Biomarkers Changes after Neoadjuvant Chemotherapy in Breast Cancer: A Seven-Year Single Institution Experience
Abstract
1. Introduction
- the concordance/discordance rate of each tumor biomarker between pre-NACT biopsy and post-NACT surgical specimen;
- the effect of biomarker changes on intrinsic subtype;
- the impact of these changes in the “real-world” of adjuvant treatment, also in view of new literature evidence.
2. Materials and Methods
2.1. Case Selection
2.2. Pathological Analysis
2.3. Statistical Analysis
3. Results
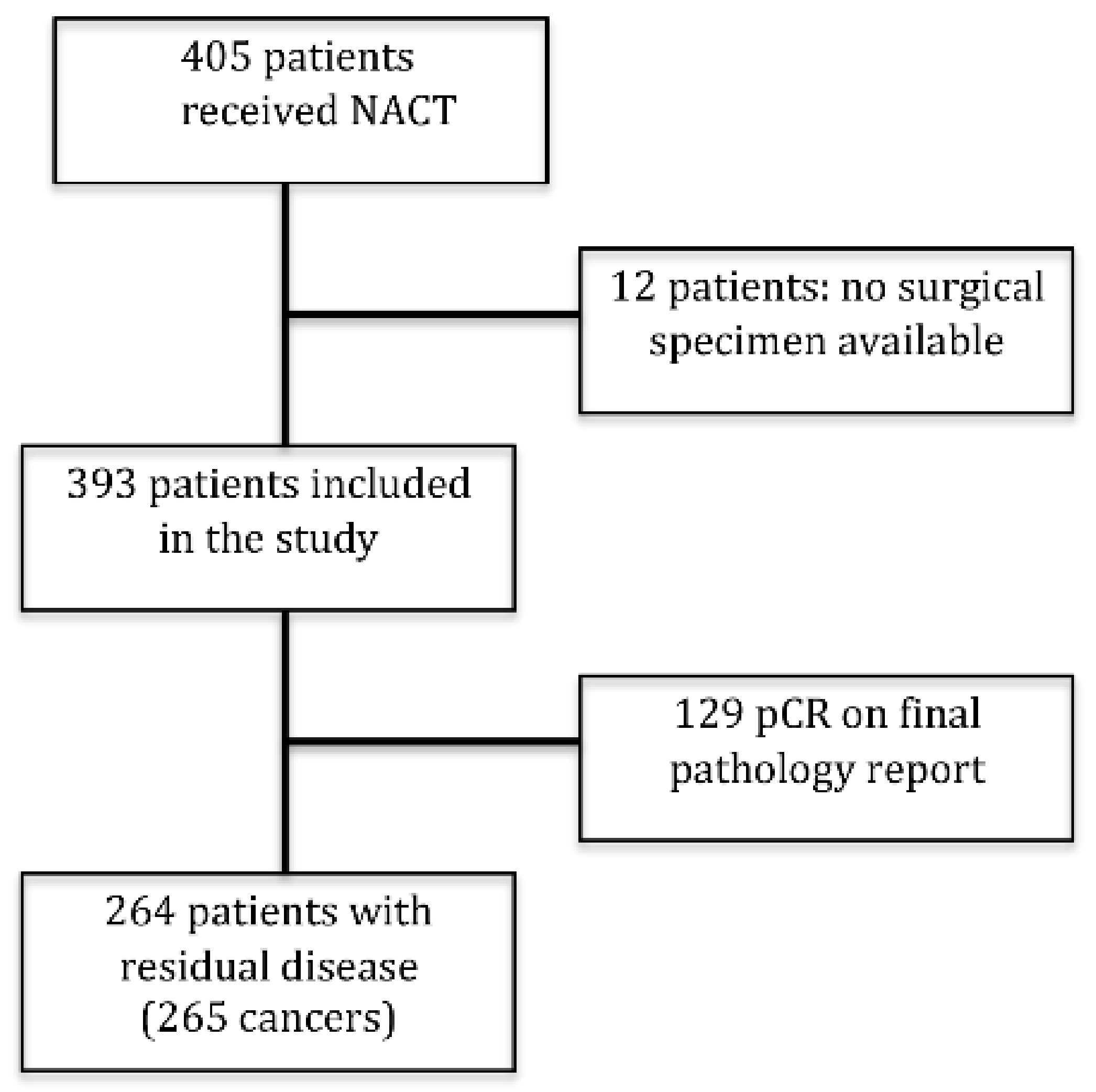
3.1. Study Cohort
3.2. Change in Biomarkers Status
3.3. Change in Intrinsic Subtype and Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaufmann, M.; von Minckwitz, G.; Bear, H.D.; Buzdar, A.; McGale, P.; Bonnefoi, H.; Colleoni, M.; Denkert, C.; Eiermann, W.; Jackesz, R.; et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: New perspectives 2006. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2007, 18, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, E.P.; Fisher, B. Preoperative (neoadjuvant) chemotherapy in patients with breast cancer. Semin. Oncol. 2001, 28, 389–399. [Google Scholar] [CrossRef]
- Van der Hage, J.A.; van de Velde, C.J.; Julien, J.P.; Tubiana-Hulin, M.; Vandervelden, C.; Duchateau, L. Preoperative chemotherapy in primary operable breast cancer: Results from the European Organization for Research and Treatment of Cancer trial 10902. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 4224–4237. [Google Scholar] [CrossRef] [PubMed]
- Kuerer, H.M.; Newman, L.A.; Smith, T.L.; Ames, F.C.; Hunt, K.K.; Dhingra, K.; Theriault, R.L.; Singh, G.; Binkley, S.M.; Sneige, N.; et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 460–469. [Google Scholar] [CrossRef]
- Chollet, P.; Amat, S.; Cure, H.; de Latour, M.; Le Bouedec, G.; Mouret-Reynier, M.A.; Ferriere, J.P.; Achard, J.L.; Dauplat, J.; Penault-Llorca, F. Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br. J. Cancer 2002, 86, 1041–1046. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Breast Cancer (Version 8.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 13 September 2021).
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Hammond, M.E.; Hayes, D.F.; Wolff, A.C.; Mangu, P.B.; Temin, S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Oncol. Pract. 2010, 6, 195–197. [Google Scholar] [CrossRef]
- Ragazzi, M.; Bisagni, A.; Gasparini, E.; Kuhn, E.; Bassano, C.; Tamagnini, I.; Foroni, M.; Bortesi, M.; Falco, G.; Ferrari, G.; et al. Impact of 2013 ASCO/CAP guidelines on HER2 determination of invasive breast cancer: A single institution experience using frontline dual-color FISH. Breast 2017, 34, 65–72. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-40617-6. [Google Scholar]
- Turashvili, G.; Brogi, E. Tumor heterogeneity in breast cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef]
- Van de Ven, S.; Smit, V.T.; Dekker, T.J.; Nortier, J.W.; Kroep, J.R. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat. Rev. 2011, 37, 422–430. [Google Scholar] [CrossRef]
- Li, P.; Liu, T.; Wang, Y.; Shao, S.; Zhang, W.; Lv, Y.; Yi, J.; Wang, Z. Influence of neoadjuvant chemotherapy on HER2/neu status in invasive breast cancer. Clin. Breast Cancer 2013, 13, 53–60. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.F.; Liao, Y.Y.; Li, L.Q.; Xie, S.R.; Xie, Y.F.; Peng, N.F. Changes in ER, PR and HER2 receptors status after neoadjuvant chemotherapy in breast cancer. Pathol. Res. Pract. 2013, 209, 797–802. [Google Scholar] [CrossRef]
- Ge, W.K.; Yang, B.; Zuo, W.S.; Zheng, G.; Dai, Y.Q.; Han, C.; Yang, L.; Zheng, M.Z. Evaluation of hormone receptor, human epidermal growth factor receptor-2 and Ki-67 with core needle biopsy and neoadjuvant chemotherapy effects in breast cancer patients. Thorac. Cancer 2015, 6, 64–69. [Google Scholar] [CrossRef]
- Qin, Q.; Gao, F.; Jiang, W.; Tan, Q.; Mo, Q.; Wei, C. Effect of neoadjuvant chemotherapy on expressions of estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2, and Ki-67 in breast cancer. Chin. Med. J. 2014, 127, 3272–3277. [Google Scholar]
- Lim, S.K.; Lee, M.H.; Park, I.H.; You, J.Y.; Nam, B.H.; Kim, B.N.; Ro, J.; Lee, K.S.; Jung, S.Y.; Kwon, Y.M.; et al. Impact of molecular Subtype conversion of breast cancers after neoadjuvant chemotherapy on clinical outcome. Cancer Res. Treat. 2016, 48, 133–141. [Google Scholar] [CrossRef]
- Xian, Z.; Quinones, A.K.; Tozbikian, G.; Zynger, D.L. Breast cancer biomarkers before and after neoadjuvant chemotherapy: Does repeat testing impact therapeutic management? Hum. Pathol. 2017, 62, 215–221. [Google Scholar] [CrossRef]
- De La Cruz, L.M.; Harhay, M.O.; Zhang, P.; Ugras, S. Impact of neoadjuvant chemotherapy on breast cancer Subtype: Does subtype change and, if so, how? IHC profile and neoadjuvant chemotherapy. Ann. Surg. Oncol. 2018, 25, 3535–3540. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, H.J.; Kim, M.; Chung, Y.R.; Kang, E.; Kim, E.K.; Kim, S.H.; Kim, Y.J.; Kim, J.H.; Kim, I.A.; et al. Negative conversion of progesterone receptor status after primary systemic therapy is associated with poor clinical outcome in patients with breast cancer. Cancer Res. Treat. 2018, 50, 1418–1432. [Google Scholar] [CrossRef] [PubMed]
- Rey-Vargas, L.; Mejía-Henao, J.C.; Sanabria-Salas, M.C.; Serrano-Gomez, S.J. Effect of neoadjuvant therapy on breast cancer biomarker profile. BMC Cancer 2020, 20, 675. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Verrico, M.; Tomao, S.; Ricci, F.; Fontana, A.; Spinelli, G.P.; Colonna, M.; Vici, P.; Tomao, F. Expression of ER, PgR, HER-2, and Ki-67 in core biopsies and in definitive histological specimens in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy. Cancer Chemother. Pharmacol. 2020, 85, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.C.; Walcott-Sapp, S.; Lee, M.K.; Srour, M.K.; Kim, S.; Amersi, F.F.; Giuliano, A.E.; Chung, A.P. Alterations in breast cancer biomarkers following neoadjuvant therapy. Ann. Surg. Oncol. 2021, 28, 5907–5917. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Hong, S.A.; Ahn, H.S.; Ahn, S.K.; Kim, M.K. Clinicopathologic factors affecting discrepancies in HER2 overexpression between core needle biopsy and surgical biopsy in breast cancer patients according to neoadjuvant treatment or not. J. Cancer 2021, 12, 4722–4728. [Google Scholar] [CrossRef]
- Provenzano, E.; Bossuyt, V.; Viale, G.; Cameron, D.; Badve, S.; Denkert, C.; MacGrogan, G.; Penault-Llorca, F.; Boughey, J.; Curigliano, G.; et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: Recommendations from an international working group. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2015, 28, 1185–1201. [Google Scholar] [CrossRef]
- Ellis, M.J.; Tao, Y.; Luo, J.; A’Hern, R.; Evans, D.B.; Bhatnagar, A.S.; Chaudri Ross, H.A.; von Kameke, A.; Miller, W.R.; Smith, I.; et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J. Natl. Cancer Inst. 2008, 100, 1380–1388. [Google Scholar] [CrossRef]
- Jones, R.L.; Salter, J.; A’Hern, R.; Nerurkar, A.; Parton, M.; Reis-Filho, J.S.; Smith, I.E.; Dowsett, M. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res. Treat. 2009, 116, 53–68. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in breast cancer: Updated recommendations from the international Ki67 in breast cancer working group. J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Wu, Y.; Scaltriti, M.; Meric-Bernstam, F.; Hunt, K.K.; Dawood, S.; Esteva, F.J.; Buzdar, A.U.; Chen, H.; Eksambi, S.; et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 7381–7388. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Y.; Sheng, S.; Yuan, H.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; et al. HER2 somatic mutations are associated with poor survival in HER2-negative breast cancers. Cancer Sci. 2017, 108, 671–677. [Google Scholar] [CrossRef]
- Guarneri, V.; Dieci, M.V.; Barbieri, E.; Piacentini, F.; Omarini, C.; Ficarra, G.; Bettelli, S.; Conte, P.F. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 2990–2994. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Conte, P.F.; Dieci, M.V.; Bisagni, G.; Laurentiis, M.D.; Tondini, C.A.; Schmid, P.; Salvo, G.L.D.; Moratello, G.; Guarneri, V. Phase III randomized study of adjuvant treatment with the ANTI-PD-L1 antibody avelumab for high-risk triple negative breast cancer patients: The A-BRAVE trial. J. Clin. Oncol. 2020, 38, TPS598. [Google Scholar] [CrossRef]
| PRE-NACT | POST-NACT | p-Value | ||
| FEATURES | RESULTS | FEATURES | RESULTS | |
| Stage | NA | |||
| IA | 4 | IA | 103 | |
| IIA | 93 | IB | 2 | |
| IIB | 93 | IIA | 76 | |
| IIIA | 59 | IIB | 24 | |
| IIIB | 16 | IIIA | 41 | |
| IIIB | 4 | |||
| IIIC | 15 | |||
| TNM | ||||
| cT1c | 5 | ypT0 | 4 * | NA |
| cT2 | 183 | ypT1 | 162 | |
| cT3 | 63 | ypT2 | 70 | |
| cT4 | 14 | ypT3 | 22 | |
| ypT4 | 6 | |||
| cN0 | 116 | ypN0 | 141 | |
| cN+ | 149 | ypN1 | 71 | |
| ypN2 | 35 | |||
| ypN3 | 15 | |||
| ypNx | 4 | |||
| Histology | ||||
| NST | 244 (92.1%) | 244 (92.1%) | 0.988 ° | |
| ILC | 16 (6.0%) | 17 (6.4%) | ||
| Metaplastic | 1 (0.4%) | 1 (0.4%) | ||
| NST + ILC | 3 (1.1%) | 2 (0.8%) | ||
| Mucinous | 1 (0.4%) | 1 (0.4%) | ||
| Grading | ||||
| G1 | 0 | 3 | 0.158 ° | |
| G2 | 148 | 137 | ||
| G3 | 117 | 125 | ||
| ER range (mean) | 0–100 (57.1 ± 43.8) | 0–100 (56.5 ± 44.3) | 0.20 ^ | |
| <1% | 77 | 84 | 0.571§ | |
| ≥1% | 188 | 181 | ||
| PR range (mean) | 0–100 (31.0 ± 37.9) | 0–100 (19.6 ± 31.7) | <0.0001 ^ | |
| <20% | 147 | 186 | 0.0006 § | |
| ≥20% | 118 | 79 | ||
| Ki67 range (mean) | 4–95 (36.9 ± 22.1) | 0–90 (27.4 ± 27.3) | <0.0001 ^ | |
| <20% | 46 | 144 | <0.0001 § | |
| ≥20% | 219 | 121 | ||
| HER2 | ||||
| POS | 105 | 91 | 0.15 § | |
| NEG | 160 | 174 | ||
| Biomarkers (Cut-Off) | Pre-NACT | Post-NACT | N (%) | % of Concordance | K [0.95 CI] |
|---|---|---|---|---|---|
| ER (≥1%) | + | + | 169 (63.8) | 88.3 | 0.723 [0.632–0.815] |
| − | − | 65 (24.5) | |||
| + | − | 19 (7.2) | |||
| − | + | 12 (4.5) | |||
| PR (≥1%) | + | + | 119 (44.9) | 83.4 | 0.67 [0.581–0.759] |
| − | − | 102 (38.5) | |||
| + | − | 36 (13.6) | |||
| − | + | 8 (3.0) | |||
| PR (≥20%) | + | + | 72 (27.2) | 80.0 | 0.582 [0.481–0.682] |
| − | − | 140 (52.8) | |||
| + | − | 46 (17.4) | |||
| − | + | 7 (2.6) | |||
| Ki67 (≥20%) | + | + | 113 (42.7) | 57.0 | 0.186 [0.073–0.299] |
| − | − | 38 (14.3) | |||
| + | − | 106 (40.0) | |||
| − | + | 8 (3.0) | |||
| HER2 | + | + | 85 (32.1) | 90.2 | 0.79 [0.714–0.887] |
| − | − | 154 (58.1) | |||
| + | − | 20 (7.5) | |||
| − | + | 6 (2.3) |
| Pre-NACT | Post-NACT | Intrinsic Subtype Agreement | ||||||
|---|---|---|---|---|---|---|---|---|
| Intrinsic Subtype | N° Cases (%) | No Change (%) | Yes Change (%) | Intrinsic Subtype (n) | Therapy Change (n) | Therapy Added | % of Concordance | K [0.95 CI] |
| Lum A | 20 (7.5) | 13 (65.0) | 7 (35.0) | Lum B (7) | 0 | None | 84.9% | 0.323 [0.129–0.516] |
| Lum B HER2- | 82 (30.9) | 51 (62.2) | 31 (37.8) | Lum A (22) Lum A HER2+ (2) Lum B HER2+ (3) TN (4) | 0 2 3 0 | None Trastuzumab Trastuzumab None | 81.1% | 0.54 [0.425–0.655] |
| Lum B HER2+ | 73 (27.6) | 45 (61.6) | 28 (38.4) | Lum A (11) Lum A HER2+ (5) Lum B HER2- (8) HER2+ (4) | 0 0 0 0 | None None None None | 86.0% | 0.62 [0.506–0.733] |
| HER2+ | 32 (12.1) | 29 (90.6) | 3 (9.4) | Lum B HER2- (2) TN (1) | 2 0 | Hormonal therapy None | 97.4% | 0.877 [0.788–0.967] |
| TN | 58 (21.9) | 55 (94.8) | 3 (5.2) | Lum B HER2- (2) Lum B HER2+ (1) | 2 1 | Hormonal therapy Trastuzumab and Hormonal therapy | 97.0% | 0.913 [0.853–0.972] |
| Total (%) | 265 (100) | 193 (72.8) | 72 (27.2) | 10 (3.8) | 72.8% | 0.656 [0.588–0.724] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coiro, S.; Gasparini, E.; Falco, G.; Santandrea, G.; Foroni, M.; Besutti, G.; Iotti, V.; Di Cicilia, R.; Foroni, M.; Mele, S.; et al. Biomarkers Changes after Neoadjuvant Chemotherapy in Breast Cancer: A Seven-Year Single Institution Experience. Diagnostics 2021, 11, 2249. https://doi.org/10.3390/diagnostics11122249
Coiro S, Gasparini E, Falco G, Santandrea G, Foroni M, Besutti G, Iotti V, Di Cicilia R, Foroni M, Mele S, et al. Biomarkers Changes after Neoadjuvant Chemotherapy in Breast Cancer: A Seven-Year Single Institution Experience. Diagnostics. 2021; 11(12):2249. https://doi.org/10.3390/diagnostics11122249
Chicago/Turabian StyleCoiro, Saverio, Elisa Gasparini, Giuseppe Falco, Giacomo Santandrea, Moira Foroni, Giulia Besutti, Valentina Iotti, Roberto Di Cicilia, Monica Foroni, Simone Mele, and et al. 2021. "Biomarkers Changes after Neoadjuvant Chemotherapy in Breast Cancer: A Seven-Year Single Institution Experience" Diagnostics 11, no. 12: 2249. https://doi.org/10.3390/diagnostics11122249
APA StyleCoiro, S., Gasparini, E., Falco, G., Santandrea, G., Foroni, M., Besutti, G., Iotti, V., Di Cicilia, R., Foroni, M., Mele, S., Ferrari, G., Bisagni, G., & Ragazzi, M. (2021). Biomarkers Changes after Neoadjuvant Chemotherapy in Breast Cancer: A Seven-Year Single Institution Experience. Diagnostics, 11(12), 2249. https://doi.org/10.3390/diagnostics11122249







