Kidney Involvement in Acute Hepatic Porphyrias: Pathophysiology and Diagnostic Implications
Abstract
1. Introduction
2. Role of the Kidney in Porphyrin Metabolism
3. Etiology of Chronic Kidney Disease in Acute Porphyria
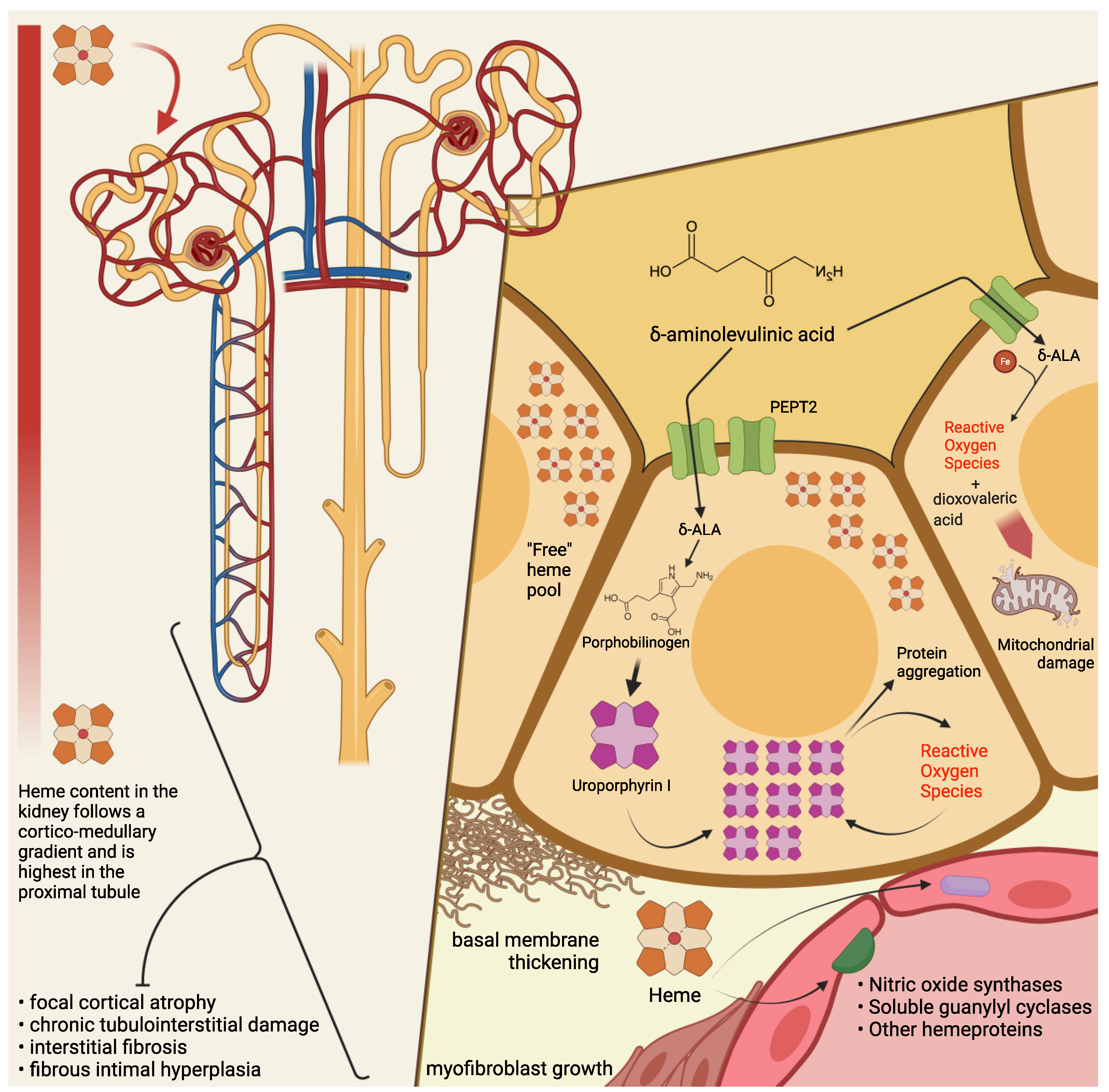
4. Pathogenesis of Kidney Damage in PAKD
5. Excretion of Heme Precursors and Kidney Transplantation in End-Stage PAKD
6. Givosiran and PAKD
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Acronyms
| AHP | Acute hepatic porphyrias |
| AIP | Acute intermittent porphyria |
| ALA | Aminolevulinic acid |
| ALAD | Aminolevulinic acid dehydratase |
| APA | Acute porphyric attack |
| CKD | Chronic kidney disease |
| DOVA | Dioxovaleric acid |
| eGFR | Estimated Glomerular Filtration Rate |
| ESRD | End-Stage Renal Disease |
| HCP | Hereditary coproporphyria |
| HMBS | Porphobilinogen-deaminase or hydroxymethylbilane-synthase |
| HREC | Human Renal Epithelial Cells |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| mRNA | Messenger RNA |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NOS | Nitric oxide synthase |
| PAKD | Porphyria-associated kidney disease |
| PBG | Porphobilinogen |
| PEPT2 | Human Peptide Transporter 2 |
| PLP | Pyridoxal phosphate |
| RISC | RNA-induced silencing complex |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| sGC | Soluble guanylyl cyclase |
| siRNA | Small interfering RNA |
| VP | Variegate porphyria |
References
- Bissell, D.M.; Anderson, K.E.; Bonkovsky, H.L. Porphyria. N. Engl. J. Med. 2017, 377, 862–872. [Google Scholar] [CrossRef]
- Souza, P.V.S.; Badia, B.M.L.; Farias, I.B.; Gonçalves, E.A.; Pinto, W.B.V.R.; Oliveira, A.S.B. Acute hepatic porphyrias for the neurologist: Current concepts and perspectives. Arq. Neuropsiquiatr. 2021, 79, 68–80. [Google Scholar] [CrossRef]
- Martinez, M.D.C.; Cerbino, G.N.; Granata, B.X.; Batlle, A.; Parera, V.E.; Rossetti, M.V. Clinical, biochemical, and genetic characterization of acute hepatic porphyrias in a cohort of Argentine patients. Mol. Genet. Genom. Med. 2021, 9, e1059. [Google Scholar] [CrossRef] [PubMed]
- Kaftory, R.; Edel, Y.; Snast, I.; Lapidoth, M.; Mamet, R.; Elis, A.; Hodak, E.; Levi, A. Greater disease burden of variegate porphyria than hereditary coproporphyria: An Israeli nationwide study of neurocutaneous porphyrias. Mol. Genet. Metab. Rep. 2021, 26, 100707. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef]
- Brun, A.; Sandberg, S. Mechanisms of photosensitivity in porphyric patients with special emphasis on erythropoietic protoporphyria. J. Photochem. Photobiol. B 1991, 10, 285–302. [Google Scholar] [CrossRef]
- Ventura, P.; Cappellini, M.D.; Rocchi, E. The acute porphyrias: A diagnostic and therapeutic challenge in internal and emergency medicine. Intern. Emerg. Med. 2009, 4, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Ventura, P. When awareness makes the difference: Diagnosing and treating the acute hepatic porphyrias. Intern. Emerg. Med. 2021, 16, 25–27. [Google Scholar] [CrossRef]
- Lissing, M.; Nowak, G.; Adam, R.; Karam, V.; Boyd, A.; Gouya, L.; Meersseman, W.; Melum, E.; Oldakowska-Jedynak, U.; Reiter, F.P.; et al. Liver transplantation for acute intermittent porphyria. Liver Transplant. 2021, 27, 491–501. [Google Scholar] [CrossRef]
- Balwani, M.; Sardh, E.; Ventura, P.; Peiró, P.A.; Rees, D.C.; St¨olzel, U.; Bissell, D.M.; Bonkovsky, H.L.; Windyga, J.; Anderson, K.E.; et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N. Eng. J. Med. 2020, 382, 2289–2301. [Google Scholar] [CrossRef]
- Ventura, P.; Bonkovsky, H.L.; Gouya, L.; Aguilera-Peiró, P.; Montgomery Bissell, D.; Stein, P.E.; Balwani, M.; Anderson, D.K.E.; Parker, C.; Kuter, D.J.; et al. Efficacy and safety of givosiran for acute hepatic porphyria: 24-month interim analysis of the randomized phase 3 ENVISION study. Liver Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.V.S.; Badia, B.M.L.; Farias, I.B.; Pinto, W.B.V.R.; Oliveira, A.S.B. Acute Hepatic Porphyria: Pathophysiological Basis of Neuromuscular Manifestations. Front. Neurosci. 2021, 15, 715523. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.F. Review of hepatocellular cancer, hypertension and renal impairment as late complications of acute porphyria and recommendations for patient follow-up. J. Clin. Pathol. 2012, 65, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Buendía-Martínez, J.; Barreda-Sánchez, M.; Rodríguez-Peña, L.; Ballesta-Martínez, M.J.; López-González, V.; Sánchez-Soler, M.J.; Serrano-Antón, A.T.; Pérez-Tomás, M.E.; Gil-Ferrer, R.; Avilés-Plaza, F.; et al. Health impact of acute intermittent porphyria in latent and non-recurrent attacks patients. Orphanet. J. Rare Dis. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Pallet, N.; Karras, A.; Thervet, E.; Gouya, L.; Karim, Z.; Puy, H. Porphyria and kidney diseases. Clin. Kidney J. 2018, 11, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Pallet, N.; Mami, I.; Schmitt, C.; Karim, Z.; Fran¸cois, A.; Rabant, M.; Nochy, D.; Gouya, L.; Deybach, J.C.; Xu-Dubois, Y.; et al. High prevalence of and potential mechanisms for chronic kidney disease in patients with acute intermittent porphyria. Kidney Int. 2015, 88, 386–395. [Google Scholar] [CrossRef]
- Bales, L.; Day, R.; Blekkenhorst, G. The clinical and biochemical features of variegate porphyria: An analysis of 300 cases studied at Groote Schuur hospital, Cape Town. Int. J. Biochem. 1980, 12, 837–853. [Google Scholar] [CrossRef]
- Campbell, J.A. The pathology of South African genetic porphyria. S. Afr. J. Lab. Clin. Med. 1963, 14, 197–203. [Google Scholar]
- Szlendak, U.; Bykowska, K.; Lipniacka, A. Clinical, biochemical and molecular characteristics of the main types of porphyria. Adv. Clin. Exp. Med. 2016, 25, 361–368. [Google Scholar] [CrossRef]
- Woods, J. Regulation of porphyrin and heme metabolism in the kidney. Semin Hematol. 1988, 25, 336–348. [Google Scholar]
- Day, R.; Eales, L.; Disler, P. Porphyrias and the kidney. Nephron 1981, 28, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Day, R.; Eales, L. Porphyrins in chronic renal failure. Nephron 1980, 26, 90–95. [Google Scholar] [CrossRef]
- Day, R.; Eales, L. Porphyrins in renal transplantation. Nephron 1982, 30, 22–27. [Google Scholar] [CrossRef]
- Martásek, P.; Jirsa, M.; Kordaˇc, V. Role of the kidneys in porphyrias. Nephron 1982, 32, 277–278. [Google Scholar] [CrossRef]
- Day, R.; Blekkenhorst, G.; Eales, L. Hepatic porphyrins in variegate porphyria. N. Eng. J. Med. 1980, 303, 1368–1369. [Google Scholar]
- Andersson, C.; Lithner, F. Hypertension and renal disease in patients with acute intermittent porphyria. J. Intern. Med. 1994, 236, 169–175. [Google Scholar] [CrossRef]
- Church, S.; Moore, M.; Youngs, G. Hypertension and renal impairment as complications of acute porphyria. Nephrol. Dial. Transplant. 1992, 7, 986–990. [Google Scholar]
- Laiwah, A.A.Y.; Mactier, R.; McColl, K.E.; Moore, M.R.; Goldberg, A. Early-onset chronic renal failure as a complication of acute intermittent porphyria. QJM 1983, 52, 92–98. [Google Scholar]
- Whitelaw, A. Acute intermittent porphyria, hypercholesterolaemia, and renal impairment. Arch. Dis. Child. 1974, 49, 406. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andersson, C.; Wikberg, A.; Stegmayr, B.; Lithner, F. Renal symptomatology in patients with acute intermittent porphyria. a population-based study. J. Intern. Med. 2000, 248, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Tchernitchko, D.; Tavernier, Q.; Lamoril, J.; Schmitt, C.; Talbi, N.; Lyoumi, S.; Robreau, A.M.; Karim, Z.; Gouya, L.; Thervet, E.; et al. A variant of peptide transporter 2 predicts the severity of porphyria-associated kidney disease. J. Am. Soc. Nephrol. 2017, 28, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Shen, H.; Teuscher, N.S.; Lorenzi, P.J.; Keep, R.F.; Smith, D.E. Role of PEPT2 in peptide/mimetic trafficking at the blood-cerebrospinal fluid barrier: Studies in rat choroid plexus epithelial cells in primary culture. J. Pharmacol. Exp. Ther. 2002, 301, 820–829. [Google Scholar] [CrossRef]
- Sobin, C.; Gutierrez, M.; Alterio, H. Polymorphisms of delta-aminolevulinic acid dehydratase (ALAD) and peptide transporter 2 (PEPT2) genes in children with low-level lead exposure. Neurotoxicology 2009, 30, 881–887. [Google Scholar] [CrossRef]
- Sobin, C.; Flores-Montoya, M.G.; Gutierrez, M.; Parisi, N.; Schaub, T. δ-aminolevulinic acid dehydratase single nucleotide polymorphism 2 (ALAD2) and peptide transporter 2*2 haplotype (hPEPT2*2) differently influence neurobehavior in low-level lead exposed children. Neurotoxicol. Teratol. 2015, 47, 137–145. [Google Scholar] [CrossRef]
- Knu¨tter, I.; Kottra, G.; Fischer, W.; Daniel, H.; Brandsch, M. High-affinity interaction of sartans with H+/peptide transporters. Drug Metab. Dispos. 2009, 37, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.E.M.; Dutra, F.; Bandy, B.; Baldini, R.L.; Gomes, S.L.; Faljoni-Alário, A.; Liria, C.W.; Miranda, M.T.M.; Bechara, E.J.H. Oxidative damage to ferritin by 5-aminolevulinic acid. Arch. Biochem. Biophys. 2003, 409, 349–356. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Bechara, E.J. 5-Aminolevulinic acid induces lipid peroxidation in cardiolipin-rich liposomes. Arch. Biochem. Biophys. 1993, 305, 282–287. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Castilho, R.F.; Valle, V.G.; Bechara, E.J.; Vercesi, A.E. Calcium-dependent mitochondrial oxidative damage promoted by 5-aminolevulinic acid. Biochim. Biophys. Acta Mol. Basis Dis. 1992, 1180, 201–206. [Google Scholar] [CrossRef]
- Vercesi, A.E.; Castilho, R.F.; Meinicke, A.R.; Valle, V.G.; Hermes-Lima, M.; Bechara, E.J. Oxidative damage of mitochondria induced by 5-aminolevulinic acid: Role of Ca2+ and membrane protein thiols. Biochim. Biophys. Acta 1994, 1188, 86–92. [Google Scholar] [CrossRef]
- Laafi, J.; Homedan, C.; Jacques, C.; Gueguen, N.; Schmitt, C.; Puy, H.; Reynier, P.; Carmen Martinez, M.; Malthièery, Y. Pro-oxidant effect of ALA is implicated in mitochondrial dysfunction of HepG2 cells. Biochimie 2014, 106, 157–166. [Google Scholar] [CrossRef]
- Mydlík, M.; Derzsiová, K. Kidney damage in acute intermittent porphyria. Prz. Lek. 2011, 68, 610–613. [Google Scholar]
- Onozato, M.; Tojo, A.; Kamijo, A.; Taniguchi, S.; Kimura, K.; Goto, A.; Fujita, T. Tubulointerstitial nephritis associated with acute intermittent porphyria. Clin. Nephrol. 2001, 55, 171–174. [Google Scholar] [PubMed]
- Marsden, J.; Chowdhury, P.; Wang, J.; Deacon, A.; Dutt, N.; Peters, T.; Macdougall, I. Acute intermittent porphyria and chronic renal failure. Clin. Nephrol. 2008, 69, 339–346. [Google Scholar] [CrossRef]
- Schley, G.; Bock, K.; Debusmann, E.; Hocevar, V.; Merguet, P.; Paar, D.; Rausch-Stroomann, J. Untersuchungen über die nierenfunktion bei der akuten intermittierenden porphyrie. Klin. Wochenschr. 1970, 48, 616–623. [Google Scholar] [CrossRef]
- Schmid, R.; Schwartz, S.; Watson, C.J. Porphyrin content of bone marrow and liver in the various forms of porphyria. Arch. Intern. Med. 1954, 93, 167–190. [Google Scholar] [CrossRef]
- Prunty, F. Sodium and chloride depletion in acute porphyria with reference to the status of adrenal cortical function. J. Clin. Investig. 1949, 28, 690–699. [Google Scholar] [CrossRef]
- Maitra, D.; Cunha, J.B.; Elenbaas, J.S.; Bonkovsky, H.L.; Shavit, J.A.; Omary, M.B. Porphyrin-induced protein oxidation and aggregation as a mechanism of porphyria-associated cell injury. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Maitra, D.; Carter, E.L.; Richardson, R.; Rittié, L.; Basrur, V.; Zhang, H.; Nesvizhskii, A.I.; Osawa, Y.; Wolf, M.W.; Ragsdale, S.W.; et al. Oxygen and conformation dependent protein oxidation and aggregation by porphyrins in hepatocytes and light-exposed cells. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 659–682. [Google Scholar] [CrossRef] [PubMed]
- Maitra, D.; Pinsky, B.M.; Soherwardy, A.; Zheng, H.; Banerjee, R.; Omary, B. Protoporphyrin-IX nanostructures modulate their protein aggregation ability via differential oxidation and protein binding. bioRxiv 2021. [Google Scholar] [CrossRef]
- Singla, A.; Griggs, N.W.; Kwan, R.; Snider, N.T.; Maitra, D.; Ernst, S.A.; Herrmann, H.; Omary, M.B. Lamin aggregation is an early sensor of porphyria-induced liver injury. J. Cell Sci. 2013, 126, 3105–3112. [Google Scholar] [CrossRef]
- Maitra, D.; Elenbaas, J.S.; Whitesall, S.E.; Basrur, V.; D’Alecy, L.G.; Omary, M.B. Ambient light promotes selective subcellular proteotoxicity after endogenous and exogenous porphyrinogenic stress. J. Biol. Chem. 2015, 290, 23711–23724. [Google Scholar] [CrossRef]
- Morehouse, K.M.; Moreno, S.N.; Mason, R.P. The one-electron reduction of uroporphyrin I by rat hepatic microsomes. Arch. Biochem. Biophys. 1987, 257, 276–284. [Google Scholar] [CrossRef]
- Morehouse, K.M.; Mason, R.P. The enzymatic one-electron reduction of por phyrins to their anion free radicals. Arch. Biochem. Biophys. 1990, 283, 306–310. [Google Scholar] [CrossRef]
- Unzu, C.; Sampedro, A.; Sardh, E.; Mauleón, I.; De Salamanca, R.E.; Prieto, J.; Salido, E.; Harper, P.; Fontanellas, A. Renal failure affects the enzymatic activities of the three first steps in hepatic heme biosynthesis in the acute intermittent porphyria mouse. PLoS ONE 2012, 7, e32978. [Google Scholar] [CrossRef][Green Version]
- Williams, H.E.; Smith, L.H., Jr. Disorders of oxalate metabolism. Am. J. Med. 1968, 45, 715–735. [Google Scholar] [CrossRef]
- Balcke, P. Pyridoxine therapy in patients with renal calcium oxalate calculi. Proc. Eur. Dial. Transplant. Assoc. 1983, 20, 417–421. [Google Scholar]
- Hoppe, B. An update on primary hyperoxaluria. Nat. Rev. Nephrol. 2012, 8, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Vitamin B6 intake and the risk of incident kidney stones. Urolithiasis 2018, 46, 265–270. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. A prospective study of the intake of vitamins C and B6, and the risk of kidney stones in men. J. Urol. 1996, 155, 1847–1851. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Speizer, F.E.; Stampfer, M.J. Intake of vitamins B6 and C and the risk of kidney stones in women. J. Am. Soc. Nephrol. 1999, 10, 840–845. [Google Scholar] [CrossRef]
- Hamfelt, A.; Wetterberg, L. Pyridoxal phosphate in acute intermittent porphyria. Ann. N. Y. Acad. Sci. 1969, 166, 361–364. [Google Scholar] [CrossRef]
- Ventura, P.; Marcacci, M.; Marchini, S.; Cuoghi, C.; Vaccari, D.; Pietrangelo, A. Is poor vitamin status a reliable target for treatment of symptomatic patients with hepatic acute porphyrias? Dig. Liver Dis. 2019, 51, e23–e24. [Google Scholar] [CrossRef]
- Lindberg, R.L.; Porcher, C.; Grandchamp, B.; Ledermann, B.; Bu¨rki, K.; Brandner, S.; Aguzzi, A.; Meyer, U.A. Porphobilinogen deaminase deficiency in mice causes a neuropathy resembling that of human hepatic porphyria. Nat. Genet. 1996, 12, 195–199. [Google Scholar] [CrossRef]
- Sardh, E.; Andersson, D.; Henrichson, A.; Harper, P. Porphyrin precursors and porphyrins in three patients with acute intermittent porphyria and end-stage renal disease under different therapy regimes. Cell. Mol. Biol. 2015, 55, 66–71. [Google Scholar]
- Gill, R.; Kolstoe, S.E.; Mohammed, F.; Al D-Bass, A.; Mosely, J.E.; Sarwar, M.; Cooper, J.B.; Wood, S.P.; Shoolingin-Jordan, P.M. Structure of human porphobilinogen deaminase at 2.8 å: The molecular basis of acute intermittent porphyria. Biochem. J. 2009, 420, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Shoolingin-Jordan, P.; Al-Dbass, A.; McNeill, L.; Sarwar, M.; Butler, D. Human porphobilinogen deaminase mutations in the investigation of the mechanism of dipyrromethane cofactor assembly and tetrapyrrole formation. Biochem. Soc. Trans. 2003, 31, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.T.; Smith, A.; Koskelo, P. The interaction of human serum albumin and hemopexin with porphyrins. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1980, 624, 271–285. [Google Scholar] [CrossRef]
- Lazareth, H.; Talbi, N.; Kamar, N.; Levi, C.; Moulin, B.; Caillard, S.; Frimat, L.; Chemouny, J.; Chatelet, V.; Vachey, C.; et al. Kidney transplantation improves the clinical outcomes of acute intermittent porphyria. Mol. Genet. Metab. 2020, 131, 259–266. [Google Scholar] [CrossRef]
- Carson, R.W.; Dunnigan, E.J.; DuBose, T.D.; Goeger, D.E.; Anderson, K.E. Removal of plasma porphyrins with high-flux hemodialysis in porphyria cutanea tarda associated with end-stage renal disease. J. Am. Soc. Nephrol. 1992, 2, 1445–1450. [Google Scholar] [CrossRef]
- Fontanellas, A.; Herrero, J.A.; Moran, M.J.; Coronel, F.; Sepulveda, P.; Barrientos, A.; De Salamanca, R.E. Efficiency of three different hemodialysis membranes for plasma porphyrin removal. Am. J. Kidney Dis. 1995, 25, 30–33. [Google Scholar] [CrossRef]
- Østergaard, M.G.; Erlandsen, E.J.; Thomsen, H.H.; Randers, E. Peritoneal dialysis resulting in discontinuance of recurring attacks of acute intermittent por phyria: A case report. Semin. Dial. 2021. [Google Scholar] [CrossRef]
- Nunez, D.; Williams, P.; Herrick, A.; Evans, D.; McColl, K. Renal transplantation for chronic renal failure in acute porphyria. Nephrol. Dial. Transplant. 1987, 2, 271–274. [Google Scholar]
- Barone, G.W.; Gurley, B.J.; Anderson, K.E.; Ketel, B.L.; Abul-Ezz, S.R. The tolerability of newer immunosuppressive medications in a patient with acute intermittent porphyria. J. Clin. Pharmacol. 2001, 41, 113–115. [Google Scholar] [CrossRef]
- El Haggan, W.; Lobbedez, T.; Ryckelynck, J.P.; de Ligny, B.H. Sirolimus tolerability in a kidney transplant recipient with acute intermittent porphyria. Nephrol. Dial. Transplant. 2002, 17, 1147. [Google Scholar] [CrossRef] [PubMed]
- Wahlin, S.; Harper, P.; Sardh, E.; Andersson, C.; Andersson, D.E.; Ericzon, B.G. Combined liver and kidney transplantation in acute intermittent porphyria. Transpl. Int. 2010, 23, e18–e21. [Google Scholar] [CrossRef]
- Ferreira, G.d.S.A.; de Oliveira, L.C.; de Sousa Ulisses, L.R.; Watanabe, A.L.C.; Medeiros, I.N.; Cardoso, H.S.S.; da Costa Alves, I.C.; de Almeida, T.M.; de Lima, L.V.; Fontoura, R.P.; et al. Combined liver and kidney transplant in acute inter mittent porphyria: A case report. Am. J. Med. Case Rep. 2020, 21, e927832-1. [Google Scholar] [CrossRef]
- NAPOS. The Drug Database for Acute Porphyria—Sulfamethoxazole and Trimethoprim. Available online: http://www.drugs-porphyria.org/monograph2.php?id=2606 (accessed on 11 November 2021).
- Gomá-Garcés, E.; Pérez-Gomez, M.V.; Ortíz, A. Givosiran for acute intermittent porphyria. N. Engl. J. Med. 2020, 383, 1989. [Google Scholar] [CrossRef]
- Lazareth, H.; Poli, A.; Bignon, Y.; Mirmiran, A.; Rabant, M.; Schmitt, C.; Puy, H.; Karras, A.; Gouya, L.; Pallet, N.; et al. Renal function decline with small interfering RNA silencing ALAS1. Kidney Int. Rep. 2021, 6, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Tracz, M.J.; Alam, J.; Nath, K.A. Physiology and pathophysiology of heme: Implications for kidney disease. J. Am. Soc. Nephrol. 2007, 18, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, D.; Sardh, E.; Harper, P.; Najafian, N.; Simon, A.; Burke, A.; Kim, J.; Garg, P.; Robbie, G.; Agarwal, S. A drug-drug interaction study to investigate the effect of givosiran on the activity of 5 major drug metabolizing CYP450 enzymes in subjects with acute intermittent porphyria who are chronic high excreters. In Proceedings of the 2019 International Congress on Porphyrins and Porphyrias, Milan, Italy, 8–11 September 2019. [Google Scholar]
- Lavandera, J.; Rodríguez, J.; Ruspini, S.; Meiss, R.; Zuccoli, J.R.; Martínez, M.D.C.; Gerez, E.; Batlle, A.; Buzaleh, A.M. Pleiotropic effects of 5-aminolevulinic acid in mouse brain. Biochem. Cell Biol. 2016, 94, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Soong, J.; Adams, M.A.; Nakatsu, K. Acute depletion of heme by succinylace- tone alters vascular responses but does not induce hypertension. Can. J. Physiol. Pharmacol. 2008, 86, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Bourque, S.L.; Benjamin, C.D.; Adams, M.A.; Nakatsu, K. Lack of hemody namic effects after extended heme synthesis inhibition by succinylacetone in rats. J. Pharmacol. Exp. Ther. 2010, 333, 290–296. [Google Scholar] [CrossRef]
- To-Figueras, J.; Wijngaard, R.; Garcí a-Villoria, J.; Aarsand, A.K.; Aguilera, P.; Deulofeu, R.; Brunet, M.; Gómez-Gómez, À.; Pozo, O.J.; Sandberg, S. Dysregulation of homocysteine homeostasis in acute intermittent porphyria patients receiving heme arginate or givosiran. J. Inherit. Metab. Dis. 2021, 44, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Marcacci, M.; Cuoghi, C.; Pietrangelo, A.; Ventura, P. Hyperhomocysteinemia in patients with acute porphyrias: A possible effect of ALAS1 modulation by siRNAm therapy and its control by vitamin supplementation. Eur. J. Intern. Med. 2021, 92, 121–123. [Google Scholar] [CrossRef]
- Petrides, P.E.; Klein, M.; Schuhmann, E.; Torkler, H.; Molitor, B.; Loehr, C.; Obermeier, Z.; Beykirch, M.K. Severe homocysteinemia in two givosiran treated porphyria patients: Is free heme deficiency the culprit? Ann. Hematol. 2021, 100, 1685–1693. [Google Scholar] [CrossRef]
- Ricci, A.; Di Pierro, E.; Marcacci, M.; Ventura, P. Mechanisms of Neuronal Damage in Acute Hepatic Porphyrias. Diagnostics 2021, 11, 2205. [Google Scholar] [CrossRef]
- Yasuda, M.; Desnick, R.J. Murine models of the human porphyrias: Contributions toward understanding disease pathogenesis and the development of new therapies. Mol. Genet. Metab. 2019, 128, 332–341. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, A.; Guida, C.C.; Manzini, P.; Cuoghi, C.; Ventura, P. Kidney Involvement in Acute Hepatic Porphyrias: Pathophysiology and Diagnostic Implications. Diagnostics 2021, 11, 2324. https://doi.org/10.3390/diagnostics11122324
Ricci A, Guida CC, Manzini P, Cuoghi C, Ventura P. Kidney Involvement in Acute Hepatic Porphyrias: Pathophysiology and Diagnostic Implications. Diagnostics. 2021; 11(12):2324. https://doi.org/10.3390/diagnostics11122324
Chicago/Turabian StyleRicci, Andrea, Claudio Carmine Guida, Paola Manzini, Chiara Cuoghi, and Paolo Ventura. 2021. "Kidney Involvement in Acute Hepatic Porphyrias: Pathophysiology and Diagnostic Implications" Diagnostics 11, no. 12: 2324. https://doi.org/10.3390/diagnostics11122324
APA StyleRicci, A., Guida, C. C., Manzini, P., Cuoghi, C., & Ventura, P. (2021). Kidney Involvement in Acute Hepatic Porphyrias: Pathophysiology and Diagnostic Implications. Diagnostics, 11(12), 2324. https://doi.org/10.3390/diagnostics11122324





