A Scoping Review of Supply Chain Management Systems for Point of Care Diagnostic Services: Optimising COVID-19 Testing Capacity in Resource-Limited Settings
Abstract
:1. Background
2. Methodology
2.1. Identification of the Research Question
2.2. Identification of Relevant Studies
2.3. Selection of Eligible Articles
2.3.1. Inclusion Criteria
- Articles reporting evidence on SCM systems of all diseases
- Articles reporting evidence of SCM systems for all POC diagnostics services at all levels of the healthcare continuum
- Articles reporting evidence of primary studies conducted in LMICs
- All reviews providing evidence of SCM systems for all POC diagnostic services
- Articles published since inception
2.3.2. Exclusion Criteria
- Articles that lacked evidence on SCM systems for all POC diagnostics services
- Articles reporting SCM systems of laboratory-based POC diagnoses
- Articles reporting evidence of primary studies conducted in high-income countries
2.4. Selection of Sources of Evidence
2.5. Charting the Data
2.6. Collating, Summarizing and Reporting the Results
2.7. Quality Appraisal
2.8. Ethical Considerations
3. Results
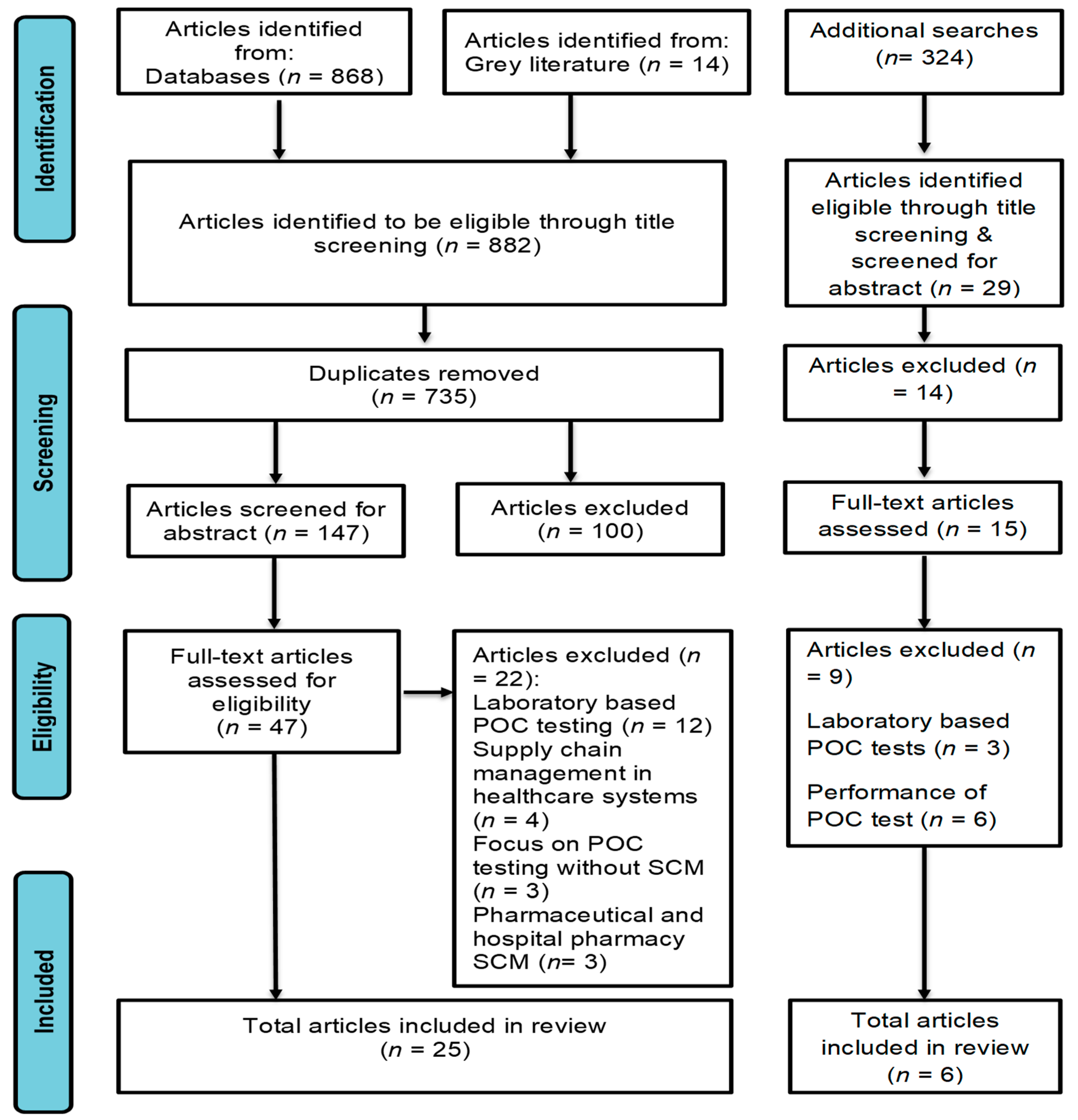
3.1. Screening Results
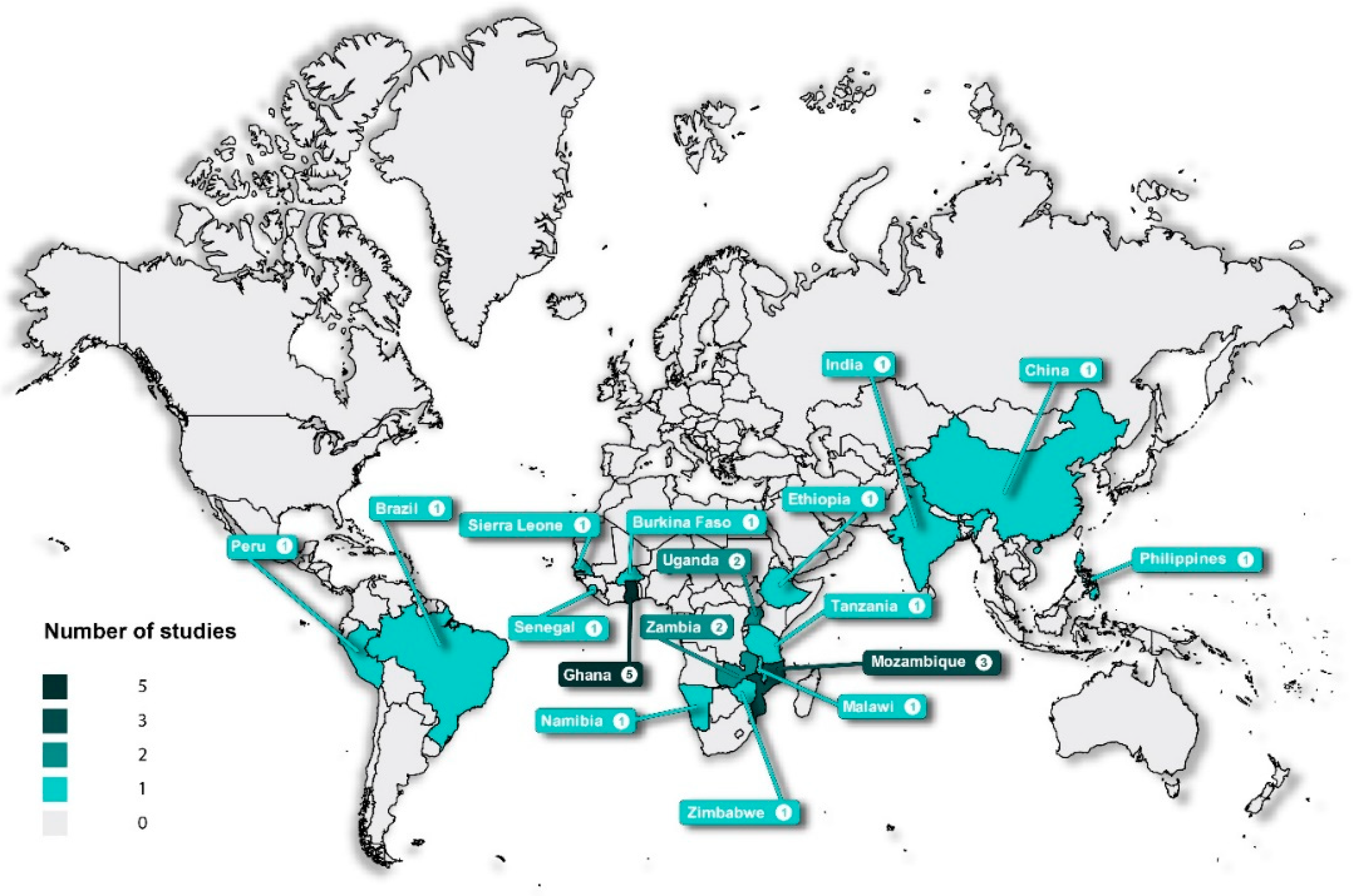
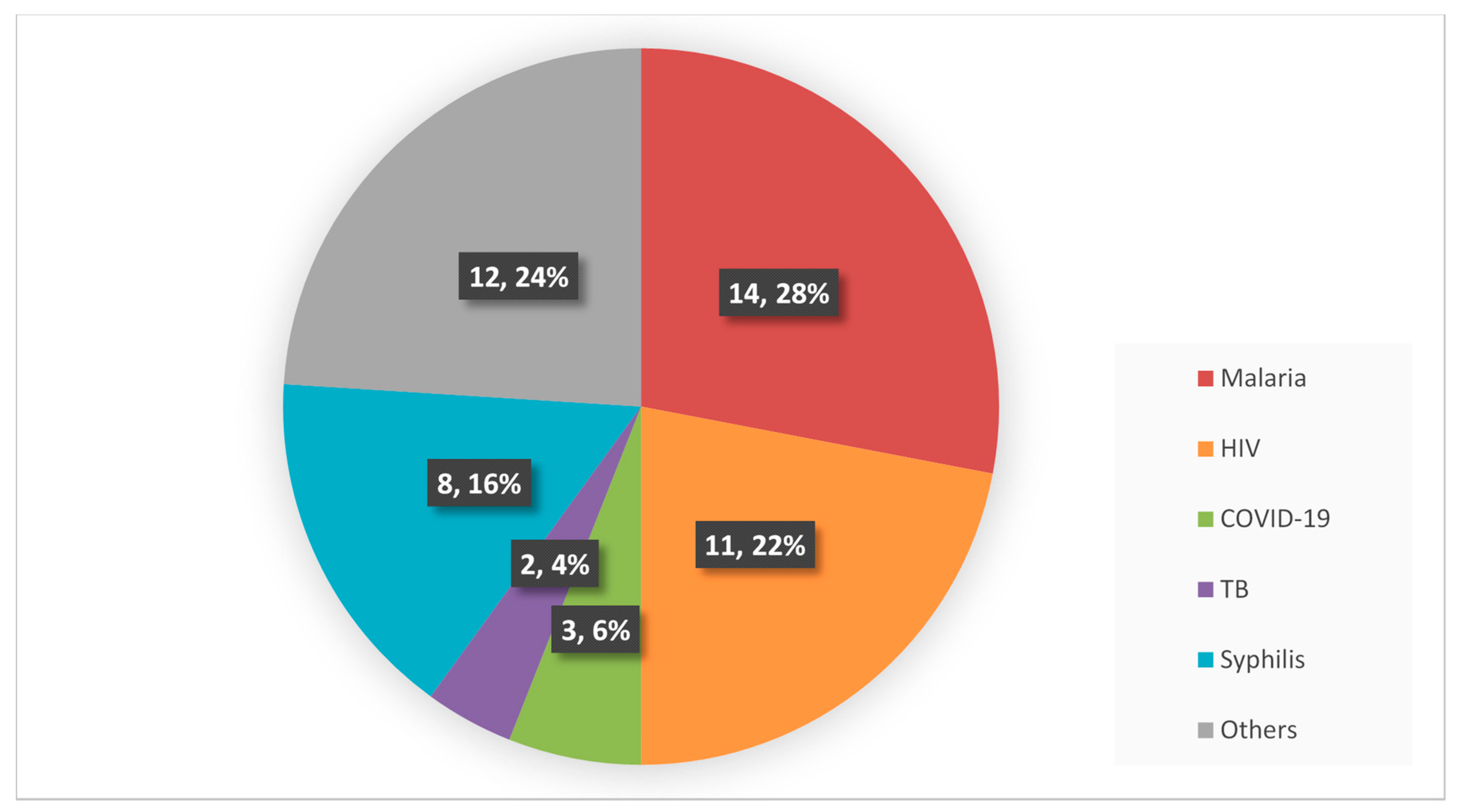
3.2. Characteristics of the Included Articles
3.3. Quality of Evidence
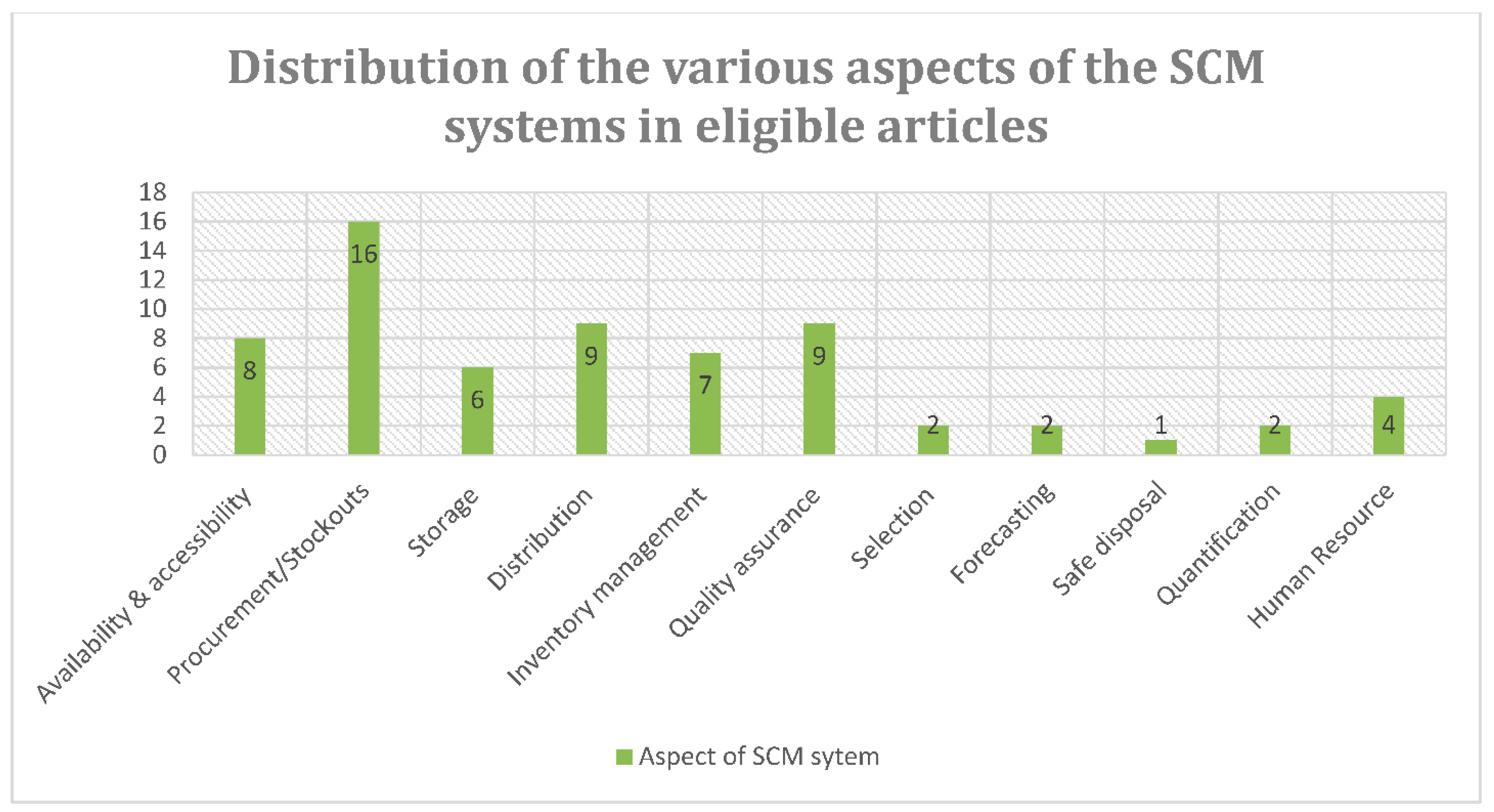
3.4. Main Findings
3.4.1. Accessibility and Availability of POC Diagnostics Services
3.4.2. Reasons for Stockouts of POC Diagnostic Tests
- Procurement of POC diagnostic tests
- Inventory management of POC diagnostic tests
- Storage of POC diagnostic tests
- Distribution of POC diagnostic tests
- Quality assurance of POC diagnostic tests
3.4.3. Human Resource Capacity in POC Diagnostic Services
4. Discussion
4.1. Implications for Practice
4.2. Implications for Research
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus type 2 |
| RT-PCR | Reverse transcription polymerase chain reaction |
| COVID-19 | Coronavirus 19 |
| WHO | World Health Organisation |
| POC | Point of care |
| SCM | Supply chain management |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping review |
| MMAT | Mixed method appraisal tool |
References
- National Department of Health. COVID-19 Antigen Testing Guidelines. Available online: https://www.nicd.ac.za/wp-content/uploads/2020/12/COVID-19-Antigen-Testing-Guidelines.pdf (accessed on 18 May 2021).
- Yan, Y.; Chang, L.; Wang, L. Laboratory testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): Current status, challenges, and countermeasures. Rev. Med. Virol. 2020, 30, e2106. [Google Scholar] [CrossRef]
- Juul, J.L.; Græsbøll, K. Are fast test results preferable to high test sensitivity in contact-tracing strategies? medRxiv 2021. [Google Scholar] [CrossRef]
- Ricco, M.; Ferraro, P.; Gualerzi, G.; Ranzieri, S.; Henry, B.M.; Said, Y.B.; Pyatigorskaya, N.V.; Nevolina, E.; Wu, J.; Bragazzi, N.L.; et al. Point-of-Care Diagnostic Tests for Detecting SARS-CoV-2 Antibodies: A Systematic Review and Meta-Analysis of Real-World Data. J. Clin. Med. 2020, 9, 1515. [Google Scholar] [CrossRef]
- Peeling, R.W.; Mabey, D. Point-of-care tests for diagnosing infections in the developing world. Clin. Microbiol. Infect. 2010, 16, 1062–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahamtan, A.; Ardebili, A. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, A.; Faron, M.L.; Yu, P.; Markham, C.; Wu, M.; Wong, S. A Rapid SARS-CoV-2 RT-PCR Assay for Low Resource Settings. Diagnostics 2020, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.P.; Papenburg, J.; Desjardins, M.; Kanjilal, S.; Quach, C.; Libman, M.; Dittrich, S.; Yansouni, C.P. Diagnostic Testing for Severe Acute Respiratory Syndrome-Related Coronavirus 2: A Narrative Review. Ann. Intern. Med. 2020, 172, 726–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeling, R.W.; Wedderburn, C.J.; Garcia, P.J.; Boeras, D.; Fongwen, N.; Nkengasong, J.; Sall, A.; Tanuri, A.; Heymann, D.L. Serology testing in the COVID-19 pandemic response. Lancet Infect. Dis. 2020, 20, e245–e249. [Google Scholar] [CrossRef]
- Kuupiel, D.; Bawontuo, V.; Mashamba-Thompson, T.P. Improving the Accessibility and Efficiency of Point-of-Care Diagnostics Services in Low- and Middle-Income Countries: Lean and Agile Supply Chain Management. Diagnostics 2017, 7, 58. [Google Scholar] [CrossRef] [Green Version]
- Lone, S.A.; Ahmad, A. COVID-19 pandemic—An African perspective. Emerg. Microbes Infect. 2020, 9, 1300–1308. [Google Scholar] [CrossRef]
- Kuupiel, D.; Bawontuo, V.; Drain, P.K.; Gwala, N.; Mashamba-Thompson, T.P. Supply chain management and accessibility to point-of-care testing in resource-limited settings: A systematic scoping review. BMC Health Serv. Res. 2019, 19, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, J.R. COVID-19: Implications for Supply Chain Management. Front. Health Serv. Manag. 2020, 37, 33–38. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Manual for Procurement of Diagnostics and Related Laboratory Items and Equipment. Available online: https://www.who.int/diagnostics_laboratory/procurement/130627_manual_for_procurement_of_diagnostics-001-june2013.pdf (accessed on 27 May 2021).
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, J.; Straus, S.; Moher, D.; Langlois, E.V.; O’Brien, K.K.; Horsley, T.; Aldcroft, A.; Zarin, W.; Garitty, C.M.; Hempel, S.; et al. Reporting scoping reviews-PRISMA ScR extension. J. Clin. Epidemiol. 2020, 123, 177–179. [Google Scholar] [CrossRef]
- Pinna, R.; Carrus, P.P.; Marras, F. Emerging Trends in Healthcare Supply Chain Management—An Italian Experience. In Applications of Contemporary Management Approaches in Supply Chains; IntechOpen: London, UK, 2015. [Google Scholar]
- Bethel, A.C.; Rogers, M.; Abbott, R. Use of a search summary table to improve systematic review search methods, results, and efficiency. J. Med. Libr. Assoc. 2021, 109, 97–106. [Google Scholar] [CrossRef]
- Hong, Q.N.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; O’Cathain, A.; et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ. Inf. 2018, 34, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Kuupiel, D.; Bawontuo, V.; Donkoh, A.; Drain, P.K.; Mashamba-Thompson, T.P. Empirical Framework for Point-of-Care Diagnostics Supply Chain Management for Accessibility and Sustainability of Diagnostic Services in Ghana’s Primary Health Care Clinics. Point Care 2019, 18, 72–75. [Google Scholar] [CrossRef]
- Kuupiel, D.; Tlou, B.; Bawontuo, V.; Drain, P.K.; Mashamba-Thompson, T.P. Poor supply chain management and stock-outs of point-of-care diagnostic tests in Upper East Region’s primary healthcare clinics, Ghana. PLoS ONE 2019, 14, e0211498. [Google Scholar] [CrossRef]
- Peeling, R.W. Diagnostics in a digital age: An opportunity to strengthen health systems and improve health outcomes. Int. Health 2015, 7, 384–389. [Google Scholar] [CrossRef] [Green Version]
- Stevens, W.; Gous, N.; Ford, N.; Scott, L.E. Feasibility of HIV point-of-care tests for resource-limited settings: Challenges and solutions. BMC Med. 2014, 12, 173. [Google Scholar] [CrossRef] [Green Version]
- Alemnji, G.; Nkengasong, J.N.; Parekh, B.S. HIV testing in developing countries: What is required? Indian J. Med Res. 2011, 134, 779–786. [Google Scholar] [CrossRef]
- Alemnji, G.; Peter, T.; Vojnov, L.; Alexander, H.; Zeh, C.; Cohn, J.; Watts, D.H.; de Lussigny, S. Building and Sustaining Optimized Diagnostic Networks to Scale-up HIV Viral Load and Early Infant Diagnosis. J. Acquir. Immune Defic. Syndr. 2020, 84, S56–S62. [Google Scholar] [CrossRef]
- Valera, E.; Jankelow, A.; Lim, J.; Kindratenko, V.; Ganguli, A.; White, K.; Kumar, J.; Bashir, R. COVID-19 Point-of-Care Diagnostics: Present and Future. ACS Nano 2021, 15, 7899–7906. [Google Scholar] [CrossRef] [PubMed]
- Benda, A.; Zerajic, L.; Ankita, A.; Cleary, E.; Park, Y.; Pandey, S. COVID-19 Testing and Diagnostics: A Review of Commercialized Technologies for Cost, Convenience and Quality of Tests. Sensors 2021, 21, 6581. [Google Scholar] [CrossRef]
- Poole, S.; Townsend, J.; Wertheim, H.; Kidd, S.P.; Welte, T.; Schuetz, P.; Luyt, C.E.; Beishuizen, A.; Jensen, J.S.; Del Castillo, J.G.; et al. How are rapid diagnostic tests for infectious diseases used in clinical practice: A global survey by the International Society of Antimicrobial Chemotherapy (ISAC). Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nehra, M.; Khurana, S.; Dilbaghi, N.; Kumar, V.; Kaushik, A.; Kim, K.H. Aspects of Point-of-Care Diagnostics for Personalized Health Wellness. Int. J. Nanomed. 2021, 16, 383–402. [Google Scholar] [CrossRef]
- Fleming, K.A.; Horton, S.; Wilson, M.L.; Atun, R.; Destigter, K.; Flanigan, J.; Sayed, S.; Adam, P.; Aguilar, B.; Andronikou, S.; et al. The Lancet Commission on diagnostics: Transforming access to diagnostics. Lancet 2021. [Google Scholar] [CrossRef]
- Bristow, C.C.; Larson, E.; Javanbakht, M.; Huang, E.; Causer, L.; Klausner, J.D. A review of recent advances in rapid point-of-care tests for syphilis. Sexual Health 2015, 12, 119–125. [Google Scholar] [CrossRef] [PubMed]
- WHO. COVID-19 Supply Chain System Procurement Considerations for COVID-19 Diagnostics. Available online: https://www.who.int/docs/default-source/coronaviruse/procurement-considerations-for-covid-19-diagnostics.pdf?sfvrsn=70a480ce_16 (accessed on 30 October 2021).
- Betran, A.P.; Bergel, E.; Griffin, S.; Melo, A.; Nguyen, M.H.; Carbonell, A.; Mondlane, S.; Merialdi, M.; Temmerman, M.; Gulmezoglu, A.M.; et al. Provision of medical supply kits to improve quality of antenatal care in Mozambique: A stepped-wedge cluster randomised trial. Lancet Glob. Health 2018, 6, E57–E65. [Google Scholar] [CrossRef] [Green Version]
- Hamer, D.H.; Brooks, E.T.; Semrau, K.; Pilingana, P.; Macleod, W.B.; Siazeele, K.; Sabin, L.L.; Thea, D.M.; Yeboah-Antwi, K. Quality and safety of integrated community case management of malaria using rapid diagnostic tests and pneumonia by community health workers. Pathog. Glob. Health 2012, 106, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Wahlfeld, C.C.; Muicha, A.; Harrison, P.; Kipp, A.M.; Claquin, G.; Silva, W.P.; Green, A.F.; Wester, C.W.; Moon, T.D. HIV Rapid Diagnostic Test Inventories in Zambézia Province, Mozambique: A Tale of 2 Test Kits. Int. J. Health Policy Manag. 2019, 8, 292–299. [Google Scholar] [CrossRef]
- Ekambaram, R.; Gomanie, N.; Mehta, K. Analysis of Failure Modes: Case study of Ruggedizing a low-cost Screening Technology in Sub-Saharan Africa. In Proceedings of the 2019 IEEE Global Humanitarian Technology Conference, Seattle, WA, USA, 17–20 October 2019; IEEE: New York, NY, USA, 2019; pp. 313–317. [Google Scholar]
- Albertini, A.; Lee, E.; Coulibaly, S.O.; Sleshi, M.; Faye, B.; Mationg, M.L.; Ouedraogo, K.; Tsadik, A.G.; Feleke, S.M.; Diallo, I.; et al. Malaria rapid diagnostic test transport and storage conditions in Burkina Faso, Senegal, Ethiopia and the Philippines. Malar. J. 2012, 11, 406. [Google Scholar] [CrossRef] [Green Version]
- Mabey, D.C.; Sollis, K.A.; Kelly, H.A.; Benzaken, A.S.; Bitarakwate, E.; Changalucha, J.; Chen, X.-S.; Yin, Y.-P.; Garcia, P.J.; Strasser, S.; et al. Point-of-Care Tests to Strengthen Health Systems and Save Newborn Lives: The Case of Syphilis. PLoS Med. 2012, 9, e1001233. [Google Scholar] [CrossRef] [Green Version]
- Palmer, T.; Aiyenigba, A.O.; Bates, I.; Okyere, D.D.; Tagbor, H.; Ampofo, G.D. Improving the effectiveness of point of care tests for malaria and anaemia: A qualitative study across three Ghanaian antenatal clinics. BMC Health Serv. Res. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Magesa, E.S.; Mitonga, K.H.; Angula, P. Factors associated with stock out of malaria test kit in Oshana Region, Namibia. J. Public Health Afr. 2019, 10, 137–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddox, B.L.P.; Wright, S.S.; Namadingo, H.; Bowen, V.B.; Chipungu, G.A.; Kamb, M.L. Assessing stakeholder perceptions of the acceptability and feasibility of national scale-up for a dual HIV/syphilis rapid diagnostic test in Malawi. Sex. Transm. Infect. 2017, 93, S59–S64. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.A.; Dandona, L.; Schellenberg, D. Public health system readiness to treat malaria in Odisha State of India. Malar. J. 2013, 12, 351. [Google Scholar] [CrossRef] [Green Version]
- Hasselback, L.; Crawford, J.; Chaluco, T.; Rajagopal, S.; Prosser, W.; Watson, N. Rapid diagnostic test supply chain and consumption study in Cabo Delgado, Mozambique: Estimating stock shortages and identifying drivers of stock-outs. Malar. J. 2014, 13, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dassah, E.T.; Adu-Sarkodie, Y.; Mayaud, P. Rollout of rapid point of care tests for antenatal syphilis screening in Ghana: Healthcare provider perspectives and experiences. BMC Health Serv. Res. 2018, 18, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asiimwe, C.; Kyabayinze, D.J.; Kyalisiima, Z.; Nabakooza, J.; Bajabaite, M.; Counihan, H.; Tibenderana, J.K. Early experiences on the feasibility, acceptability, and use of malaria rapid diagnostic tests at peripheral health centres in Uganda-insights into some barriers and facilitators. Implement. Sci. 2012, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Blanas, D.A.; Ndiaye, Y.; Nichols, K.; Jensen, A.; Siddiqui, A.; Hennig, N. Barriers to community case management of malaria in Saraya, Senegal: Training, and supply-chains. Malaria J. 2013, 12, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boadu, N.Y.; Amuasi, J.; Ansong, D.; Einsiedel, E.; Menon, D.; Yanow, S.K. Challenges with implementing malaria rapid diagnostic tests at primary care facilities in a Ghanaian district: A qualitative study. Malaria J. 2016, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, B.; Cunningham, B.; Boeras, D.I.; Mafaune, P.; Simbi, R.; Peeling, R.W. Data connectivity: A critical tool for external quality assessment. Afr. J. Lab. Med. 2016, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- United Nations. The 17 Goals Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 18 May 2021).
- FIND. Diagnosis for All. Available online: https://www.finddx.org/ (accessed on 30 October 2021).
- Ng, W.Y.; Tan, T.-E.; Movva, P.V.H.; Fang, A.H.S.; Yeo, K.-K.; Ho, D.; Foo, F.S.S.; Xiao, Z.; Sun, K.; Wong, T.Y.; et al. Blockchain applications in health care for COVID-19 and beyond: A systematic review. Lancet Digit. Health 2021. [Google Scholar] [CrossRef]
- Da Cunha, P.R.; Soja, P.; Themistocleous, M. Blockchain for Development: Preliminary Insights from a Literature Review. 2020. Available online: https://aisel.aisnet.org/amcis2020/adv_info_systems_research/adv_info_systems_research/10 (accessed on 19 August 2021).
- Mashamba-Thompson, T.P.; Crayton, E.D. Blockchain and Artificial Intelligence Technology for Novel Coronavirus Disease-19 Self-Testing. Diagnostics 2020, 10, 198. [Google Scholar] [CrossRef] [Green Version]
- USAID. Technical Brief: Stock Visibility System. Available online: https://www.ghsupplychain.org/sites/default/files/2020-12/GHSC-TA%20Technical%20Brief_Stock%20Visibility%20System_2019.09.16%20V.F_508%20compliant.pdf (accessed on 19 August 2021).
- Koshta, N.; Devi, Y.; Patra, S. Aerial Bots in the Supply Chain: A New Ally to Combat COVID-19. Technol. Soc. 2021, 66, 101646. [Google Scholar] [CrossRef]
- Green, D.R.; Karachok, A.R.; Gregory, B.J. The Potential of Drone Technology in Pandemics. In COVID-19 Pandemic, Geospatial Information, and Community Resilience; CRC Press: Boca Raton, FL, USA, 2021; pp. 69–78. [Google Scholar]
- Khan, H.; Kushwah, K.K.; Singh, S.; Urkude, H.; Maurya, M.R.; Sadasivuni, K.K. Smart technologies driven approaches to tackle COVID-19 pandemic: A review. 3 Biotech 2021, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, E.; Serwaa, D. The use of zipline drones technology for COVID-19 samples transportation in Ghana. HighTech Innov. J. 2020, 1, 67–71. [Google Scholar] [CrossRef]
- Ayamga, M.; Akaba, S.; Nyaaba, A.A. Multifaceted applicability of drones: A review. Technol. Forecast. Soc. Chang. 2021, 167, 120677. [Google Scholar] [CrossRef]
- Moshref-Javadi, M.; Winkenbach, M. Applications and Research avenues for drone-based models in logistics: A classification and review. Expert Syst. Appl. 2021, 177, 114854. [Google Scholar] [CrossRef]
| Population | Point of Care (POC) diagnostic services: Diagnostic services that use innovative medical technologies that enable near-patient disease diagnosis [10]. |
| Concept | Supply Chain Management (SCM) systems: Resources and processes needed to deliver goods and services to consumers with complete satisfaction in a cost-optimized manner [13,18]. |
| Context | Globally |
| Author and Year of Publication | Title of Study | Aim of Study | Country | Study Design | Study Setting | Study Population | Type of POC Test Investigated | Stage of SCM Investigated | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| Kuupiel et al., 2017 [10] | Improving the Accessibility and Efficiency of Point-of-Care Diagnostics Services in Low and Middle-Income Countries: Lean and Agile Supply Chain Management | The review provides an overview of the impact of POC diagnostics on healthcare outcomes and factors that contribute to the accessibility of POC diagnostics in LMICs | Global | Review | Low and middle income | General population | HIV, Syphilis and Malaria rapid diagnostic test | Accessibility and availability of POC tests, Test production, selection, quantification, procurement, storage, distribution, quality assurance, inventory management | Barriers to supply chain: Irregular supply, poor forecasting, selection of appropriate diagnostics, unclear procurement systems, delay distribution systems, poor maintenance of quality assurance, and inadequate stock affect existing diagnostics |
| Kuupiel et al., 2019 [12] | Supply chain management and accessibility to point-of-care testing in resource-limited settings: a systematic scoping review | The study aimed to map evidence on SCM of and accessibility to POC testing focusing on availability and use of POC tests in LMICs. | Global | Review | Low and middle income | General population | Malaria, Syphilis, HIV, Diabetes, Dyslipidaemia, RST and Hepatitis B virus rapid diagnostic tests | Availability of tests, Stockouts, Quantification, Forecasting, Inventory management, Distribution and Storage | Challenges reported: weak procurement, inventory and stock management, and human resource capacity for SCM resulted in test stockouts as well as, declined use of POC tests. Availability of adequate quality POC diagnostic tests increases access to POC testing and improved healthcare. Need to strengthen quantification and forecasting, procurement, inventory management, distribution systems, quality management systems, and human resource capacity to prevent test stockouts, sustain POC testing services, and maximize the benefits of implementing POC testing programmes in LMICs. |
| Kuupiel et al., 2019 [21] | Empirical Framework for Point-of-Care Diagnostics Supply Chain Management for Accessibility and Sustainability of Diagnostic Services in Ghana’s Primary Health Care Clinics | The aim of the review is to describe the significance of supply chain management in relation to POC diagnostic tests in rural PHC clinics. | Ghana | Review | Low income | General population | Not specified | Stockouts, Distribution, Storage, Inventory management | SCM challenges reported: poor inventory management, clinic managers mostly do not have the autonomy to purchase medical consumables or supplies such as POC tests on their own, clinic managers mostly request POC tests either from centralised regional/provincial medical stores or from their respective district health directorates, and the timely supply of tests is mostly dependent on availability of the test. Even when the POC tests are made available at various medical stores, supply chain management challenges arise: storage, transportation, quality management, inventory management challenges, and human resource capacity for POC testing may be weak and could threaten the sustainability of the service at the PHC level. |
| Kuupiel et al., 2019 [22] | Poor supply chain management and stockouts of point-of-care diagnostic tests in Upper East Region’s primary healthcare clinics, Ghana | The aim of the study is to audit the supply chain management for POC diagnostic tests in rural Upper East Region’s (UER) PHC clinics, Ghana to determine the reasons for POC tests deficiencies | Ghana | Review | Low income | General population | Haemoglobin, HIV, Syphilis, Hepatitis B, Blood glucose, Malaria, Urine pregnancy and Urine protein | Inventory management, Selection, Distribution, Stock levels | Inventory management: responsibility of the clinic supervisor/manager within the clinic. Test selection: responsibility of higher authorities at the District, Regional, and National levels (ASSURED guidelines). Distribution: responsibility of the health authorities at the Regional medical store and District Health Directorate upon request by the PHC clinics. Stockouts: due to inadequate inventory management and test stockout at the Regional Medical Store/District Health Directorates |
| Peeling 2015 [23] | Diagnostics in a digital age: an opportunity to strengthen health systems and improve health outcomes | Re-examine the Achilles heel and explore the promises and challenges of diagnostics in a digital age. | Global | Review | Global coverage | General population | Malaria, HIV and Syphilis rapid diagnostic tests | Access to testing, Quality Assurance, Stockouts | Enablers of SCM: Real-time data monitoring via electronic readers to improve coordination. Data on stocks, device usage and condition can be uploaded via Wi-Fi or cellular networks and transmitted to central databases. By linking the data to SCM software, stockouts can be avoided, health system efficiency improved. |
| Stevens et al., 2014 [24] | Feasibility of HIV point-of-care tests for resource-limited settings: challenges and solutions | The aim of the study is to outline challenges and solution in the implementation of HIV point of care tests in resource-limited settings | Global | Review | Low and middle income | General population | HIV rapid diagnostic test | Procurement, Production and Quality Assurance | Raises challenges with reimbursement, quality monitoring, lack guideline and regulations |
| Alemnji et al., 2011 [25] | HIV testing in developing countries: What is required? | This article highlights some of the challenges being faced during decentralization of testing facilities in developing countries and some thoughtful considerations for improving infrastructure and quality systems | Global | Review | Low and middle income | General population | HIV rapid diagnostic test | Procurement, Inventory management | Enablers of SCM: efficient procurement, reagent inventory and stock maintenance, cold chain and establishment of equipment service contracts to ensure uninterrupted, timely and quality testing. |
| Alemnji et al., 2020 [26] | Building and Sustaining Optimized Diagnostic Networks to Scale-up HIV Viral Load and Early Infant Diagnosis | Reviewing the set of frameworks identified for the effective use of both POC-based and laboratory-based technologies in large-scale VL and EID testing programs among countries, implementing partners, and donors. | Global | Review | Global coverage | General population | HIV rapid diagnostic test | Procurement | The daily challenges that commonly limit the functioning of testing centres: reagent stockouts, inadequate quality assurance and waste management. Moving from laboratory to POC testing does not reduce these challenges, many of which are associated with procurement and supply chain systems. Instead, they may be exacerbated because of the need to manage a larger number of testing sites. |
| Valera et al., 2021 [27] | COVID-19 Point-of-Care Diagnostics: Present and Future | To analyse the current state of POC technologies for the diagnosis and monitoring of COVID-19 infection and discuss future challenges inCOVID-19 diagnostics | Global | Review | Global coverage | General population | COVID-19 rapid test | Availability of POC Accessibility of POC Procurement Quality assurance | Stakeholders injecting funds to speed up the development of rapid and widely accessible COVID-19 testing. Stakeholders to increase the testing capacity at the POC or at home with new molecular and antigen devices authorized for OTC, at-home testing, the challenges will be to ensure adequate sample collection (to ensure the quality of the test), correct collection technique (to avoid patient harm), and a price that allows continuous access to available tests. |
| Benda et al., 2021 [28] | COVID-19 Testing and Diagnostics: A Review of Commercialized Technologies for Cost, Convenience and Quality of Tests | Our objective here is to review the commercialized in vitro diagnostic tests for the detection of SARS-CoV-2, primarily focusing on tests granted Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA). | Global | Review | Global coverage | General population | COVID-19 rapid test | Accessibility and availability of POC Selection | Despite commendable efforts, the pandemic continues to rattle several parts of the world, especially the low- and middle-income households where testing sites are inaccessible and test kits are cost-prohibitive or in limited supply. Conventional test-trace-isolate strategies for SARS-CoV-2 may eventually be replaced by at-home, low-cost, self-testing based on personal preferences. This requires making COVID-19 testing resources easily accessible, affordable, scalable, quicker, and convenient for the general population. At present, it is paramount to ramp up population-scale testing in low and middle-income countries by building a sustainable supply chain logistics. |
| Poole et al., 2021 [29] | How are rapid diagnostic tests for infectious diseases used in clinical practice: a global survey by the International Society of Antimicrobial Chemotherapy (ISAC) | The study aims to assess the current patterns of use around the world, identify issues for successful implementation and suggest best practice advice on how to introduce new tests. | Global | Review | Global coverage | General population | Influenza, HIV, Hepatitis B, Hepatitis C and Meningitis rapid tests | Availability Quality control | Lower-income countries reported a lower proportion in the availability of rapid tests, but HIV and hepatitis testing were available in greater proportions. HIV and Hepatitis are prevalent in LMICs and are given high priority. The cost of each test is likely to also be a factor in the difference of availability, with multiplexed assays generally being considerably more expensive and requiring more complex logistical support. Methods for reducing the costs of many RDTs are lacking, which limit their availability in low-income settings. There are several existing international regulatory processes for drugs and medications, providing safeguards for their safety and efficacy, they are often lacking for RDTs. As a result, diagnostic tests are often sold and used without any evidence of effectiveness. |
| Kumar et al., 2021 [30] | Aspects of Point-of-Care Diagnostics for Personalized Health Wellness | The review focuses on practical scenarios associated with miniaturized analytical diagnostic devices at POC application for targeted disease diagnostics smartly and efficiently. | Global | Review | Global coverage | General population | Dengue, TB, HIV, Hepatitis B and COVID-19 rapid tests | Human resource capacity | The major concern for POC testing is to achieve the improvement in accuracy and precision of diagnosis at various stages. To prevent wastages of POC tests appropriate sample handling approaches are required to reduce errors during sampling and measurement. |
| Fleming et al., 2021 [31] | The Lancet Commission on diagnostics: transforming access to diagnostics | In this Commission, we analyse the current status of diagnostics with the use of the six WHO building blocks of health systems, namely health service delivery, health workforce, health information systems, access to diagnostics (analogous to essential medicines), financing, and leadership and governance, as the basis. | Global | Review | Global coverage | General population | Diabetes, Hypertension, HIV, TB, Hepatitis B, Syphilis, COVID-19 rapid tests | Accessibility Quality assurance | Forty-seven percent of the global population has little to no access to diagnostics. Diagnostics are central and fundamental to quality health care. This notion is under recognised, leading to underfunding and inadequate resources at all levels. The level of primary health care is the diagnostic so-called last mile and particularly affects poor, rural, and marginalised communities globally; appropriate access is essential for equity and social justice. The COVID-19 pandemic has emphasised the crucial role of diagnostics in health care and that without access to diagnostics, delivery of universal health coverage, antimicrobial resistance mitigation, and pandemic preparedness cannot be achieved. Innovations within the past 15 years in many areas (e.g., in financing, technology, and workforce) can reduce the diagnostic gap, improve access, and democratise diagnostics to empower patients. As an example of the potential impact, 1.1 million premature deaths in low-income and middle-income countries could be avoided annually by reducing the diagnostic gap for six priority conditions: diabetes, hypertension, HIV, and tuberculosis in the overall population, and hepatitis B virus infection and syphilis for pregnant women. |
| Bristow et al., 2015 [32] | A review of recent advances in rapid point-of-care tests for syphilis | The objective of this paper is to assess recent performance data, summarize the latest developments in rapid, point-of care syphilis testing technology and discuss strategies and future directions in the implementation and use of this technology for the prevention and control of syphilis. | Global | Review | Global coverage | Antenatal care clinics | Syphilis rapid diagnostic test | Accessibility, quality assurance | Decentralisation of testing using POC tests can result in increased case finding and treatment for those who need it. The introduction of syphilis rapid tests increased the proportion of antenatal care attendees screened for syphilis to over 90% in all regions that had previously had some testing. WHO helps to make rapid tests available in the places that they are needed through their prequalification program and bulk procurement system. For point-of-care rapid tests to be effective, training on the use and interpretation of tests must be properly provided, and supply chains must be able to sustain access to tests and effective treatment. |
| WHO 2021 [33] | COVID-19 Supply Chain System Procurement Considerations for COVID-19 Diagnostics | This document aims to bring clarity on the process of requesting and receiving globally sourced COVID-19 critical diagnostics supplies | Global | Website | Low and middle income | General population | COVID-19 rapid test | Procurement Selection Availability of POC | Low- and middle-income countries, as well as some high-income small island developing states, have continued to experience restrictions in test access due to competition for limited volumes with high-income countries. Manufacturers have also faced challenges scaling up manufacturing to meet all testing needs. Prices for diagnostic products remain high and some national governments continue to face restricted access to tests. To access POC tests, purchasers may place orders directly with the companies or utilize one of the available multilateral procurement channels. The funded demand and requests are being followed closely to determine whether these tests may be constrained in volume availability. If they become constrained, an allocation model using the same principles as above will be implemented to ensure equitable distribution. |
| Betran et al., 2018 [34] | Provision of medical supply kits to improve quality of antenatal care in Mozambique: a stepped-wedge cluster randomised trial | The formative research component of the study assessed factors affecting the implementation of evidence-based antenatal care service | Mozambique | Randomised controlled trial | Low income | Antenatal care clinics | Proteinuria, Anaemia, malaria, HIV, syphilis rapid diagnostic test | Procurement, Distribution, accessibility | Limitations of the health system: packaging of all required supplies and timely delivery of these kits at the clinics addressed weaknesses in the procurement and supply systems. Poor procurement process at higher levels. Scaling up and sustainability were important considerations. Scaling up means assessing the cost effectiveness and ensuring accessibility/availability of the POC test. |
| Hamer et al., 2012 [35] | Quality and safety of integrated community case management of malaria using rapid diagnostic tests and pneumonia by community health workers | To assess the quality and safety of having community health workers (CHWs) in rural Zambia use rapid diagnostic tests (RDTs) and provide integrated management of malaria and pneumonia. | Zambia | Randomised controlled trial | Low income | Child clinic | Malaria rapid diagnostic test | Inventory management Human resource | Enablers of SCM: transparency provided by well-organized record keeping by CHWs and the engagement of study supervisors to ensure adequate supplies. To strengthen stock management, daily registers and periodic reconciliation of stocks was performed to assess commodity use and ensure that none have passed their expiration dates. |
| Wahlfeld et al., 2019 [36] | HIV Rapid Diagnostic Test Inventories in Zambézia Province, Mozambique: A Tale of 2 Test Kits | The aim of was to evaluate the inventory levels of HIV RDT kits at healthcare facilities in Zambézia province, Mozambique by identifying patterns of threatened inventory levels and/or stockouts of the RDTs. | Mozambique | Cross-sectional | Low income | General healthcare facilities | HIV rapid diagnostic test | Procurement, Storage, Distribution, Inventory management | Disparities in inventory levels were reported at 3 districts. Barriers to supply chain: insufficient access to, and communication with, the provincial warehouse to be able to avoid the high levels of stockouts that were reported. |
| Ekambaram et al., 2019 [37] | Analysis of Failure Modes: Case study of Ruggedizing a low-cost Screening Technology in Sub-Saharan Africa | The article outlines the steps taken by the Ukweli Test Strips venture to ensure the quality of the UTI and preeclampsia urinalysis screening strips in Sierra Leone. | Sierra Leone | Case study | Low income | General health facilities | UTI and Preeclampsia urinalysis screening strips | Quality assurance | Potential quality control problems throughout the supply chain (1) original equipment manufacturer: manufacturing standards (2) Boat transport: humidity and temperature (3) Warehouse storage: human error and storage issues (4) Truck transport: humidity, temperature and travel issues (5) District storage: human error and storage issues (6) Motorcycle transport: humidity, temperature and storage issues (7) Clinics and community health care workers: humidity, light, temperature, human error and storage issues |
| Albertini et al., 2012 [38] | Malaria rapid diagnostic test transport and storage conditions in Burkina Faso, Senegal, Ethiopia and the Philippines | This study aimed to gather data on actual temperatures and humidity levels, in different climatic zones, to which RDTs are subjected as they move through the supply chains that typically serve malaria-endemic countries. | Burkina Faso, Senegal, Philippines and Ethiopia | Cohort study | Low and middle income | General population | Malaria rapid diagnostic test | Storage, Distribution | Storage conditions: high temperatures were recorded at central storage facilities in some countries, conditions were inappropriate for many of the RDTs on the market and frequently exceeded common pharmaceutical storage standards. |
| Mabey et al., 2012 [39] | Point-of-Care Tests to Strengthen Health Systems and Save Newborn Lives: The Case of Syphilis | Our goal was to determine the feasibility of introducing POCTs into different settings in countries with different health systems and cultural and socio-economic contexts. | Brazil, Peru, Tanzania, Uganda, China and Zambia | Mixed methods | Low and middle income | Antenatal care clinics | Syphilis rapid diagnostic test | Quality assurance, availability of POC | For quality assurance, supervisors were provided with proficiency panels prepared by a reference health care facility. Syphilis POCTs were provided to health facilities through the normal supply chain (training in stock management, record keeping, and quality control) to allow the PIs to monitor supply chain problems and provide sustainable solutions in case of stockouts. |
| Palmer et al., 2020 [40] | Improving the effectiveness of point of care tests for malaria and anaemia: a qualitative study across three Ghanaian antenatal clinics | This study utilises qualitative interviews to identify the current practice of POCT use, to explore the enablers and barriers to effective implementation of POCT, and to determine how relationships between each of the stakeholder groups may impact on POCT use. | Ghana | Interviews and Focus Group Discussions | Low income | Pregnant women | Malaria and Anaemia rapid diagnostic test | Stockouts, Human resource capacity | Barriers of SCM: lack of consistency in the supply of both mRDTs and HCS to the healthcare facilities, regular stockouts of mRDTs, HCS was unavailable in all three facilities (they were available and consistently supplied only during the trial period), concerns about procurement and regular supply of HCS kits (after the trial period). Health workers reported often having to resort to purchasing mRDTs privately. |
| Magesa et al., 2020 [41] | Factors associated with stock out of malaria test kit in Oshana region, Namibia | This study focuses its attention on factors associates with stockout of mRDT | Namibia | Mixed methods | Middle income | General health facilities | Malaria rapid diagnostic test | Stockouts, Distribution, Storage | Four themes arose from the study. Theme 1: Stock out of mRDT. Theme 2: Medicine policy and decision makers Theme 3: Shortage of knowledge/training in supply chain logistics. Theme 4: Delays in transportation. |
| Maddox et al., 2017 [42] | Assessing stakeholder perceptions of the acceptability and feasibility of national scale-up for a dual HIV/syphilis rapid diagnostic test in Malawi | This evaluation explores stakeholder perceptions of a novel, dual HIV/syphilis rapid diagnostic test and potential barriers to national scale-up of the dual test in Malawi. | Malawi | Interviews | Low income | Stakeholders involved in provision of antenatal care | HIV and Syphilis rapid diagnostic test | Stockouts, Procurement | Perceived reasons for HIV and syphilis test kit stockouts: low baseline supply of tests given limited funding, expired test kits or staff unwillingness to conduct the tests because they have not received training. Syphilis test kits were stocked out because they were expired, and people wanted to be trained to use the test kits |
| Hussain et al., 2013 [43] | Public health system readiness to treat malaria in Odisha State of India | The study attempted to evaluate the system readiness to deploy RDTs and ACT for malaria control across the State through health facility surveys and interviews with community workers. | India | Mixed methods | Middle income | General healthcare facilities and stakeholders in malaria control | Malaria rapid diagnostic test | Stockouts | Readiness was assessed in terms of the availability of trained human resources, drugs and diagnostics. Despite a high level of knowledge about how best to diagnose and treat malaria, the ability of the peripheral health workers to optimize fever management and malaria diagnosis was compromised by a failure of the supply chain (poor availability of POC tests due to poor communication/procurement system) |
| Hasselback et al., 2014 [44] | Rapid diagnostic test supply chain and consumption study in Cabo Delgado, Mozambique: estimating stock shortages and identifying drivers of stockouts | The aim of the study is to estimate malaria RDT stock shortages and the percentage of overall need met by the existing stock in the Cabo Delgado province of Mozambique | Mozambique | Mixed methods | Low income | General health facilities | Malaria rapid diagnostic test | Procurement, stockouts | SCM challenges: procurement for tests was donor supported and requisition-based supply chain has been associated with supply dysfunction (stockouts followed by periods of excessive stock). Supply chains need to respond to timely consumption data to ensure that inventory is appropriately stocked with respect to demand. Stockouts: poor data control and consumption tracking, system responded to an underestimate of the true demand thereby positioning lower inventory than needed in the supply chain. |
| Dassah et al., 2018 [45] | Rollout of rapid point of care tests for antenatal syphilis screening in Ghana: healthcare provider perspectives and experiences | The aim of the study is to present the perspective of healthcare providers in public health facilities in selected regions of Ghana in relation to their experiences and challenges following a national rollout of rapid syphilis POCTs in Ghana. | Ghana | Mixed methods | Low income | Antenatal care clinics | Syphilis rapid diagnostic test | Stockouts | Interruptions in the supply of syphilis POCTs and penicillin: lack of clear communication channels and poor monitoring and supervision adversely affected implementation of the programme, expired tests kits and failure to replenish stocks, healthcare providers and programme coordinators blamed each other for stockouts. |
| Blanas et al., 2013 [47] | Barriers to community case management of malaria in Saraya, Senegal: training, and supply-chains | The study evaluates communities’ perceptions of a new community case management of malaria programme in Senegal | Senegal | Mixed methods | Low income | General population | Malaria rapid diagnostic test | Availability, Stockouts, human resource capacity | Stockouts were reported: attributed to inaccurate record-keeping and ignored supply requests, procurement system failures and inadequate central stores. |
| Boadu et al., 2016 [48] | Challenges with implementing malaria rapid diagnostic tests at primary care facilities in a Ghanaian district: a qualitative study | The aim of the study was to determine which factors influenced RDT implementation for routine malaria management at primary care facilities in the study district. | Ghana | Interviews, focus group discussions, direct observation | Low income | Primary health care facilities | Malaria rapid diagnostic test | Storage, Quality Assurance, Distribution Accessibility | RDT supplies from the district health directorate to their facilities were often insufficient and sporadic. This challenge was more pronounced at remote facilities solely dependent on government supplies and a major hindrance to routine malaria testing at all facilities. At the time of the study four facilities had limited RDTs while two had none. Stockouts were common, sometimes lasting several months, making providers hesitant to use limited quantities when available. Malaria testing at public facilities dependent on government RDT supplies was interrupted due to frequent and prolonged RDT stock outs. Some private providers mentioned purchasing RDTs from independent sources when available. Others abandoned RDT use altogether, citing the economic and technical advantages of microscopy over RDTs. |
| Asiimwe et al., 2012 [46] | Early experiences on the feasibility, acceptability, and use of malaria rapid diagnostic tests at peripheral health centres in Uganda-insights into some barriers and facilitators | This study and other operational research were conceived and carried out to facilitate evidence-based policy formulation and high-quality implementation of mRDT-led, parasite-based diagnosis. | Uganda | Qualitative Cross-sectional | Low income | General health facilities | Malaria rapid diagnostic test | Selection, Quality Assurance and Safe Disposal | Test selection: Given the predominance of P. falciparum as the cause of malaria in this setting, it was decided to use a histidine rich protein-2 (HRP2) type of mRDT. In deciding the mRDT brand to use, a basic assessment of ease of- use was carried out on four brands amongst nine health workers at a health centre not involved in this study. The brand was chosen on the basis of packaging and labelling, ease of performance, readability of the results, cost, heat stability data, and reported sensitivity and specificity. |
| Cheng et al., 2016 [49] | Data connectivity: A critical tool for external quality assessment | The aim of the study is to address challenges in training and quality assurance when embedding connectivity in their new POC diagnostic instruments or providing some form of channel for electronic result exchange. | Zimbabwe | Review | Low income | General health facilities | HIV rapid diagnostic test | Quality assurance, Stockouts | Connectivity has shown that it is possible for Ministries of Health to have up-to-the-hour information on testing and test results across the country. These systems can also be twinned to supply chain management software to monitor supplies at each site, providing an automated system for alerts to avoid stockouts. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maluleke, K.; Musekiwa, A.; Kgarosi, K.; Gregor, E.M.; Dlangalala, T.; Nkambule, S.; Mashamba-Thompson, T. A Scoping Review of Supply Chain Management Systems for Point of Care Diagnostic Services: Optimising COVID-19 Testing Capacity in Resource-Limited Settings. Diagnostics 2021, 11, 2299. https://doi.org/10.3390/diagnostics11122299
Maluleke K, Musekiwa A, Kgarosi K, Gregor EM, Dlangalala T, Nkambule S, Mashamba-Thompson T. A Scoping Review of Supply Chain Management Systems for Point of Care Diagnostic Services: Optimising COVID-19 Testing Capacity in Resource-Limited Settings. Diagnostics. 2021; 11(12):2299. https://doi.org/10.3390/diagnostics11122299
Chicago/Turabian StyleMaluleke, Kuhlula, Alfred Musekiwa, Kabelo Kgarosi, Emily Mac Gregor, Thobeka Dlangalala, Sphamandla Nkambule, and Tivani Mashamba-Thompson. 2021. "A Scoping Review of Supply Chain Management Systems for Point of Care Diagnostic Services: Optimising COVID-19 Testing Capacity in Resource-Limited Settings" Diagnostics 11, no. 12: 2299. https://doi.org/10.3390/diagnostics11122299
APA StyleMaluleke, K., Musekiwa, A., Kgarosi, K., Gregor, E. M., Dlangalala, T., Nkambule, S., & Mashamba-Thompson, T. (2021). A Scoping Review of Supply Chain Management Systems for Point of Care Diagnostic Services: Optimising COVID-19 Testing Capacity in Resource-Limited Settings. Diagnostics, 11(12), 2299. https://doi.org/10.3390/diagnostics11122299







