Bolus Intravenous Procainamide in Patients with Frequent Ventricular Ectopics during Cardiac Magnetic Resonance Scanning: A Way to Ensure High Quality Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
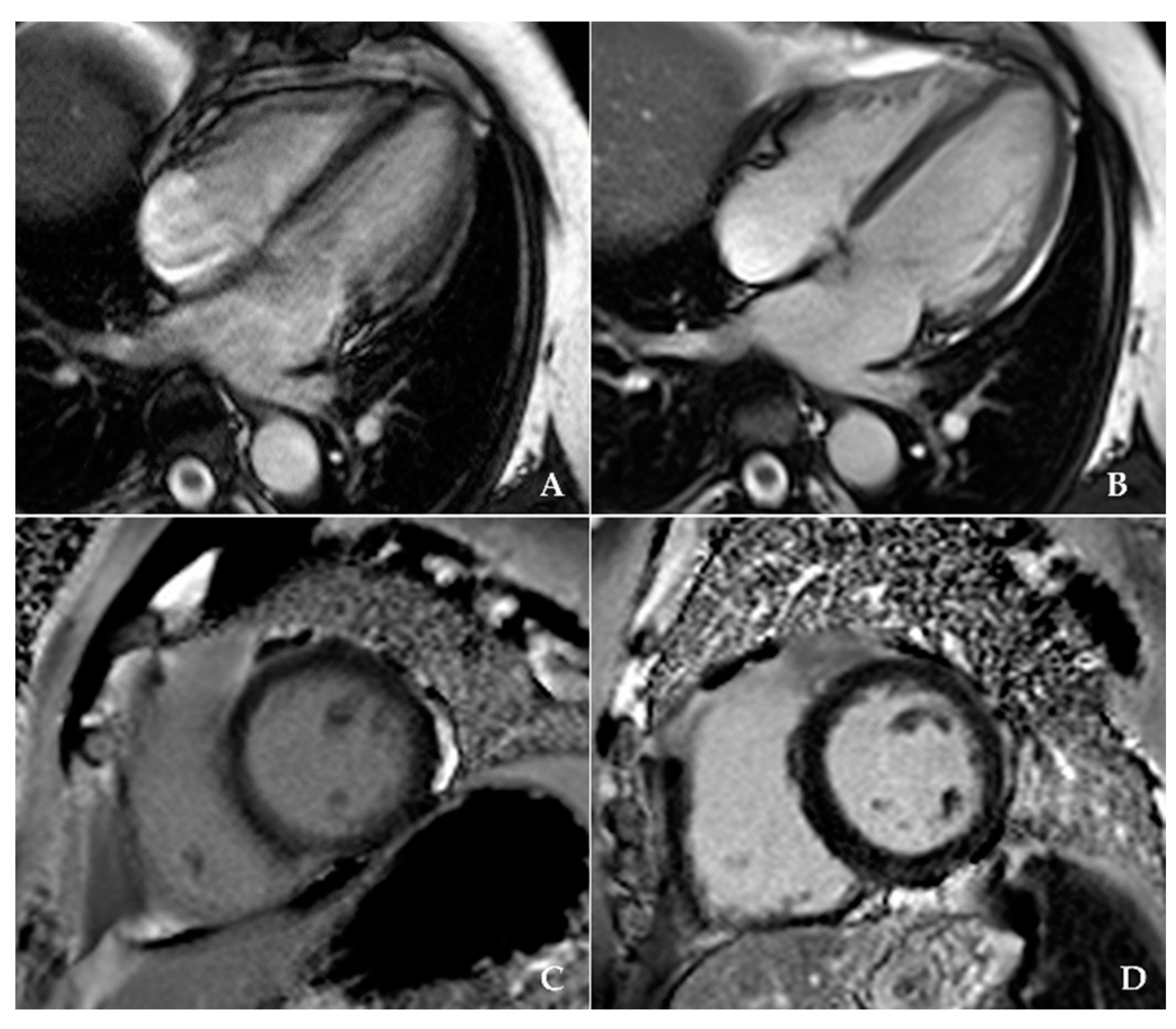
2.2. CMR Protocol
2.3. Procainamide Administration
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baggiano, A.; Torto, A.D.; Guglielmo, M.; Muscogiuri, G.; Fusini, L.; Babbaro, M.; Collevecchio, A.; Mollace, R.; Scafuri, S.; Mushtaq, S.; et al. Role of CMR Mapping Techniques in Cardiac Hypertrophic Phenotype. Diagnostics 2020, 10, 770. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial Tissue Characteriza-tion and Fibrosis by Imaging. JACC Cardiovasc. Imaging 2020, 13, 1221–1234. [Google Scholar] [CrossRef]
- Muser, D.; Santangeli, P.; Castro, S.A.; Arroyo, R.C.; Maeda, S.; Benhayon, D.A.; Liuba, I.; Liang, J.J.; Sadek, M.M.; Chahal, A.; et al. Risk Stratification of Patients with Apparently Idiopathic Premature Ventricular Contractions: A Multicenter International CMR Registry. JACC Clin. Electrophysiol. 2020, 6, 722–735. [Google Scholar] [CrossRef]
- Muser, D.; Nucifora, G.; Pieroni, M.; Castro, S.A.; Arroyo, R.C.; Maeda, S.; Benhayon, D.A.; Liuba, I.; Sadek, M.; Magnani, S.; et al. Prognostic Value of Non-Ischemic Ring-Like Left Ventricular Scar in Patients with Apparently Idiopathic Non-Sustained Ventricular Arrhythmias. Circulation 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, G.; Muser, D.; Masci, P.G.; Barison, A.; Rebellato, L.; Piccoli, G.; Daleffe, E.; Toniolo, M.; Zanuttini, D.; Facchin, D.; et al. Prevalence and prognostic value of concealed structural abnormali-ties in patients with apparently idiopathic ventricular arrhythmias of left versus right ventricular origin: A magnetic resonance imaging study. Circ. Arrhythm. Electrophysiol. 2014, 7, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Nacif, M.S.; Zavodni, A.; Kawel, N.; Choi, E.-Y.; Lima, J.A.C.; Bluemke, D.A. Cardiac magnetic resonance imaging and its electrocardiographs (ECG): Tips and tricks. Int. J. Cardiovasc. Imaging 2012, 28, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, B.; Thompson, H. Procainamide. [Updated 24 May 2020]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557788/ (accessed on 20 December 2020).
- Somani, R.; Krahn, A.D.; Healey, J.S.; Chauhan, V.S.; Birnie, D.H.; Champagne, J.; Sanatani, S.; Angaran, P.; Gow, R.M.; Chakrabarti, S.; et al. Procainamide infusion in the evaluation of unexplained cardiac ar-rest: From the Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (CASPER). Heart Rhythm 2014, 11, 1047–1054. [Google Scholar] [CrossRef]
- Bucciarelli-Ducci, C.; Baritussio, A.; Auricchio, A. Cardiac MRI Anatomy and Function as a Substrate for Ar-rhythmias. EP Eur. 2016, 18, iv130–iv135. [Google Scholar]
- De Maria, E.; Aldrovandi, A.; Borghi, A.; Modonesi, L.; Cappelli, S. Cardiac magnetic resonance imaging: Which information is use-ful for the arrhythmologist? World J. Cardiol. 2017, 9, 773–786. [Google Scholar] [CrossRef]
- Andreini, D.; Dello Russo, A.; Pontone, G.; Mushtaq, S.; Conte, E.; Perchinunno, M.; Guglielmo, M.; Santos, A.C.; Magatelli, M.; Baggiano, A.; et al. CMR for Identifying the Substrate of Ventricular Arrhythmia in Patients With Normal Echocardiography. JACC Cardiovasc. Imaging 2020, 13, 410–421. [Google Scholar] [CrossRef]
- Ridgway, J.P. Cardiovascular magnetic resonance physics for clinicians: Part I. J. Cardiovasc. Magn. Res. 2010, 12, 71. [Google Scholar] [CrossRef]
- Menchón-Lara, R.-M.; Simmross-Wattenberg, F.; Casaseca-De-La-Higuera, P.; Martín-Fernández, M.; Alberola-López, C. Reconstruction techniques for cardiac cine MRI. Insights Imaging 2019, 10, 100. [Google Scholar] [CrossRef]
- Axel, L.; Otazo, R. Accelerated MRI for the assessment of cardiac function. Br. J. Radiol. 2016, 89, 20150655. [Google Scholar] [CrossRef]
- Kido, T.; Kido, T.; Nakamura, M.; Watanabe, K.; Schmidt, M.; Forman, C.; Mochizuki, T. Compressed sensing real-time cine cardiovascular magnetic resonance: Accurate assessment of left ventricular function in a single-breath-hold. J. Cardiovasc. Magn. Res. 2016, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Piehler, K.M.; Wong, T.C.; Puntil, K.S.; Zareba, K.M.; Lin, K.; Harris, D.M.; Deible, C.R.; Lacomis, J.M.; Czeyda-Pommersheim, F.; Cook, S.C.; et al. Free-Breathing, Motion-Corrected Late Gadolinium Enhancement Is Robust and Extends Risk Stratification to Vulnerable Patients. Circ. Cardiovasc. Imaging 2013, 6, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Captur, G.; Lobascio, I.; Ye, Y.; Culotta, V.; Boubertakh, R.; Xue, H.; Kellman, P.; Moon, J.C. Motion-corrected free-breathing LGE delivers high quality imaging and reduces scan time by half: An independent validation study. Int. J. Cardiovasc. Imaging 2019, 35, 1893–1901. [Google Scholar] [CrossRef]
- Giardina, E.G. Procainamide: Clinical Pharmacology and Efficacy against Ventricular Arrhythmias. Ann. N. Y. Acad. Sci. 1984, 432, 177–188. [Google Scholar] [CrossRef]
- Giardina, E.G.; Heissenbuttel, R.H.; Bigger, J.T., Jr. Intermittent Intravenous Procaine Amide to Treat Ventricular Arrhythmias. Correlation of plasma concentration with effect on arrhythmia, electrocardiogram, and blood pressure. Ann. Intern. Med. 1973, 78, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.; Martín, A.; Arribas, F.; Coll-Vinent, B.; Del Arco, C.; Peinado, R.; Almendral, J. Randomized comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: The PROCAMIO study. Eur. Heart J. 2017, 38, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Komura, S.; Chinushi, M.; Furushima, H.; Hosaka, Y.; Izumi, D.; Iijima, K.; Watanabe, H.; Yagihara, N.; Aizawa, Y. Efficacy of procainamide and lidocaine in terminating sustained monomorphic ventricular tachycardia. Circ. J. 2010, 74, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, G.K.; Pastromas, S.; Tzeis, S. Flecainide: Current status and perspectives in arrhythmia management. World J. Cardiol. 2015, 7, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [PubMed]
- Lawson, D.H.; Jick, H. Adverse reactions to procainamide. Br. J. Clin. Pharmacol. 1977, 4, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Dukes, J.W.; Dewland, T.A.; Vittinghoff, E.; Mandyam, M.C.; Heckbert, S.R.; Siscovick, D.S.; Stein, P.K.; Psaty, B.M.; Sotoodehnia, N.; Gottdiener, J.S.; et al. Ventricular Ectopy as a Predictor of Heart Failure and Death. J. Am. Coll. Cardiol. 2015, 66, 101–109. [Google Scholar] [CrossRef] [PubMed]

| Clinical Characteristics | |
| Patients (n) | 50 |
| Female, n (%) | 26 (52%) |
| Age (years) | 53.0 [42.0–58.0] |
| Body Surface Area (BSA), m2 | 1.9 ± 0.2 |
| Clinical indication | |
| Premature ventricular contractions (PVCs), n (%) | 44 (88%) |
| Non-sustained ventricular tachycardia (NSVT), n (%) | 6 (12%) |
| CMR Measurements | |
| LV end diastolic volume (mL) | 175 ± 58 |
| LV end systolic volume (mL) | 83 ± 49 |
| LV ejection fraction (%) | 55 ± 9 |
| RV end diastolic volume (mL) | 154 ± 42 |
| RV end systolic volume (mL) | 64 ± 31 |
| RV ejection fraction (%) | 60 ± 8 |
| CMR Diagnosis | |
| Normal, n (%) | 21 (42%) |
| Non-ischemic cardiomyopathy, n (%) | 13 (26%) |
| PVC-related cardiomyopathy, n (%) | 8 (16%) |
| Previous myocarditis, n (%) | 7 (14%) |
| Dual pathology (DCM and MI), n (%) | 1 (2%) |
| Procainamide Administration | Before | After | p-Value |
| Mean procainamide dose (mg): 567 ± 197 Range (mg): 200–1000 | - | - | - |
| Systolic blood pressure (mmHg) | 133 ± 19 | 121 ± 17 | <0.001 |
| Diastolic blood pressure (mmHg) | 68 ± 14 | 64 ± 12 | 0.012 |
| Heart rate (bpm) | 75 ± 12 | 74 ± 13 | 0.24 |
| Image Quality (1 bad, 2 moderate, 3 very good, 4 excellent) | 1.62 ± 0.49 | 3.46 ± 0.51 | <0.001 |
| Result | No. of Patients (%) | ||
| Complete PVCs suppression | 20 (40%) | ||
| Significant PVCs reduction | 21 (42%) | ||
| Minimal PVCs suppression | 7 (14%) | ||
| No response | 2 (4%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaidou, C.; Kouskouras, K.; Fragakis, N.; Vassilikos, V.P.; Karvounis, H.; Karamitsos, T.D. Bolus Intravenous Procainamide in Patients with Frequent Ventricular Ectopics during Cardiac Magnetic Resonance Scanning: A Way to Ensure High Quality Imaging. Diagnostics 2021, 11, 178. https://doi.org/10.3390/diagnostics11020178
Nikolaidou C, Kouskouras K, Fragakis N, Vassilikos VP, Karvounis H, Karamitsos TD. Bolus Intravenous Procainamide in Patients with Frequent Ventricular Ectopics during Cardiac Magnetic Resonance Scanning: A Way to Ensure High Quality Imaging. Diagnostics. 2021; 11(2):178. https://doi.org/10.3390/diagnostics11020178
Chicago/Turabian StyleNikolaidou, Chrysovalantou, Konstantinos Kouskouras, Nikolaos Fragakis, Vassilios P. Vassilikos, Haralambos Karvounis, and Theodoros D. Karamitsos. 2021. "Bolus Intravenous Procainamide in Patients with Frequent Ventricular Ectopics during Cardiac Magnetic Resonance Scanning: A Way to Ensure High Quality Imaging" Diagnostics 11, no. 2: 178. https://doi.org/10.3390/diagnostics11020178
APA StyleNikolaidou, C., Kouskouras, K., Fragakis, N., Vassilikos, V. P., Karvounis, H., & Karamitsos, T. D. (2021). Bolus Intravenous Procainamide in Patients with Frequent Ventricular Ectopics during Cardiac Magnetic Resonance Scanning: A Way to Ensure High Quality Imaging. Diagnostics, 11(2), 178. https://doi.org/10.3390/diagnostics11020178








