Socio-Organizational Impact of Confocal Laser Endomicroscopy in Neurosurgery and Neuropathology: Results from a Process Analysis and Expert Survey
Abstract
:1. Introduction
2. Methods
2.1. Process Analysis
2.2. Expert Survey
3. Results
3.1. Process Analysis (Results for RQ1)
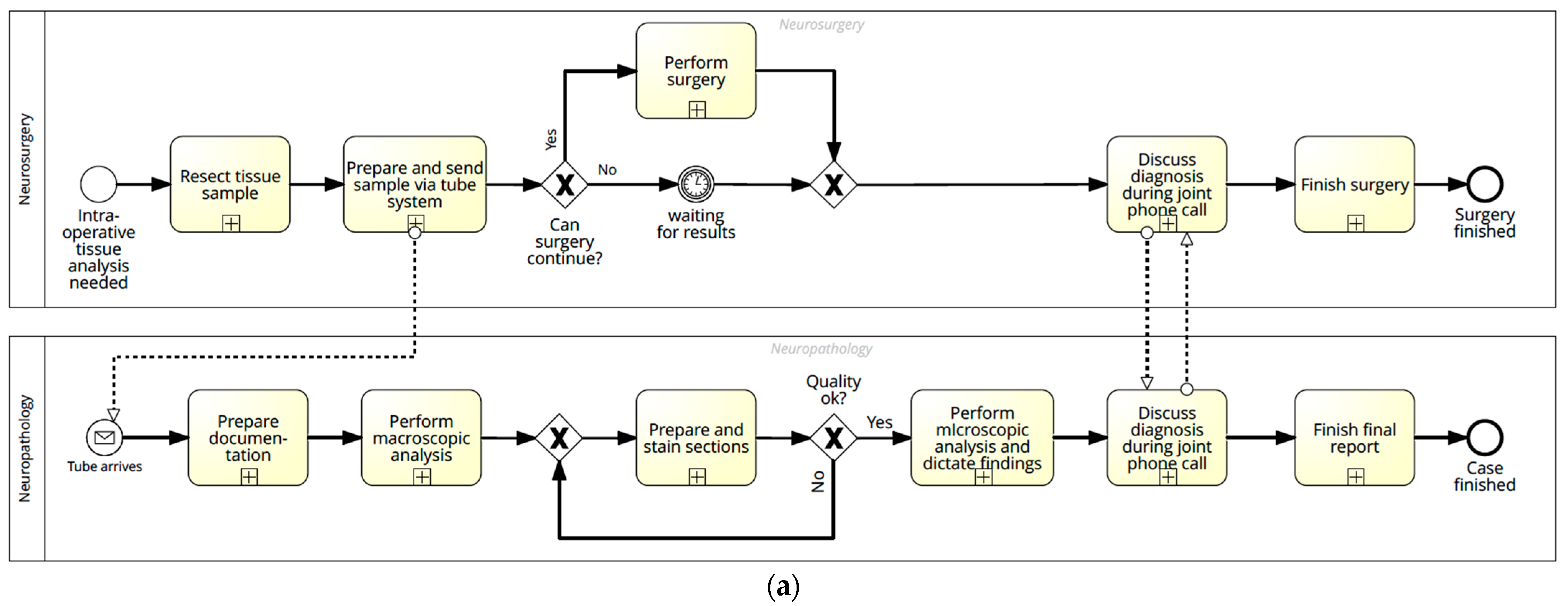
3.1.1. Current Process
3.1.2. CLE Process
3.1.3. Process Comparison
3.2. Expert Survey (Results for RQ2)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breuskin, D.; Szczygielski, J.; Urbschat, S.; Kim, Y.-J.; Oertel, J. Confocal Laser Endomicroscopy in Neurosurgery-An Alternative to Instantaneous Sections? World Neurosurg. 2017, 100, 180–185. [Google Scholar] [CrossRef]
- Sankar, T.; Delaney, P.M.; Ryan, R.W.; Eschbacher, J.; Abdelwahab, M.; Nakaji, P.; Coons, S.W.; Scheck, A.C.; Smith, K.A.; Spetzler, R.F.; et al. Miniaturized handheld confocal microscopy for neurosurgery: Results in an experimental glioblastoma model. Neurosurgery 2010, 66, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Eschbacher, J.; Hattendorf, G.; Coons, S.W.; Preul, M.C.; Smith, K.A.; Nakaji, P.; Spetzler, R.F. Intraoperative confocal microscopy for brain tumors: A feasibility analysis in humans. Neurosurgery 2011, 68, ons282–ons290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belykh, E.; Cavallo, C.; Gandhi, S.; Zhao, X.; Veljanoski, D.; Izady Yazdanabadi, M.; Martirosyan, N.L.; Byvaltsev, V.A.; Eschbacher, J.; Preul, M.C.; et al. Utilization of intraoperative confocal laser endomicroscopy in brain tumor surgery. J. Neurosurg. Sci. 2018, 62, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Charalampaki, P.; Javed, M.; Daali, S.; Heiroth, H.-J.; Igressa, A.; Weber, F. Confocal Laser Endomicroscopy for Real-time Histomorphological Diagnosis: Our Clinical Experience With 150 Brain and Spinal Tumor Cases. Neurosurgery 2015, 62, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Plesec, T.P.; Prayson, R.A. Frozen section discrepancy in the evaluation of central nervous system tumors. Arch. Pathol. Lab. Med. 2007, 131, 1532–1540. [Google Scholar] [CrossRef]
- Belykh, E.; Zhao, X.; Ngo, B.; Farhadi, D.S.; Byvaltsev, V.A.; Eschbacher, J.M.; Nakaji, P.; Preul, M.C. Intraoperative Confocal Laser Endomicroscopy Ex Vivo Examination of Tissue Microstructure During Fluorescence-Guided Brain Tumor Surgery. Front. Oncol. 2020, 10, 599250. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, N.L.; Eschbacher, J.M.; Kalani, M.Y.; Turner, J.D.; Belykh, E.; Spetzler, R.F.; Nakaji, P.; Preul, M.C. Prospective evaluation of the utility of intraoperative confocal laser endomicroscopy in patients with brain neoplasms using fluorescein sodium: Experience with 74 cases. Neurosurg. Focus 2016, 40, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlov, V.; Meyronet, D.; Meyer-Bisch, V.; Armoiry, X.; Pikul, B.; Dumot, C.; Beuriat, P.-A.; Signorelli, F.; Guyotat, J. Intraoperative Probe-Based Confocal Laser Endomicroscopy in Surgery and Stereotactic Biopsy of Low-Grade and High-Grade Gliomas: A Feasibility Study in Humans. Neurosurgery 2016, 79, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Eschbacher, J.; Martirosyan, N.L.; Nakaji, P.; Sanai, N.; Preul, M.C.; Smith, K.A.; Coons, S.W.; Spetzler, R.F. In vivo intraoperative confocal microscopy for real-time histopathological imaging of brain tumors. J. Neurosurg. 2012, 116, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Acerbi, F.; Pollo, B.; De Laurentis, C.; Restelli, F.; Falco, J.; Vetrano, I.G.; Broggi, M.; Schiariti, M.; Tramacere, I.; Ferroli, P.; et al. Ex Vivo Fluorescein-Assisted Confocal Laser Endomicroscopy (CONVIVO® System) in Patients With Glioblastoma: Results From a Prospective Study. Front. Oncol. 2020, 10, 606574. [Google Scholar] [CrossRef] [PubMed]
- Restelli, F.; Pollo, B.; Vetrano, I.G.; Cabras, S.; Broggi, M.; Schiariti, M.; Falco, J.; de Laurentis, C.; Raccuia, G.; Ferroli, P.; et al. Confocal Laser Microscopy in Neurosurgery: State of the Art of Actual Clinical Applications. J. Clin. Med. 2021, 10, 2035. [Google Scholar] [CrossRef]
- Goetz, M.; Kiesslich, R. Advances of endomicroscopy for gastrointestinal physiology and diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G797–G806. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansour, M.R.; Caycedo-Marulanda, A.; Davis, B.R.; Alawashez, A.; Docimo, S.; Qureshi, A.; Tsuda, S. SAGES TAVAC safety and efficacy analysis confocal laser endomicroscopy. Surg. Endosc. 2021, 35, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.P.; Liao, J.C. Confocal laser endomicroscopy of bladder and upper tract urothelial carcinoma: A new era of optical diagnosis? Curr. Urol. Rep. 2014, 15, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, F.S.; Zirlik, S.; Hildner, K.; Schubert, J.; Vieth, M.; Neurath, M.F. Confocal laser endomicroscopy for diagnosing lung cancer in vivo. Eur. Respir. J. 2013, 41, 1401–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sievert, M.; Auberville, M.; Oetter, N.; Stelzle, F.; Maier, A.; Mantsopoulos, K.; Iro, H.; Goncalves, M. Konfokale Laser-Endomikroskopie des Kopf-Hals-Plattenepithelkarzinoms: Eine systematische Übersicht. [Confocal laser endomicroscopy of head and neck squamous cell carcinoma: A systematic review]. Laryngorhinootologie 2021, 100, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Ammenwerth, E.; Hackl, W.O. IT-Assisted Process Management in Healthcare. Stud. Health Technol. Inform. 2020, 274, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Sooter, L.J.; Hasley, S.; Lario, R.; Rubin, K.S.; Hasić, F. Modeling a Clinical Pathway for Contraception. Appl. Clin. Inform. 2019, 10, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Peres Penteado, A.; Molina Cohrs, F.; Diniz Hummel, A.; Erbs, J.; Maciel, R.F.; Feijó Ortolani, C.L.; de Aguiar Roza, B.; Torres Pisa, I. Kidney Transplantation Process in Brazil Represented in Business Process Modeling Notation. Transplant. Proc. 2015, 47, 963–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fotteler, M.; Heninger, L.; Holl, F.; Schlegel, J.; Swoboda, W. Confocal Laser Endomicroscopy for Intraoperative Tumor Assessment: Development of a Conceptual Model for an Evaluation Study. Stud. Health Technol. Inform. 2019, 262, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Höhne, J.; Schebesch, K.-M.; Zoubaa, S.; Proescholdt, M.; Riemenschneider, M.J.; Schmidt, N.O. Intraoperative imaging of brain tumors with fluorescein: Confocal laser endomicroscopy in neurosurgery. Clinical and user experience. Neurosurg. Focus FOC 2021, 50, E19. [Google Scholar] [CrossRef]
- Eschbacher, J.M.; Abramov, I.; Belykh, E.; Dru, A.; Kris, S.; Porter, R.; Little, A.; Lawton, M.; Preul, M. Correlation of Intraoperative Confocal Laser Endomicroscopy Imaging to Pathology for Brain Tumors. In Proceedings of the 97th Annual Meeting of the American Association of Neuropathologists, St. Louis, MO, USA, 10–13 June 2021; pp. 558–607. [Google Scholar]
- Object Management Group. Business Process Model and Notation. Available online: https://www.bpmn.org/ (accessed on 31 August 2021).
- Martirosyan, N.L.; Georges, J.; Kalani, M.Y.; Nakaji, P.; Spetzler, R.F.; Feuerstein, B.G.; Preul, M.C. Handheld confocal laser endomicroscopic imaging utilizing tumor-specific fluorescent labeling to identify experimental glioma cells in vivo. Surg. Neurol. Int. 2016, 7, S995–S1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childers, C.P.; Maggard-Gibbons, M. Understanding Costs of Care in the Operating Room. JAMA Surgery 2018, 153, e176233. [Google Scholar] [CrossRef] [PubMed]
- Foersch, S.; Heimann, A.; Ayyad, A.; Spoden, G.A.; Florin, L.; Mpoukouvalas, K.; Kiesslich, R.; Kempski, O.; Goetz, M.; Charalampaki, P. Confocal laser endomicroscopy for diagnosis and histomorphologic imaging of brain tumors in vivo. PLoS ONE 2012, 7, e41760. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Georges, J.; Eschbacher, J.M.; Cavalcanti, D.D.; Elhadi, A.M.; Abdelwahab, M.G.; Scheck, A.C.; Nakaji, P.; Spetzler, R.F.; Preul, M.C. Potential application of a handheld confocal endomicroscope imaging system using a variety of fluorophores in experimental gliomas and normal brain. Neurosurg. Focus 2014, 36, E16. [Google Scholar] [CrossRef]
- Dietz, R.L.; Hartman, D.J.; Pantanowitz, L. Systematic Review of the Use of Telepathology During Intraoperative Consultation. Am. J. Clin. Pathol. 2020, 153, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Charalampaki, P.; Liu, Y.; Yang, G.-Z.; Giannarou, S. Context aware decision support in neurosurgical oncology based on an efficient classification of endomicroscopic data. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1187–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziebart, A.; Stadniczuk, D.; Roos, V.; Ratliff, M.; von Deimling, A.; Hänggi, D.; Enders, F. Deep Neural Network for Differentiation of Brain Tumor Tissue Displayed by Confocal Laser Endomicroscopy. Front. Oncol. 2021, 11, 668273. [Google Scholar] [CrossRef] [PubMed]
- Belykh, E.; Miller, E.J.; Patel, A.A.; Yazdanabadi, M.I.; Martirosyan, N.L.; Yağmurlu, K.; Bozkurt, B.; Byvaltsev, V.A.; Eschbacher, J.M.; Nakaji, P.; et al. Diagnostic Accuracy of a Confocal Laser Endomicroscope for In Vivo Differentiation Between Normal Injured And Tumor Tissue During Fluorescein-Guided Glioma Resection: Laboratory Investigation. World Neurosurg. 2018, 115, e337–e348. [Google Scholar] [CrossRef] [PubMed]

| Section | Questions |
|---|---|
| 1. General information | How old are you? What is your gender? What is your position? How long have you been working in this position? |
| 2. Your work with the device | Conditional question depending on position: How many operations have you conducted/assisted with using the device?/What is the number of operations using the device you analyzed images of? 1 What tasks do you conduct with the device? (multiple choice) How does your workload change when using the device? Conditional question: Please explain how your workload increased/decreased. 1 |
| 3. Your opinion of the device | How do you rate the following indicators on a scale from 1–3: usability, integration, reliability, data/image quality, medicinal use, patient safety, data protection? Do you think it makes sense to use the device for standard diagnostics? Conditional question: Please comment why you do not think that this makes sense. 1 Do you think, surgery duration could be shortened using the device? What is your opinion on telepathology? How satisfied are you overall with the device? |
| 4. Final questions | What do you think are the biggest advantages/chances of the device? What do you think are the biggest disadvantages/risks of the device? |
| Current Process | CLE Process | |
|---|---|---|
| Parties | 2 | 2 |
| Staff members | 6–7 | 3 |
| Tasks total | 42 | 26 |
| Tasks neurosurgery | 12 | 17 |
| Tasks neuropathology | 30 | 9 |
| Transfers between parties | 2 | 2 |
| Medium of transfer | tube (paper, sample), phone | digital, phone |
| Time until results are ready | ~18:02 min | immediately (continuous communication) |
| Total | Satisfaction with CLE Device (N) 1 | ||
|---|---|---|---|
| N (%) | Satisfied | Neutral | |
| Age | |||
| Below 30 years | 3 (25.0) | 3 | 0 |
| 30–59 years | 8 (66.7) | 6 | 2 |
| 60 years or older | 1 (8.3) | 1 | 0 |
| Gender | |||
| Female | 5 (41.7) | 4 | 1 |
| Male | 7 (58.3) | 6 | 1 |
| Position | |||
| Neurosurgeon | 6 (50.0) | 5 | 1 |
| Neuropathologist | 3 (25.0) | 2 | 1 |
| Medical student | 3 (25.0) | 3 | 0 |
| Experience | |||
| <5 years | 3 (25.0) | 3 | 0 |
| 5–10 years | 5 (41.7) | 4 | 1 |
| >10 years | 4 (33.3) | 3 | 1 |
| Number of operations with CLE | |||
| <5 | 1 (8.3) | 0 | 1 |
| 5–20 | 4 (33.3) | 4 | 0 |
| >20 | 7 (58.3) | 6 | 1 |
| Advantages | Disadvantages |
|---|---|
| Training and experience needed (N = 6) Imprecise diagnosis, risk of misinterpretation (N = 4) User dependent (N = 2) Additional effort for neurosurgery (N = 2) Price (N = 1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotteler, M.L.; Liesche-Starnecker, F.; Brielmaier, M.C.; Schobel, J.; Gempt, J.; Schlegel, J.; Swoboda, W. Socio-Organizational Impact of Confocal Laser Endomicroscopy in Neurosurgery and Neuropathology: Results from a Process Analysis and Expert Survey. Diagnostics 2021, 11, 2128. https://doi.org/10.3390/diagnostics11112128
Fotteler ML, Liesche-Starnecker F, Brielmaier MC, Schobel J, Gempt J, Schlegel J, Swoboda W. Socio-Organizational Impact of Confocal Laser Endomicroscopy in Neurosurgery and Neuropathology: Results from a Process Analysis and Expert Survey. Diagnostics. 2021; 11(11):2128. https://doi.org/10.3390/diagnostics11112128
Chicago/Turabian StyleFotteler, Marina L., Friederike Liesche-Starnecker, Maria C. Brielmaier, Johannes Schobel, Jens Gempt, Jürgen Schlegel, and Walter Swoboda. 2021. "Socio-Organizational Impact of Confocal Laser Endomicroscopy in Neurosurgery and Neuropathology: Results from a Process Analysis and Expert Survey" Diagnostics 11, no. 11: 2128. https://doi.org/10.3390/diagnostics11112128
APA StyleFotteler, M. L., Liesche-Starnecker, F., Brielmaier, M. C., Schobel, J., Gempt, J., Schlegel, J., & Swoboda, W. (2021). Socio-Organizational Impact of Confocal Laser Endomicroscopy in Neurosurgery and Neuropathology: Results from a Process Analysis and Expert Survey. Diagnostics, 11(11), 2128. https://doi.org/10.3390/diagnostics11112128







