DGEMRIC in the Assessment of Pre-Morphological Cartilage Degeneration in Rheumatic Disease: Rheumatoid Arthritis vs. Psoriatic Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MR Imaging
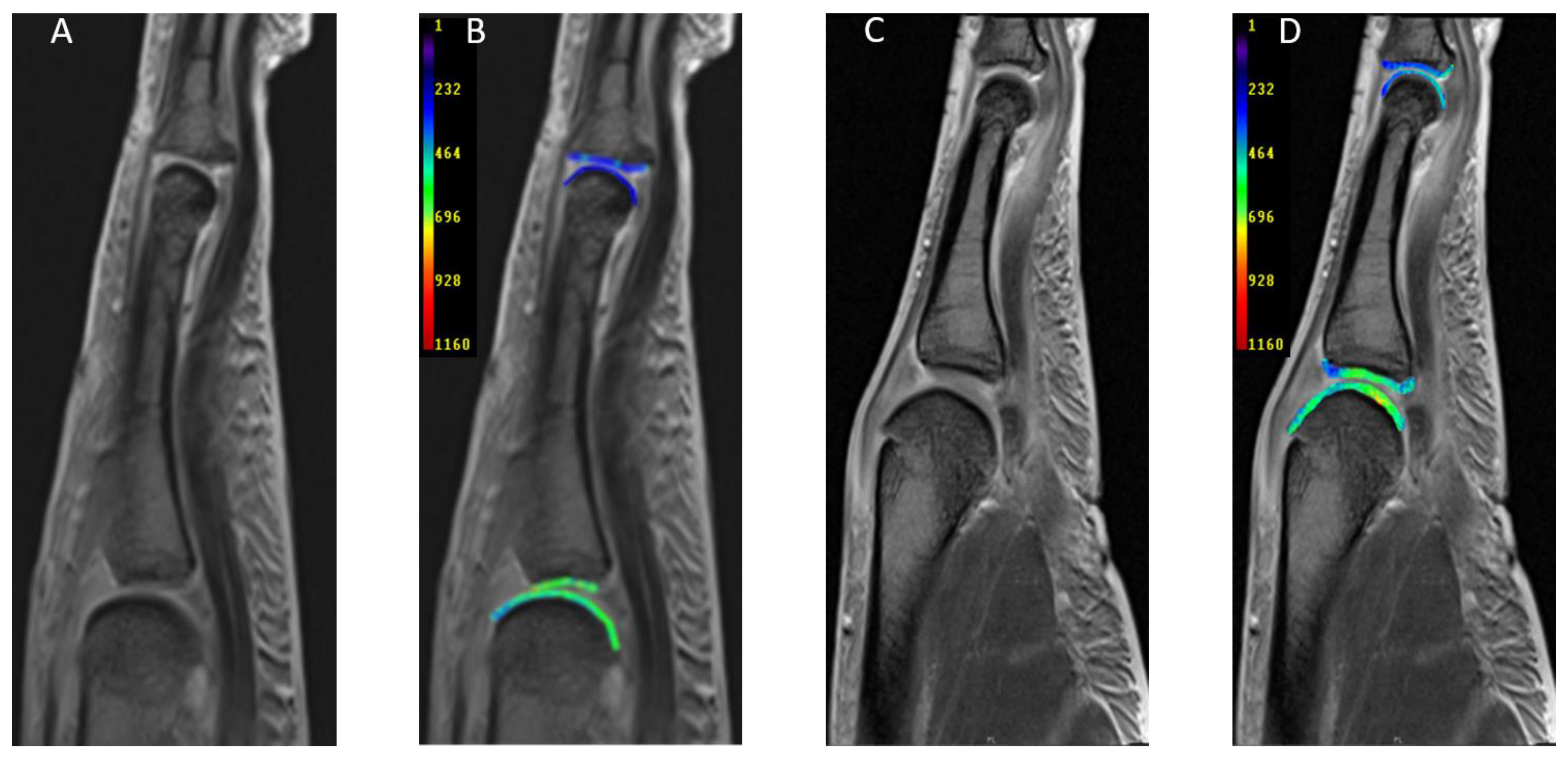
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schett, G.; Coates, L.C.; Ash, Z.R.; Finzel, S.; Conaghan, P.G. Structural damage in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: Traditional views, novel insights gained from TNF blockade, and concepts for the future. Arthritis Res. Ther. 2011, 13, S4. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, M.; Maśliński, W.; Prochorec-Sobieszek, M.; Nieciecki, M.; Sudoł-Szopińska, I. Cartilage and bone damage in rheumatoid arthritis. Reumatologia 2018, 56, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Buchbender, C.; Ostendorf, B.; Mattes-György, K.; Wittsack, H.J.; Quentin, M.; Specker, C.; Schneider, M.; Antoch, G.; Müller, H.W.; Scherer, A. Synovitis and bone inflammation in early rheumatoid arthritis: High-resolution multi-pinhole SPECT versus MRI. Diagn. Interv. Radiol. 2013, 19, 20–24. [Google Scholar] [PubMed]
- Bugatti, S.; Caporali, R.; Manzo, A.; Vitolo, B.; Pitzalis, C.; Montecucco, C. Involvement of subchondral bone marrow in rheumatoid arthritis: Lymphoid neogenesis and in situ relationship to subchondral bone marrow osteoclast recruitment. Arthritis Rheum. 2005, 52, 3448–3459. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Boj, E.; Redlich, K.; Türk, B.; Hanslik-Schnabel, B.; Wanivenhaus, A.; Chott, A.; Smolen, J.S.; Schett, G. Interaction between Synovial Inflammatory Tissue and Bone Marrow in Rheumatoid Arthritis. J. Immunol. 2005, 175, 2579–2588. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Steiner, G. Does damage cause inflammation? Revisiting the link between joint damage and inflam-mation. Ann. Rheum. Dis. 2009, 68, 159–162. [Google Scholar] [CrossRef]
- Aletaha, D.; Funovits, J.; Smolen, J.S. Physical disability in rheumatoid arthritis is associated with cartilage damage rather than bone destruction. Ann. Rheum. Dis. 2011, 70, 733–739. [Google Scholar] [CrossRef]
- Hayer, S.; Bauer, G.; Willburger, M.; Sinn, K.; Alasti, F.; Plasenzotti, R.; Shvets, T.; Niederreiter, B.; Aschauer, C.; Steiner, G.; et al. Cartilage damage and bone erosion are more prominent determinants of functional impairment in longstanding experimental arthritis than synovial inflammation. Dis. Model. Mech. 2016, 9, 1329–1338. [Google Scholar] [CrossRef]
- Bhattaram, P.; Chandrasekharan, U.M. The joint synovium: A critical determinant of articular cartilage fate in inflammatory joint diseases. Semin. Cell Dev. Biol. 2017, 62, 86–93. [Google Scholar] [CrossRef]
- Bartosińska, J.; Michalak-Stoma, A.; Juszkiewicz-Borowiec, M.; Kowal, M.; Chodorowska, G. The Assessment of Selected Bone and Cartilage Biomarkers in Psoriatic Patients from Poland. Mediat. Inflamm. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Chandran, V.; Abji, F.; Perruccio, A.V.; Gandhi, R.; Li, S.; Cook, R.J.; Gladman, D.D. Serum-based soluble markers differentiate psoriatic arthritis from osteoarthritis. Ann. Rheum. Dis. 2019, 78, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Farouk, H.M.; Mostafa, A.A.A.; Youssef, S.S.; Elbeblawy, M.M.S.; Assaf, N.Y.; Elokda, E.S.E. Value of Entheseal Ultrasonography and Serum Cartilage Oligomeric Matrix Protein in the Preclinical Diagnosis of Psoriatic Arthritis. Clin. Med. Insights: Arthritis Musculoskelet. Disord. 2010, 3, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Mortezavi, M.; Thiele, R.; Ritchlin, C. The joint in psoriatic arthritis. Clin. Exp. Rheumatol. 2015, 2015, 20–25. [Google Scholar]
- McGonagle, D.; Tan, A.L.; Møller Døhn, U.; Ostergaard, M.; Benjamin, M. Microanatomic studies to define predictive factors for the topography of peri-articular erosion formation in inflammatory arthritis. Arthritis Rheum. 2009, 60, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Tan, A. The enthesis in psoriatic arthritis. Clin. Exp. Rheumatol. 2015, 33, 36–39. [Google Scholar]
- McGonagle, D.; Lories, R.J.U.; Tan, A.L.; Benjamin, M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. 2007, 56, 2482–2491. [Google Scholar] [CrossRef]
- Coates, L.C. Treating to target in psoriatic arthritis. Curr. Opin. Rheumatol. 2015, 27, 107–110. [Google Scholar] [CrossRef]
- Van Vollenhoven, R. Treat-to-target in rheumatoid arthritis—are we there yet? Nat. Rev. Rheumatol. 2019, 15, 180–186. [Google Scholar] [CrossRef]
- Van den Bosch, F.; Coates, L. Clinical management of psoriatic arthritis. Lancet 2018, 391, 2285–2294. [Google Scholar] [CrossRef]
- Møller-Bisgaard, S.; Hørslev-Petersen, K.; Ejbjerg, B.; Hetland, M.L.; Ørnbjerg, L.M.; Glinatsi, D.; Møller, J.; Boesen, M.; Christensen, R.; Stengaard-Pedersen, K. Effect of Magnetic Resonance Imaging vs Conventional Treat-to-Target Strategies on Disease Activity Remission and Radiographic Progression in Rheumatoid Arthritis: The IMAGINE-RA Randomized Clinical Trial. JAMA 2019, 321, 461–472. [Google Scholar] [CrossRef]
- Miese, F.R.; Ostendorf, B.; Wittsack, H.-J.; Reichelt, D.C.; Mamisch, T.C.; Zilkens, C.; Lanzman, R.S.; Schneider, M.; Scherer, A. Metacarpophalangeal Joints in Rheumatoid Arthritis: Delayed Gadolinium-enhanced MR Imaging of Cartilage—A Feasibility Study. Radiology 2010, 257, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Miese, F.; Buchbender, C.; Scherer, A.; Wittsack, H.-J.; Specker, C.; Schneider, M.; Antoch, G.; Ostendorf, B. Molecular imaging of cartilage damage of finger joints in early rheumatoid arthritis with delayed gadolinium-enhanced magnetic resonance imaging. Arthritis Rheum. 2012, 64, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Van Tiel, J.; Kotek, G.; Reijman, M.; Bos, P.K.; Bron, E.E.; Klein, S.; Nasserinejad, K.; van Osch, G.J.; Verhaar, J.A.; Krestin, G.P.; et al. Is T1ρ Mapping an Alternative to Delayed Gadolinium-enhanced MR Imaging of Car-tilage in the Assessment of Sulphated Glycosaminoglycan Content in Human Osteoarthritic Knees? An in Vivo Validation Study. Radiology 2016, 279, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Schleich, C.; Schleich, C.; Sewerin, P.; Ostendorf, B.; Buchbender, C.; Schneider, M.; Antoch, G.; Miese, F. Intra-individual assessment of inflammatory severity and cartilage composition of finger joints in rheumatoid arthritis. Skelet. Radiol. 2014, 44, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Sewerin, P.; Müller-Lutz, A.; Abrar, D.B.; Odendahl, S.; Eichner, M.; Schneider, M.; Ostendorf, B.; Schleich, C. Prevention of the progressive biochemical cartilage destruction under methotrexate therapy in early rheumatoid arthritis. Clin. Exp. Rheumatol. 2018, 37, 179–185. [Google Scholar]
- Abrar, D.B.; Schleich, C.; Nebelung, S.; Frenken, M.; Radke, K.L.; Vordenbäumen, S.; Brinks, R.; Schneider, M.; Ostendorf, B.; McGonagle, D.; et al. High-resolution MRI of flexor tendon pulleys using a 16-channel hand coil: Disease detection and differentiation of psoriatic and rheumatoid arthritis. Arthritis Res. 2020, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, M.; Peterfy, C.G.; Bird, P.; Gandjbakhch, F.; Glinatsi, D.; Eshed, I.; Haavardsholm, E.A.; Lillegraven, S.; Bøyesen, P.; Ejbjerg, B.; et al. The OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging (MRI) Scoring System: Updated Recommendations by the OMERACT MRI in Arthritis Working Group. J. Rheumatol. 2017, 44, 1706–1712. [Google Scholar] [CrossRef]
- Østergaard, M.; McQueen, F.; Wiell, C.; Bird, P.; Bøyesen, P.; Ejbjerg, B.; Peterfy, C.; Gandjbakhch, F.; Duer-Jensen, A.; Coates, L.; et al. The OMERACT Psoriatic Arthritis Magnetic Resonance Imaging Scoring System (PsAMRIS): Definitions of Key Pathologies, Suggested MRI Sequences, and Preliminary Scoring System for PsA Hands. J. Rheumatol. 2009, 36, 1816–1824. [Google Scholar] [CrossRef]
- Herz, B.; Albrecht, A.; Englbrecht, M.; Welsch, G.H.; Uder, M.; Renner, N.; Schlechtweg, P.M.; Paul, D.; Lauer, L.; Engelke, K.; et al. Osteitis and synovitis, but not bone erosion, is associated with proteoglycan loss and microstructure damage in the cartilage of patients with rheumatoid arthritis. Ann. Rheum. Dis. 2013, 73, 1101–1106. [Google Scholar] [CrossRef]
- Miese, F.; Kröpil, P.; Ostendorf, B.; Scherer, A.; Buchbender, C.; Quentin, M.; Lanzman, R.S.; Blondin, D.; Schneider, M.; Bittersohl, B.; et al. Motion correction improves image quality of dGEMRIC in finger joints. Eur. J. Radiol. 2011, 80, e427–e431. [Google Scholar] [CrossRef]
- Merola, J.; Espinoza, L.R.; Fleischmann, R. Distinguishing rheumatoid arthritis from psoriatic arthritis. RMD Open 2018, 4, e000656. [Google Scholar] [CrossRef] [PubMed]
- Braum, L.S.; McGonagle, D.; Bruns, A.; Philipp, S.; Hermann, S.; Aupperle, K.; Tan, A.L.; Diekhoff, T.; Hamm, B.; Hermann, K.-G. Characterisation of hand small joints arthropathy using high-resolution MRI—Limited discrimination between osteoarthritis and psoriatic arthritis. Eur. Radiol. 2013, 23, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Wu, D.; Tam, L.-S. The role of imaging in early diagnosis and prevention of joint damage in inflammatory arthritis. Expert Rev. Clin. Immunol. 2018, 14, 499–511. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Hermann, K.-G.A.; Tan, A.L. Differentiation between osteoarthritis and psoriatic arthritis: Implications for pathogenesis and treatment in the biologic therapy era. Rheumatology 2015, 54, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sankowski, A.J.; Lebkowska, U.M.; Cwikła, J.; Walecka, I.; Walecki, J. Psoriatic arthritis. Pol. J. Radiol. 2013, 78, 7–17. [Google Scholar]
- Harrison, S.H. The proximal interphalangeal joint in rheumatoid arthritis. Hand 1971, 3, 125–130. [Google Scholar] [CrossRef]
- Heidari, B. Rheumatoid Arthritis: Early diagnosis and treatment outcomes. Casp. J. Intern. Med. 2011, 2, 161–170. [Google Scholar]
- Gladman, D.D. Early Psoriatic Arthritis. Rheum. Dis. Clin. North. Am. 2012, 38, 373–386. [Google Scholar] [CrossRef]
- Sewerin, P.; Ostendorf, B.; Schleich, C. MRT-Diagnostik bei entzündlichen Gelenk- und Wirbelsäulenerkrankungen: Protokolle und Spezialsequenzen: Wann und wozu? Z. Rheumatol. 2018, 77, 538–548. [Google Scholar] [CrossRef]
- Kanda, T.; Nakai, Y.; Hagiwara, A.; Oba, H.; Toyoda, K.; Furui, S. Distribution and chemical forms of gadolinium in the brain: A review. Br. J. Radiol. 2017, 90. [Google Scholar] [CrossRef]
- Zilkens, C.; Miese, F.; Kim, Y.-J.; Jäger, M.; Mamisch, T.C.; Hosalkar, H.; Antoch, G.; Krauspe, R.; Bittersohl, B. Direct comparison of intra-articular versus intravenous delayed gadolinium-enhanced MRI of hip joint cartilage. J. Magn. Reson. Imaging 2014, 39, 94–102. [Google Scholar] [CrossRef] [PubMed]
| dGEMRIC [ms] | D2 | D3 | D4 | D5 | ||||
|---|---|---|---|---|---|---|---|---|
| MCP | PIP | MCP | PIP | MCP | PIP | MCP | PIP | |
| PsA | ||||||||
| Mean | 523.68 | 420.81 | 516.2 | 338.62 | 552.07 | 407.88 | 537.35 | 450.01 |
| SD | 180.69 | 113.73 | 147.05 | 98.52 | 123.11 | 125.44 | 143.22 | 118.0 |
| Minimum | 272.0 | 202.9 | 259.3 | 190.6 | 348.3 | 235.5 | 327.3 | 277.2 |
| Maximum | 951.4 | 639.6 | 717.0 | 49.4 | 806.4 | 678.7 | 861.9 | 674.3 |
| RA | ||||||||
| Mean | 519.27 | 399.53 | 545.12 | 415.59 | 576.81 | 439.39 | 537.52 | 435.97 |
| SD | 106.11 | 146.95 | 117.0 | 139.65 | 126.65 | 106.33 | 99.82 | 85.97 |
| Minimum | 349.1 | 225.6 | 335.5 | 249.2 | 298.7 | 312.6 | 366.3 | 307.8 |
| Maximum | 679.6 | 777.9 | 764.9 | 671.2 | 834.1 | 644.3 | 739.3 | 607.8 |
| JSW [mm] | D2 | D3 | D4 | D5 | ||||
|---|---|---|---|---|---|---|---|---|
| PsA | RA | PsA | RA | PsA | RA | PsA | RA | |
| MCP | ||||||||
| Mean | 1.59 | 1.65 | 1.57 | 1.44 | 1.34 | 1.31 | 1.44 | 1.35 |
| SD | 0.19 | 0.33 | 0.26 | 0.22 | 0.24 | 0.24 | 0.23 | 0.26 |
| Minimum | 1.30 | 1.13 | 1.15 | 1.13 | 0.92 | 1.02 | 1.08 | 0.98 |
| Maximum | 1.96 | 2.55 | 2.03 | 1.86 | 1.80 | 1.90 | 1.80 | 1.88 |
| p-value | 0.536 | 0.109 | 0.640 | 0.271 | ||||
| PIP | ||||||||
| Mean | 1.09 | 0.88 | 1.14 | 0.88 | 0.95 | 0.85 | 0.90 | 0.82 |
| SD | 0.33 | 0.14 | 0.30 | 0.17 | 0.35 | 0.16 | 0.30 | 0.24 |
| Minimum | 0.68 | 0.56 | 0.66 | 0.65 | 0.54 | 0.59 | .049 | 0.54 |
| Maximum | 1.72 | 1.12 | 1.62 | 1.19 | 1.75 | 1.17 | 1.59 | 1.59 |
| p-value | 0.005 | 0.005 | 0.272 | 0.364 | ||||
| dGEMRIC [ms] PsA vs. RA | D2 | D3 | D4 | D5 | ||||
|---|---|---|---|---|---|---|---|---|
| MCP | PIP | MCP | PIP | MCP | PIP | MCP | PIP | |
| Mean difference | 4.41 | 21.28 | −28.92 | −76.97 | −24.74 | −31.51 | −0.17 | 14.03 |
| 95% CI lower limit | −98.71 | −74.57 | −124.16 | −168.54 | −113.91 | −116.74 | −86.7 | −165.89 |
| 95% CI upper limit | 107.54 | 117.14 | 66.33 | 14.6 | 64.43 | 53.72 | 86.36 | 129.04 |
| p-value | 0.931 | 0.653 | 0.540 | 0.096 | 0.576 | 0.456 | 0.997 | 0.798 |
| dGEMRIC. MCP vs. PIP | D2 | D3 | D4 | D5 | ||||
|---|---|---|---|---|---|---|---|---|
| PsA | RA | PsA | RA | PsA | RA | PsA | RA | |
| Mean difference | 102.87 | 119.74 | 177.58 | 129.53 | 144.19 | 137.41 | 87.34 | 101.54 |
| 95% CI lower limit | −10.05 | 31.97 | 82.82 | 36.88 | 51.23 | 55.13 | −13.06 | 38.44 |
| 95% CI upper limit | 215.79 | 207.51 | 272.34 | 222.18 | 237.15 | 219.69 | 187.74 | 164.65 |
| p-value | 0.073 | 0.009 | 0.001 | 0.008 | 0.004 | 0.002 | 0.086 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrar, D.B.; Schleich, C.; Frenken, M.; Vordenbäumen, S.; Richter, J.; Schneider, M.; Ostendorf, B.; Nebelung, S.; Sewerin, P. DGEMRIC in the Assessment of Pre-Morphological Cartilage Degeneration in Rheumatic Disease: Rheumatoid Arthritis vs. Psoriatic Arthritis. Diagnostics 2021, 11, 147. https://doi.org/10.3390/diagnostics11020147
Abrar DB, Schleich C, Frenken M, Vordenbäumen S, Richter J, Schneider M, Ostendorf B, Nebelung S, Sewerin P. DGEMRIC in the Assessment of Pre-Morphological Cartilage Degeneration in Rheumatic Disease: Rheumatoid Arthritis vs. Psoriatic Arthritis. Diagnostics. 2021; 11(2):147. https://doi.org/10.3390/diagnostics11020147
Chicago/Turabian StyleAbrar, Daniel B., Christoph Schleich, Miriam Frenken, Stefan Vordenbäumen, Jutta Richter, Matthias Schneider, Benedikt Ostendorf, Sven Nebelung, and Philipp Sewerin. 2021. "DGEMRIC in the Assessment of Pre-Morphological Cartilage Degeneration in Rheumatic Disease: Rheumatoid Arthritis vs. Psoriatic Arthritis" Diagnostics 11, no. 2: 147. https://doi.org/10.3390/diagnostics11020147
APA StyleAbrar, D. B., Schleich, C., Frenken, M., Vordenbäumen, S., Richter, J., Schneider, M., Ostendorf, B., Nebelung, S., & Sewerin, P. (2021). DGEMRIC in the Assessment of Pre-Morphological Cartilage Degeneration in Rheumatic Disease: Rheumatoid Arthritis vs. Psoriatic Arthritis. Diagnostics, 11(2), 147. https://doi.org/10.3390/diagnostics11020147







