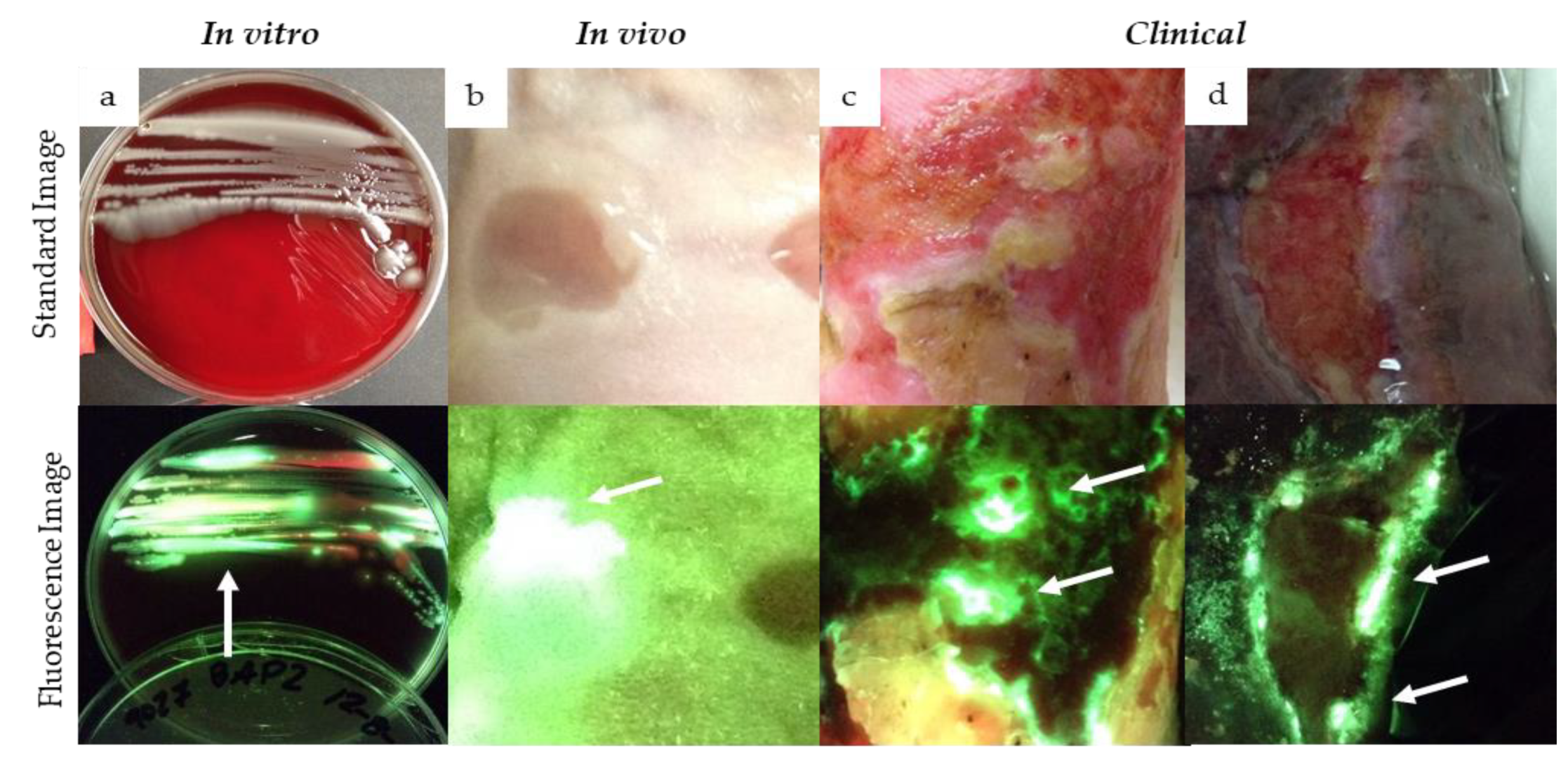
| Question | Example Image |
|---|---|
| Does the fluorescent signature have a glowing white center with a blue/green border? Dashed circle outlines regions of glowing cyan/white in the wound. |  |
| Do any sharp edges match biological landmarks observed in the standard image? The bright cyan fluorescence observed (white arrows) does not correspond to any specific landmark or tissue structure on the standard image (yellow arrow). | 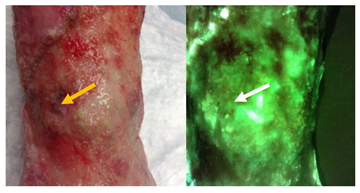 |
| How does it compare to the color of the surrounding skin? The surrounding skin tissue will appear a dull green (yellow arrow) compared to the bright white/cyan fluorescence indicative of bacterial burden (white arrows). | 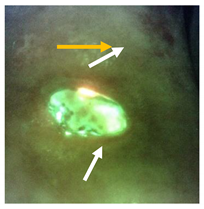 |
| Patient Demographics | No. (% of Total) |
|---|---|
| Sex (male) | 16 (57.1) |
| Wound Type | |
| VLU | 17 (60.7%) |
| DFU | 4 (14.3%) |
| Surgical site | 2 (7.1%) |
| Pressure ulcer | 1 (3.6%) |
| Lymphedema | 1 (3.6%) |
| Rheumatoid wound | 1 (3.6%) |
| Other | 2 (7.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raizman, R.; Little, W.; Smith, A.C. Rapid Diagnosis of Pseudomonas aeruginosa in Wounds with Point-Of-Care Fluorescence Imaing. Diagnostics 2021, 11, 280. https://doi.org/10.3390/diagnostics11020280
Raizman R, Little W, Smith AC. Rapid Diagnosis of Pseudomonas aeruginosa in Wounds with Point-Of-Care Fluorescence Imaing. Diagnostics. 2021; 11(2):280. https://doi.org/10.3390/diagnostics11020280
Chicago/Turabian StyleRaizman, Rose, William Little, and Allie Clinton Smith. 2021. "Rapid Diagnosis of Pseudomonas aeruginosa in Wounds with Point-Of-Care Fluorescence Imaing" Diagnostics 11, no. 2: 280. https://doi.org/10.3390/diagnostics11020280
APA StyleRaizman, R., Little, W., & Smith, A. C. (2021). Rapid Diagnosis of Pseudomonas aeruginosa in Wounds with Point-Of-Care Fluorescence Imaing. Diagnostics, 11(2), 280. https://doi.org/10.3390/diagnostics11020280





