Associations among Heavy Metals and Proteinuria and Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Recruitment
2.2. Collection of Demographic, Medical, and Laboratory Data
2.3. Measurement of Blood and Urine Heavy Metals
2.4. Definition of Proteinuria and CKD
2.5. Ethics Statement
2.6. Statistical Analysis
3. Results
3.1. Determinants of Proteinuria
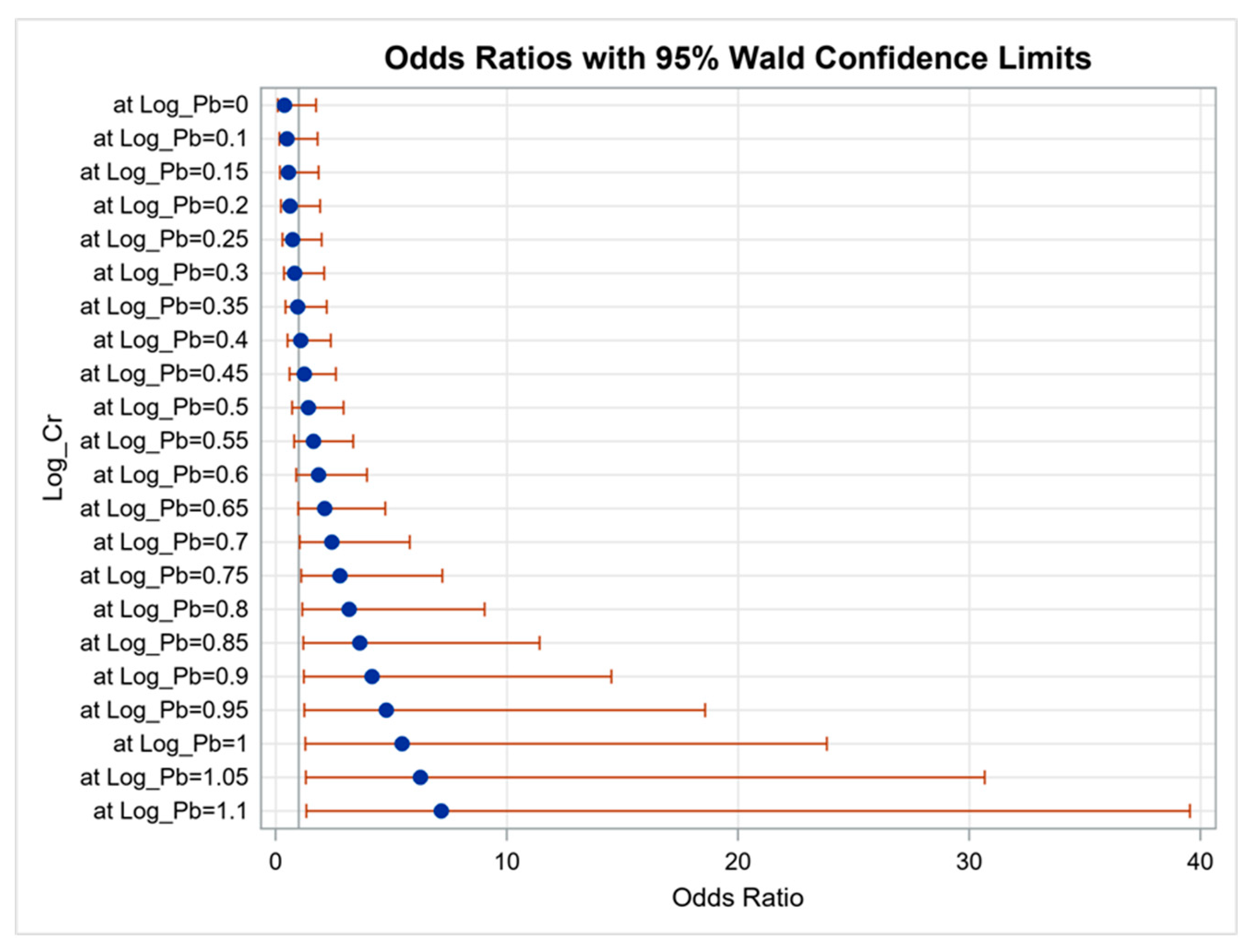
3.2. Determinants of CKD
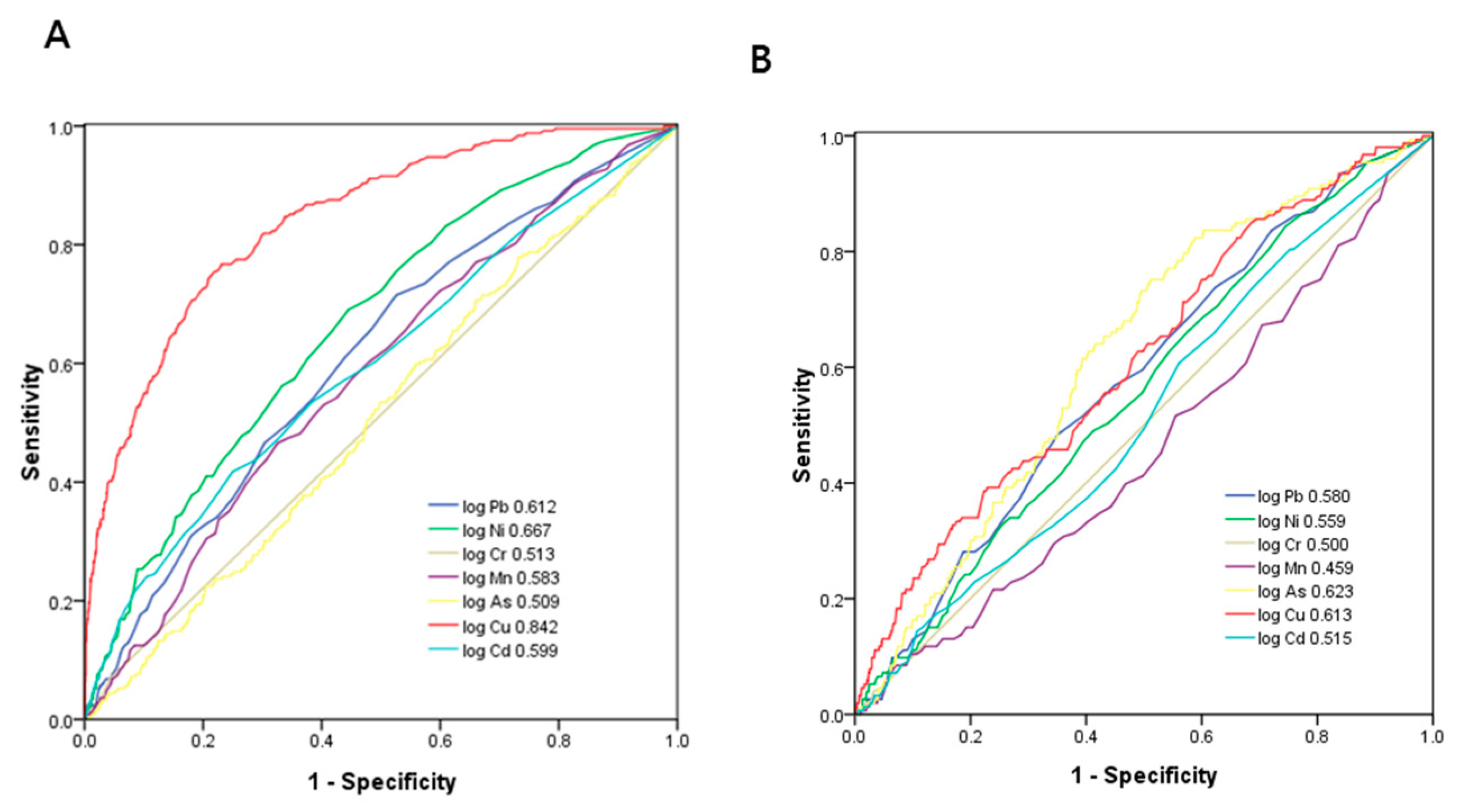
3.3. ROC Curve Analysis for Heavy Metals in Identifying Proteinuria and eGFR < 60 mL/min/1.73 m2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Landrigan, P.J.; Sly, J.L.; Ruchirawat, M.; Silva, E.R.; Huo, X.; Diaz-Barriga, F.; Zar, H.J.; King, M.; Ha, E.H.; Asante, K.A.; et al. Health Consequences of Environmental Exposures: Changing Global Patterns of Exposure and Disease. Ann. Glob. Health 2016, 82, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Sly, P.D.; Carpenter, D.O.; Berg, M.V.D.; Stein, R.T.; Landrigan, P.J.; Brune-Drisse, M.-N.; Suk, W. Health Consequences of Environmental Exposures: Causal Thinking in Global Environmental Epidemiology. Ann. Glob. Health 2016, 82, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Radke, E.G.; Braun, J.M.; Nachman, R.M.; Cooper, G.S. Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environ. Int. 2020, 137, 105408. [Google Scholar] [CrossRef]
- Grant, M.K.; Goldizen, B.F.C.; Sly, P.; Brune, M.M.-N.; Neira, M.; Berg, M.V.D.; Norman, R.E. Health consequences of exposure to e-waste: A systematic review. Lancet Glob. Health 2013, 1, e350–e361. [Google Scholar] [CrossRef]
- Verhulst, S.L.; Nelen, V.; Hond, E.D.; Koppen, G.; Beunckens, C.; Vael, C.; Schoeters, G.; Desager, K. Intrauterine Exposure to Environmental Pollutants and Body Mass Index during the First 3 Years of Life. Environ. Health Perspect. 2009, 117, 122–126. [Google Scholar] [CrossRef]
- Carpenter, D.O.; Bushkin-Bedient, S. Exposure to Chemicals and Radiation during Childhood and Risk for Cancer Later in Life. J. Adolesc. Health 2013, 52, S21–S29. [Google Scholar] [CrossRef] [PubMed]
- Sall, M.L.; Diaw, A.K.D.; Gningue-Sall, D.; Aaron, S.E.; Aaron, J.-J. Toxic heavy metals: Impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ. Sci. Pollut. Res. 2020, 27, 29927–29942. [Google Scholar] [CrossRef]
- Orr, S.; Bridges, C.C. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef]
- Scammell, M.K.; Sennett, C.M.; Petropoulos, Z.E.; Kamal, J.; Kaufman, J.S. Environmental and Occupational Exposures in Kidney Disease. Semin. Nephrol. 2019, 39, 230–243. [Google Scholar] [CrossRef]
- Soderland, P.; Lovekar, S.; Weiner, D.E.; Brooks, D.R.; Kaufman, J.S. Chronic Kidney Disease Associated With Environmental Toxins and Exposures. Adv. Chronic Kidney Dis. 2010, 17, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Cheng, T.Y.D.; Tsai, M.K.; Chang, Y.C.; Chan, H.T.; Tsai, S.P.; Chiang, P.H.; Hsu, C.C.; Sung, P.K.; Hsu, Y.H.; et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008, 371, 2173–2182. [Google Scholar] [CrossRef]
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of Chronic Kidney Disease in the United States. JAMA 2007, 298, 2038–2047. [Google Scholar] [CrossRef]
- Imai, E.; Horio, M.; Iseki, K.; Yamagata, K.; Watanabe, T.; Hara, S.; Ura, N.; Kiyohara, Y.; Hirakata, H.; Moriyama, T.; et al. Prevalence of chronic kidney disease (CKD) in the Japanese general population predicted by the MDRD equation modified by a Japanese coefficient. Clin. Exp. Nephrol. 2007, 11, 156–163. [Google Scholar] [CrossRef]
- Manns, B.; Hemmelgarn, B.; Tonelli, M.; Au, F.; Chiasson, T.C.; Dong, J.; Klarenbach, S. Population based screening for chronic kidney disease: Cost effectiveness study. BMJ 2010, 341, c5869. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Levey, A.S.; De Jong, P.E.; Coresh, J.; Nahas, M.E.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.-U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Levey, A.S.; Pandya, K.; Stoycheff, N.; Okparavero, A.; Greene, T. Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Early Change in Proteinuria as a Surrogate End Point for Kidney Disease Progression: An Individual Patient Meta-analysis. Am. J. Kidney Dis. 2014, 64, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-J.; Tsai, J.-C.; Chen, H.-C. Epidemiology, impact and preventive care of chronic kidney disease in Taiwan. Nephrology 2010, 15 (Suppl. S2), 3–9. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Xu, X.; Nie, S.; Ding, H.; Hou, F.F. Environmental pollution and kidney diseases. Nat. Rev. Nephrol. 2018, 14, 313–324. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Initiative KDOQ K/DOQI. Clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Harari, F.; Sallsten, G.; Christensson, A.; Petkovic, M.; Hedblad, B.; Forsgard, N.; Melander, O.; Nilsson, P.M.; Borné, Y.; Engström, G.; et al. Blood Lead Levels and Decreased Kidney Function in a Population-Based Cohort. Am. J. Kidney Dis. 2018, 72, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B.A.; DuVal, G. Effects of lead on the kidney: Roles of high-affinity lead-binding proteins. Environ. Health Perspect. 1991, 91, 77–80. [Google Scholar] [CrossRef]
- Evans, M.; Elinder, C.G. Chronic renal failure induced by lead. Kidney Int. 2011, 79, 688–689. [Google Scholar] [CrossRef] [PubMed]
- Afsar, B.; Afsar, R.E.; Kanbay, A.; Covic, A.; Ortiz, A.; Kanbay, M. Air pollution and kidney disease: Review of current evidence. Clin. Kidney J. 2019, 12, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, Z.-K.; Wang, Z.-Y.; Yang, D.-B.; Liu, Z.-P.; Wang, L. Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch. Toxicol. 2016, 90, 1193–1209. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Lin, J.L.; Lin-Tan, D.T. Environmental exposure to lead and progression of chronic renal diseases: A four-year pro-spective longitudinal study. J. Am. Soc. Nephrol. 2004, 15, 1016–1022. [Google Scholar] [CrossRef]
- Lei, R.; Wu, C.; Yang, B.; Ma, H.; Shi, C.; Wang, Q.; Wang, Q.; Yuan, Y.; Liao, M. Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: A rapid in vivo screening method for nanotoxicity. Toxicol. Appl. Pharmacol. 2008, 232, 292–301. [Google Scholar] [CrossRef]
- Kumar, V.; Kalita, J.; Bora, H.K.; Misra, U.K. Relationship of antioxidant and oxidative stress markers in different organs following copper toxicity in a rat model. Toxicol. Appl. Pharmacol. 2016, 293, 37–43. [Google Scholar] [CrossRef]
- Yang, F.; Yi, X.; Guo, J.; Xu, S.; Xiao, Y.; Huang, X.; Duan, Y.; Luo, D.; Xiao, S.; Huang, Z.; et al. Association of plasma and urine metals levels with kidney function: A population-based cross-sectional study in China. Chemosphere 2019, 226, 321–328. [Google Scholar] [CrossRef]
- Jung, J.; Park, J.Y.; Kim, Y.C.; Lee, H.; Kim, E.; Kim, Y.S.; Lee, J.P.; Kim, H.; Clinical Research Center for End-Stage Renal Disease (CRC for ESRD) Investigators Clinical Research Center for End-Stage Renal Disease (CRC for ESRD) Investigators; Kim, Y.; et al. Long-Term Effects of Air Pollutants on Mortality Risk in Patients with End-Stage Renal Disease. Int. J. Environ. Res. Public Health 2020, 17, 546. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, C.; Lopez-Chaves, C.; Gomez-Aracena, J.; Galindo, P.; Aranda, P.; Llopis, J. Association of plasma man-ganese levels with chronic renal failure. J. Trace Elem. Med. Biol. 2015, 31, 78–84. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Xiao, Y.; Li, Y.; Yu, Y.; Mo, T.; Jiang, H.; Li, X.; Yang, H.; Xu, C.; et al. Associations of plasma metal concentrations with the decline in kidney function: A longitudinal study of Chinese adults. Ecotoxicol. Environ. Saf. 2020, 189, 110006. [Google Scholar] [CrossRef]
- Shen, Y.; Yin, Z.; Lv, Y.; Luo, J.; Shi, W.; Fang, J.; Shi, X. Plasma element levels and risk of chronic kidney disease in elderly populations (>/= 90 Years old). Chemosphere 2020, 254, 126809. [Google Scholar] [CrossRef]
- Guo, H.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Wang, X.; Wu, B.; Chen, K.; Deng, J. Modulation of the PI3K/Akt Pathway and Bcl-2 Family Proteins Involved in Chicken’s Tubular Apoptosis Induced by Nickel Chloride (NiCl2). Int. J. Mol. Sci. 2015, 16, 22989–23011. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-C.; Wu, C.-L.; Kor, C.-T.; Lian, I.-B.; Chang, C.-H.; Chang, T.-H.; Chang, C.-C.; Chiu, P.-F. Prospective associations between environmental heavy metal exposure and renal outcomes in adults with chronic kidney disease. Nephrology 2018, 23, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. BioMetals 2010, 23, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Goyer, R.A. Toxic and Essential Metal Interactions. Annu. Rev. Nutr. 1997, 17, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Vesey, D.A. Transport pathways for cadmium in the intestine and kidney proximal tubule: Focus on the interaction with essential metals. Toxicol. Lett. 2010, 198, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Jenkitkasemwong, S.; Wang, C.-Y.; MacKenzie, B.; Knutson, M.D. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. BioMetals 2012, 25, 643–655. [Google Scholar] [CrossRef]
- Wilbur, S.; Abadin, H.; Fay, M.; Yu, D.; Tencza, B.; Ingerman, L.; Klotzbach, J.; James, S. Toxicological Profile for Chromium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012. [Google Scholar]
- Wang, T.; Jia, G.; Zhang, J.; Ma, Y.; Feng, W.; Liu, L.; Zhang, N.; Yan, L.; Wang, X.; Zhang, X.; et al. Renal impairment caused by chronic occupational chromate exposure. Int. Arch. Occup. Environ. Health 2011, 84, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-L.; Kuo, C.-C.; Pan, W.-H.; Chung, Y.-T.; Chen, C.-Y.; Wu, T.-N.; Wang, S.-L. The decline in kidney function with chromium exposure is exacerbated with co-exposure to lead and cadmium. Kidney Int. 2017, 92, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Kulathunga, M.R.D.L.; Wijayawardena, M.A.A.; Naidu, R.; Wijeratne, A.W. Chronic kidney disease of unknown aetiology in Sri Lanka and the exposure to environmental chemicals: A review of literature. Environ. Geochem. Health 2019, 41, 2329–2338. [Google Scholar] [CrossRef]
- Eastmond, D.A.; MacGregor, J.T.; Slesinski, R.S. Trivalent Chromium: Assessing the Genotoxic Risk of an Essential Trace Element and Widely Used Human and Animal Nutritional Supplement. Crit. Rev. Toxicol. 2008, 38, 173–190. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Barnes, C.; Yedjou, C.; Velma, V.R.; Tchounwou, P.B. Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ. Toxicol. 2008, 24, 66–73. [Google Scholar] [CrossRef]
- Jia, Q.; Ha, X.; Yang, Z.; Hui, L.; Yang, X. Oxidative stress: A possible mechanism for lead-induced apoptosis and nephrotoxicity. Toxicol. Mech. Methods 2012, 22, 705–710. [Google Scholar] [CrossRef]
- Sahu, B.D.; Koneru, M.; Bijargi, S.R.; Kota, A.; Sistla, R. Chromium-induced nephrotoxicity and ameliorative effect of carvedilol in rats: Involvement of oxidative stress, apoptosis and inflammation. Chem. Interact. 2014, 223, 69–79. [Google Scholar] [CrossRef] [PubMed]
- White, S.L.; Yu, R.; Craig, J.C.; Polkinghorne, K.R.; Atkins, R.C.; Chadban, S.J. Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am. J. Kidney Dis. 2010, 58, 19–28. [Google Scholar] [CrossRef]

| Characteristics | All (n = 2447) | Without Proteinuria (n = 2194) | With Proteinuria (n = 253) | p |
|---|---|---|---|---|
| Age (year) | 55.1 ± 13.2 | 54.6 ± 13.0 | 59.7 ± 14.0 | <0.001 |
| Male gender (%) | 39.9 | 39.0 | 48.2 | 0.004 |
| DM (%) | 10.5 | 8.4 | 28.5 | <0.001 |
| Hypertension (%) | 25.3 | 23.0 | 45.5 | <0.001 |
| BMI (kg/m2) | 25.0 ± 4.0 | 24.8 ± 3.9 | 26.2 ± 4.6 | <0.001 |
| SBP (mmHg) | 132.1 ± 19.8 | 131.1 ± 19.2 | 140.0 ± 22.4 | <0.001 |
| DBP (mmHg) | 77.5 ± 11.7 | 77.2 ± 11.4 | 80.1 ± 13.6 | 0.001 |
| Occupation (%) | 0.041 | |||
| Agriculture, forestry, fishing, and animal husbandr | 4.9 | 4.9 | 5.1 | |
| Commerce | 20.8 | 21.1 | 18.4 | |
| Industry | 11.3 | 11.9 | 6.1 | |
| Government employees | 23.0 | 22.9 | 23.5 | |
| Service industry | 6.2 | 6.3 | 4.6 | |
| None | 33.8 | 32.8 | 42.3 | |
| Living environment (%) | ||||
| Oil-painted in the past six months | 6.3 | 6.2 | 7.2 | 0.556 |
| House decoration in the past six months | 4.1 | 4.4 | 1.4 | 0.041 |
| Burned incense | 74.2 | 73.9 | 76.9 | 0.350 |
| Laboratory parameters | ||||
| Fasting glucose (mg/dL) | 99.9 ± 27.4 | 97.6 ± 23.8 | 119.7 ± 43.4 | <0.001 |
| Triglyceride (mg/dL) | 105.0 (73.0–150.0) | 102.0 (72.0–146.0) | 129.0 (90.0–192.5) | <0.001 |
| Total cholesterol (mg/dL) | 199.6 ± 37.4 | 200.2 ± 37.2 | 194.9 ± 39.4 | 0.043 |
| HDL-cholesterol (mg/dL) | 53.0 ± 13.6 | 53.3 ± 13.6 | 49.9 ± 13.9 | <0.001 |
| LDL-cholesterol (mg/dL) | 119.2 ± 34.0 | 119.8 ± 34.0 | 113.5 ± 34.0 | 0.005 |
| Hemoglobin (g/dL) | 14.0 ± 1.6 | 14.0 ± 1.6 | 14.0 ± 1.9 | 0.887 |
| eGFR (mL/min/1.73 m2) | 89.1 ± 16.3 | 90.2 ± 15.0 | 79.3 ± 22.8 | <0.001 |
| Uric acid (mg/dL) | 5.7 ± 1.6 | 5.7 ± 1.5 | 6.2 ± 1.7 | <0.001 |
| Heavy metals | ||||
| Blood | ||||
| Pb (μg/dL) | 1.6 (1.0–2.2) | 1.5 (1.0–2.2) | 1.9 (1.3–2.7) | <0.001 |
| Urine | ||||
| Ni (μg/L) | 2.4 (1.5–3.7) | 2.3 (1.5–3.5) | 3.3 (2.3–5.5) | <0.001 |
| Cr (μg/L) | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.134 |
| Mn (μg/L) | 1.7 (0.9–3.0) | 1.7 (0.9–2.9) | 2.2 (1.2–3.5) | <0.001 |
| As (μg/L) | 78.9 (45.6–142.0) | 78.2 (45.3–142.5) | 82.7 (48.6–138.9) | 0.760 |
| Cu (μg/dL) | 1.5 (1.0–2.0) | 1.4 (1.0–1.8) | 2.4 (1.9–3.5) | <0.001 |
| Cd (μg/L) | 0.8 (0.5–1.4) | 0.8 (0.3–1.3) | 1.1 (0.6–2.0) | <0.001 |
| Heavy Metals | Multivariable | |
|---|---|---|
| OR (95% CI) | p | |
| Blood | ||
| Pb (log per 1 μg/dL) | 3.089 (1.630–5.853) | 0.001 |
| Urine | ||
| Ni (log per 1 μg/L) | 3.642 (2.285–5.807) | <0.001 |
| Cr (log per 1 μg/L) | 1.810 (0.793–4.128) | 0.159 |
| Mn (log per 1 μg/L) | 2.443 (1.649–3.619) | <0.001 |
| As (log per 1 μg/L) | 0.765 (0.473–1.239) | 0.277 |
| Cu (log per 0.1 μg/dL) | 1.945 (1.750–2.162) | <0.001 |
| Cd (log per 1 μg/L) | 2.671 (1.733–4.118) | <0.001 |
| Characteristics | eGFR ≥ 60 (n = 2292) | eGFR < 60 (n = 155) | p |
|---|---|---|---|
| Heavy metals | |||
| Blood | |||
| Pb (μg/dL) | 1.5 (1.0–2.2) | 1.8 (1.2–2.5) | 0.002 |
| Urine | |||
| Ni (μg/L) | 2.4 (1.5–3.7) | 2.7 (1.8–4.3) | <0.001 |
| Cr (μg/L) | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.371 |
| Mn (μg/L) | 1.7 (0.9–3.0) | 1.6 (0.7–2.7) | 0.249 |
| As (μg/L) | 76.3 (44.1–139.0) | 107.4 (73.0–177.1) | <0.001 |
| Cu (μg/dL) | 1.4 (1.0–1.9) | 1.7 (1.3–2.4) | <0.001 |
| Cd (μg/L) | 0.8 (0.5–1.4) | 0.8 (0.5–1.5) | 0.352 |
| Heavy Metals | Multivariable | |
|---|---|---|
| OR (95% CI) | p | |
| Blood | ||
| Pb (log per 1 μg/dL) | 3.727 (1.207–11.510) | 0.022 |
| Urine | ||
| Ni (log per 1 μg/L) | 1.315 (0.779–2.220) | 0.305 |
| Cr (log per 1 μg/L) | 0.653 (0.128–3.329) | 0.608 |
| Mn (log per 1 μg/L) | 0.894 (0.539–1.482) | 0.663 |
| As (log per 1 μg/L) | 0.775 (0.369–1.629) | 0.502 |
| Cu (log per 0.1 μg/dL) | 1.163 (1.038–1.303) | 0.009 |
| Cd (log per 1 μg/L) | 0.758 (0.404–1.423) | 0.389 |
| Heavy Metals | AUC (95% Confidence Interval) | Cutoff Value | Sensitivity (%) | Specificity (%) | Youden Index |
|---|---|---|---|---|---|
| Pb | 0.612 (0.575–0.648) * | 0.217 | 61.0 | 56.1 | 0.171 |
| Ni | 0.667 (0.633–0.701) * | 0.455 | 60.6 | 62.5 | 0.231 |
| Cr | 0.513 (0.474–0.551) | −0.850 | 6.8 | 95.7 | 0.025 |
| Mn | 0.583 (0.546–0.620) * | 0.267 | 57.4 | 54.9 | 0.123 |
| As | 0.509 (0.472–0.547) | 1.907 | 51.4 | 51.4 | 0.028 |
| Cu | 0.842 (0.816–0.867) * | 0.271 | 76.7 | 76.9 | 0.536 |
| Cd | 0.599 (0.560–0.638) * | −0.023 | 57.0 | 56.4 | 0.134 |
| Heavy Metals | AUC (95% Confidence Interval) | Cutoff Value | Sensitivity (%) | Specificity (%) | Youden Index |
|---|---|---|---|---|---|
| Pb | 0.580 (0.535–0.624) * | 0.217 | 56.9 | 55.0 | 0.119 |
| Ni | 0.559 (0.514–0.603) * | 0.407 | 52.9 | 53.3 | 0.062 |
| Cr | 0.500 (0.453–0.547) | −0.850 | 4.6 | 95.5 | 0.001 |
| Mn | 0.459 (0.413–0.506) | 0.217 | 45.1 | 47.1 | −0.078 |
| As | 0.623 (0.582–0.665) * | 1.984 | 60.8 | 60.8 | 0.216 |
| Cu | 0.613 (0.568–0.659) * | 0.192 | 55.6 | 56.8 | 0.124 |
| Cd | 0.515 (0.469–0.561) | −0.071 | 49.7 | 49.8 | −0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-J.; Hung, C.-H.; Wang, C.-W.; Tu, H.-P.; Li, C.-H.; Tsai, C.-C.; Lin, W.-Y.; Chen, S.-C.; Kuo, C.-H. Associations among Heavy Metals and Proteinuria and Chronic Kidney Disease. Diagnostics 2021, 11, 282. https://doi.org/10.3390/diagnostics11020282
Tsai H-J, Hung C-H, Wang C-W, Tu H-P, Li C-H, Tsai C-C, Lin W-Y, Chen S-C, Kuo C-H. Associations among Heavy Metals and Proteinuria and Chronic Kidney Disease. Diagnostics. 2021; 11(2):282. https://doi.org/10.3390/diagnostics11020282
Chicago/Turabian StyleTsai, Hui-Ju, Chih-Hsing Hung, Chih-Wen Wang, Hung-Pin Tu, Chiu-Hui Li, Chun-Chi Tsai, Wen-Yi Lin, Szu-Chia Chen, and Chao-Hung Kuo. 2021. "Associations among Heavy Metals and Proteinuria and Chronic Kidney Disease" Diagnostics 11, no. 2: 282. https://doi.org/10.3390/diagnostics11020282
APA StyleTsai, H.-J., Hung, C.-H., Wang, C.-W., Tu, H.-P., Li, C.-H., Tsai, C.-C., Lin, W.-Y., Chen, S.-C., & Kuo, C.-H. (2021). Associations among Heavy Metals and Proteinuria and Chronic Kidney Disease. Diagnostics, 11(2), 282. https://doi.org/10.3390/diagnostics11020282






