Diagnostic Value of CEUS LI-RADS Version 2017 in Differentiating AFP-Negative Hepatocellular Carcinoma from Other Primary Malignancies of the Liver
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Instruments and Methods
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
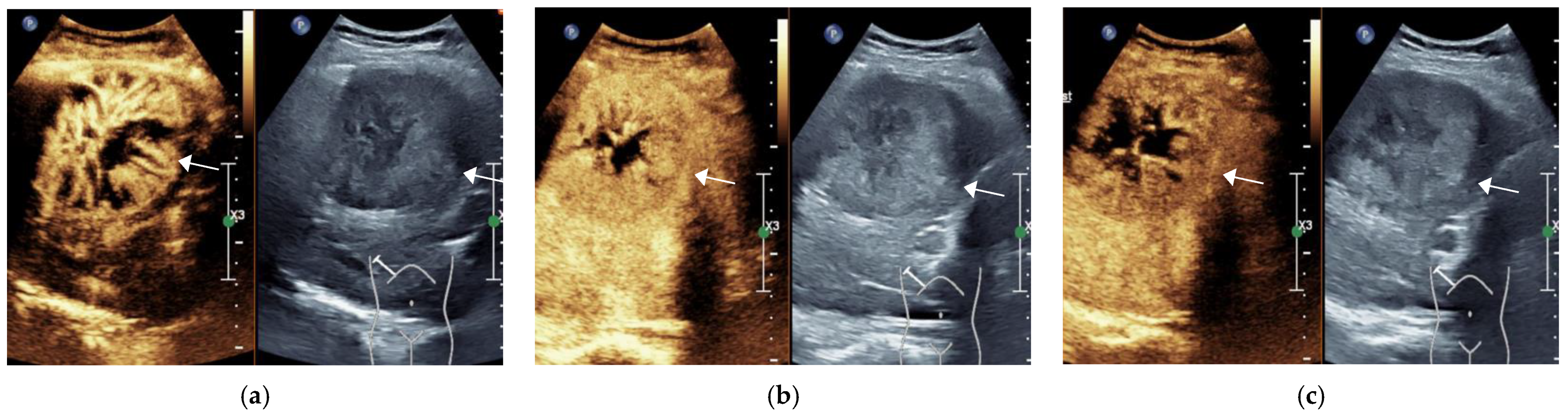
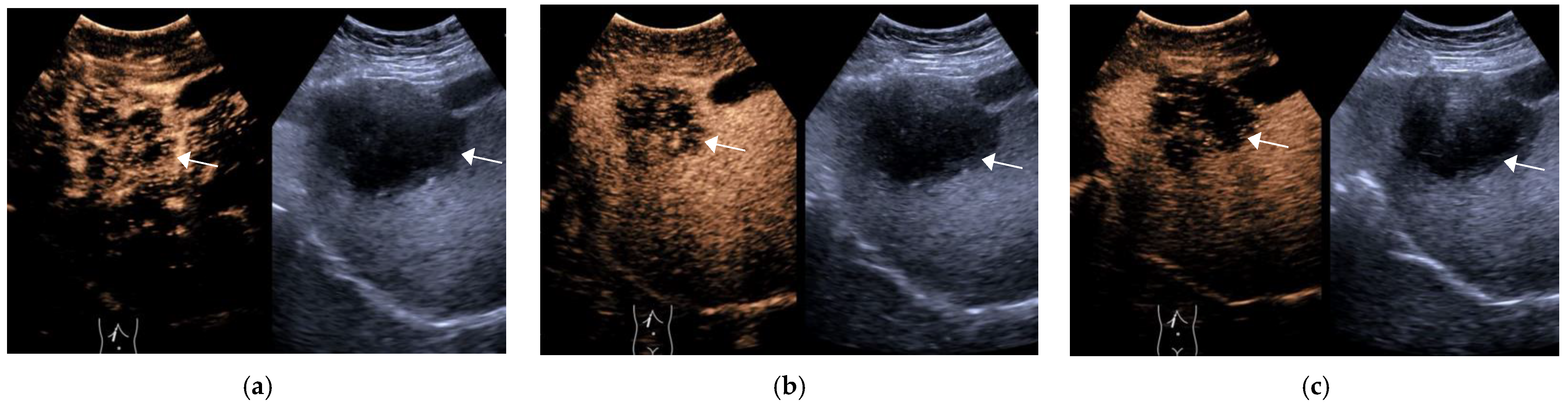
3.1. General Information of the Patient and CEUS Features of the Lesions
3.2. Final Results of CEUS LI-RADS Category of Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of Liver Diseases in the World. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global Epidemiology of Hepatocellular Carcinoma: An Emphasis on Demographic and Regional Variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef] [Green Version]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the Diagnosis and Management of Intrahepatic Cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef] [Green Version]
- Ayuso, C.; Rimola, J.; Vilana, R.; Burrel, M.; Darnell, A.; García-Criado, A.; Bianchi, L.; Belmonte, E.; Caparroz, C.; Barrufet, M.; et al. Diagnosis and Staging of Hepatocellular Carcinoma (HCC): Current Guidelines. Eur. J. Radiol. 2018, 101, 72–81. [Google Scholar] [CrossRef]
- Burkhart, R.A.; Pawlik, T.M. Staging and Prognostic Models for Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017, 24, 1073274817729235. [Google Scholar] [CrossRef]
- Medical Administration of the Health and Family Planning Commission of the People’s Republic of China. Treatment Specification for Primary Liver Cancer (2017 Edition). Chin. J. Dig. Surg. 2017, 16, 635–647. [Google Scholar]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Kono, Y.; Lyshchik, A.; Cosgrove, D.; Dietrich, C.F.; Jang, H.J.; Kim, T.K.; Piscaglia, F.; Willmann, J.K.; Wilson, S.R.; Santillan, C.; et al. Contrast Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS®): The Official Version by the American College of Radiology (ACR). Ultraschall Med. 2017, 38, 85–86. [Google Scholar] [CrossRef] [Green Version]
- Berretta, M.; Cavaliere, C.; Alessandrini, L.; Stanzione, B.; Facchini, G.; Balestreri, L.; Perin, T.; Canzonieri, V. Serum and Tissue Markers in Hepatocellular Carcinoma and Cholangiocarcinoma: Clinical and Prognostic Implications. Oncotarget 2017, 8, 14192–14220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohle, W.; Clemens, P.U.; Heubach, T.; Zoller, W.G. Contrast-Enhanced Ultrasound (CEUS) for Differentiating between Hepatocellular and Cholangiocellular Carcinoma. Ultraschall Med. 2012, 33, E191–E195. [Google Scholar] [CrossRef] [PubMed]
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsøe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver-Update 2012: A WFUMB-EFSUMB Initiative in Cooperation with Representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med. Biol. 2013, 39, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, V.; Marschner, C.; Volckers, W.; De Figueiredo, G.N.; Rübenthaler, J.; Clevert, D.-A. The Diagnostic Performance of Contrast-Enhanced Ultrasound (CEUS) for Evaluating Hepatocellular Carcinoma (HCC) Juxtaposed to MRI Findings; a Retrospective Single-Center Analysis of 292 Patients. Clin. Hemorheol. Microcirc. 2020, 76, 155–160. [Google Scholar] [CrossRef]
- Terzi, E.; Iavarone, M.; Pompili, M.; Veronese, L.; Cabibbo, G.; Fraquelli, M.; Riccardi, L.; De Bonis, L.; Sangiovanni, A.; Leoni, S.; et al. Contrast Ultrasound LI-RADS LR-5 Identifies Hepatocellular Carcinoma in Cirrhosis in a Multicenter Restropective Study of 1006 Nodules. J. Hepatol. 2018, 68, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, Q.; Zou, X.B.; Wang, J.W.; Han, F.; Li, F.; Huang, L.S.; Li, A.H.; Zhou, J.H. Evaluation of Contrast-Enhanced US LI-RADS Version 2017: Application on 2020 Liver Nodules in Patients with Hepatitis B Infection. Radiology 2020, 294, 299–307. [Google Scholar] [CrossRef]
- Loria, F.; Loria, G.; Basile, S.; Crea, G.; Randazzo, D.; Frosina, L. Contrast-Enhanced Ultrasound of Hepatocellular Carcinoma: Correlation between Enhancement Pattern and Cellular Differentiation on Histopathlogy. Updates Surg. 2012, 64, 247–255. [Google Scholar] [CrossRef]
- Feng, Y.; Qin, X.C.; Luo, Y.; Li, Y.Z.; Zhou, X. Efficacy of Contrast-Enhanced Ultrasound Washout Rate in Predicting Hepatocellular Carcinoma Differentiation. Ultrasound Med. Biol. 2015, 41, 1553–1560. [Google Scholar] [CrossRef]
- Honda, H.; Tajima, T.; Kajiyama, K.; Kuroiwa, T.; Yoshimitsu, K.; Irie, H.; Aibe, H.; Shimada, M.; Masuda, K. Vascular Changes in Hepatocellular Carcinoma: Correlation of Radiologic and Pathologic Findings. AJR Am. J. Roentgenol. 1999, 173, 1213–1217. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Xie, X.; Lin, Y.; Liu, B.; Wang, W.; Huang, X.; Huang, G. Imaging Features of Combined Hepatocellular-Cholangiocarcinoma on Contrast-Enhanced Ultrasound: Correlation with Clinicopathological Findings. Clin. Radiol. 2018, 73, 237–243. [Google Scholar] [CrossRef]
- Calame, P.; Tyrode, G.; Verhoeven, D.W.; Félix, S.; Klompenhouwer, A.J.; di Martino, V.; Delabrousse, E.; Thévenot, T. Clinical Characteristics and Outcomes of Patients with Hepatic Angiomyolipoma: A literature Review. World J. Gastroenterol. 2021, 27, 2299–2311. [Google Scholar] [CrossRef]
- Zou, M.H.; Huang, Q.; Zou, Q.; Jiang, Y.; Ju, J.X.; Zhou, H.C.; Jiao, J.; Zheng, R.Q. Clinical and Contrast-Enhanced Ultrasound Characteristics of Epithelioid and Classic Hepatic Angiomyolipoma: Comparison with Alpha-Fetoprotein-Negative Hepatocellular Carcinoma. Ultrasound Med. Biol. 2021, 47, 446–453. [Google Scholar] [CrossRef] [PubMed]


| Group | Num. | Gender | Age/Years | Location | Maximum Diameter/cm | |||||
| Male | Female | Left Lobe | Right Lobe | <3 | ≥3 | |||||
| HCC | 61 | 50 (82.0) | 11 (18.0%) | 55.540 ± 10.096 | 15 (24.6%) | 46 (75.4%) | 23 (37.7%) | 38 (62.3%) | ||
| OM | 38 | 24 (63.2%) | 14 (36.8%) | 58.530 ± 11.215 | 11 (28.9%) | 27 (71.1%) | 3 (7.9%) | 35 (92.1%) | ||
| X2/t | - | 4.389 | −1.338 | 0.230 | 10.744 | |||||
| p | - | 0.036 | 0.185 | 0.632 | 0.001 | |||||
| Group | Num. | Enhancement Pattern in AP | Degree of Wash-Out | Time to Wash Out/s | ||||||
| Non-Rim APHE | Rim APHE | Non APHE | Mild | Marked | Non | <60 | ≥60 | Non-Wash Out | ||
| HCC | 61 | 58 (95.1%) | 2 (3.3%) | 1 (1.6%) | 54 (88.5%) | 3 (4.9%) | 4 (6.6%) | 11 (18.0%) | 46 (75.4%) | 4 (6.3%) |
| OM | 38 | 14 (36.8%) | 22 (57.9%) | 2 (5.3%) | 13 (34.2%) | 25 (65.8%) | 0 (0%) | 32 (84.2%) | 6 (15.8%) | 0 (0%) |
| X2/t | - | 40.745 | 43.373 | 41.946 | ||||||
| p | - | <0.001 | <0.001 | <0.001 | ||||||
| Group | Sen | Spe | PPV | NPV | DCR | |
|---|---|---|---|---|---|---|
| non- rim APHE | HCC | 95.1 (58/61) | 63.2 (24/38) | 80.6 (58/72) | 88.9 (24/27) | 82.8 (82/99) |
| rim APHE | OM | 57.9 (22/38) | 96.7 (59/61) | 91.7 (22/24) | 78.7 (59/75) | 81.8 (81/99) |
| mild wash-out | HCC | 88.5 (54/61) | 65.8 (25/28) | 80.6 (54/67) | 78.1 (25/32) | 79.8 (79/99) |
| marked wash-out | OM | 65.8 (25/38) | 95.1 (58/61) | 89.3 (25/28) | 81.7 (58/71) | 83.8 (83/99) |
| late wash-out | HCC | 75.4 (46/61) | 84.2 (32/38) | 88.5 (46/52) | 68.1 (32/47) | 78.8 (78/99) |
| early wash-out | OM | 84.2 (32/38) | 82.0 (50/61) | 74.4 (32/43) | 89.3 (50/56) | 82.8 (82/99) |
| LR-4 | HCC | 21.3 (13/61) | 97.4 (38/39) | 100.0 (13/13) | 44.2 (38/86) | 51.5 (51/99) |
| LR-5 | HCC | 62.3 (38/61) | 92.1 (35/38) | 92.7 (38/41) | 60.3 (35/58) | 73.7 (73/99) |
| LR-M | OM | 92.1 (35/38) | 83.6 (51/61) | 77.8 (35/45) | 94.4 (51/54) | 86.9 (86/99) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Nie, F.; Dong, T.; Yang, D.; Liu, T.; Wang, G. Diagnostic Value of CEUS LI-RADS Version 2017 in Differentiating AFP-Negative Hepatocellular Carcinoma from Other Primary Malignancies of the Liver. Diagnostics 2021, 11, 2250. https://doi.org/10.3390/diagnostics11122250
Wang P, Nie F, Dong T, Yang D, Liu T, Wang G. Diagnostic Value of CEUS LI-RADS Version 2017 in Differentiating AFP-Negative Hepatocellular Carcinoma from Other Primary Malignancies of the Liver. Diagnostics. 2021; 11(12):2250. https://doi.org/10.3390/diagnostics11122250
Chicago/Turabian StyleWang, Peihua, Fang Nie, Tiantian Dong, Dan Yang, Ting Liu, and Guojuan Wang. 2021. "Diagnostic Value of CEUS LI-RADS Version 2017 in Differentiating AFP-Negative Hepatocellular Carcinoma from Other Primary Malignancies of the Liver" Diagnostics 11, no. 12: 2250. https://doi.org/10.3390/diagnostics11122250
APA StyleWang, P., Nie, F., Dong, T., Yang, D., Liu, T., & Wang, G. (2021). Diagnostic Value of CEUS LI-RADS Version 2017 in Differentiating AFP-Negative Hepatocellular Carcinoma from Other Primary Malignancies of the Liver. Diagnostics, 11(12), 2250. https://doi.org/10.3390/diagnostics11122250






