Detection of 2-Hydroxyglutarate by 3.0-Tesla Magnetic Resonance Spectroscopy in Gliomas with Rare IDH Mutations: Making Sense of “False-Positive” Cases
Abstract
:1. Introduction
2. Materials and Methods
3. Results
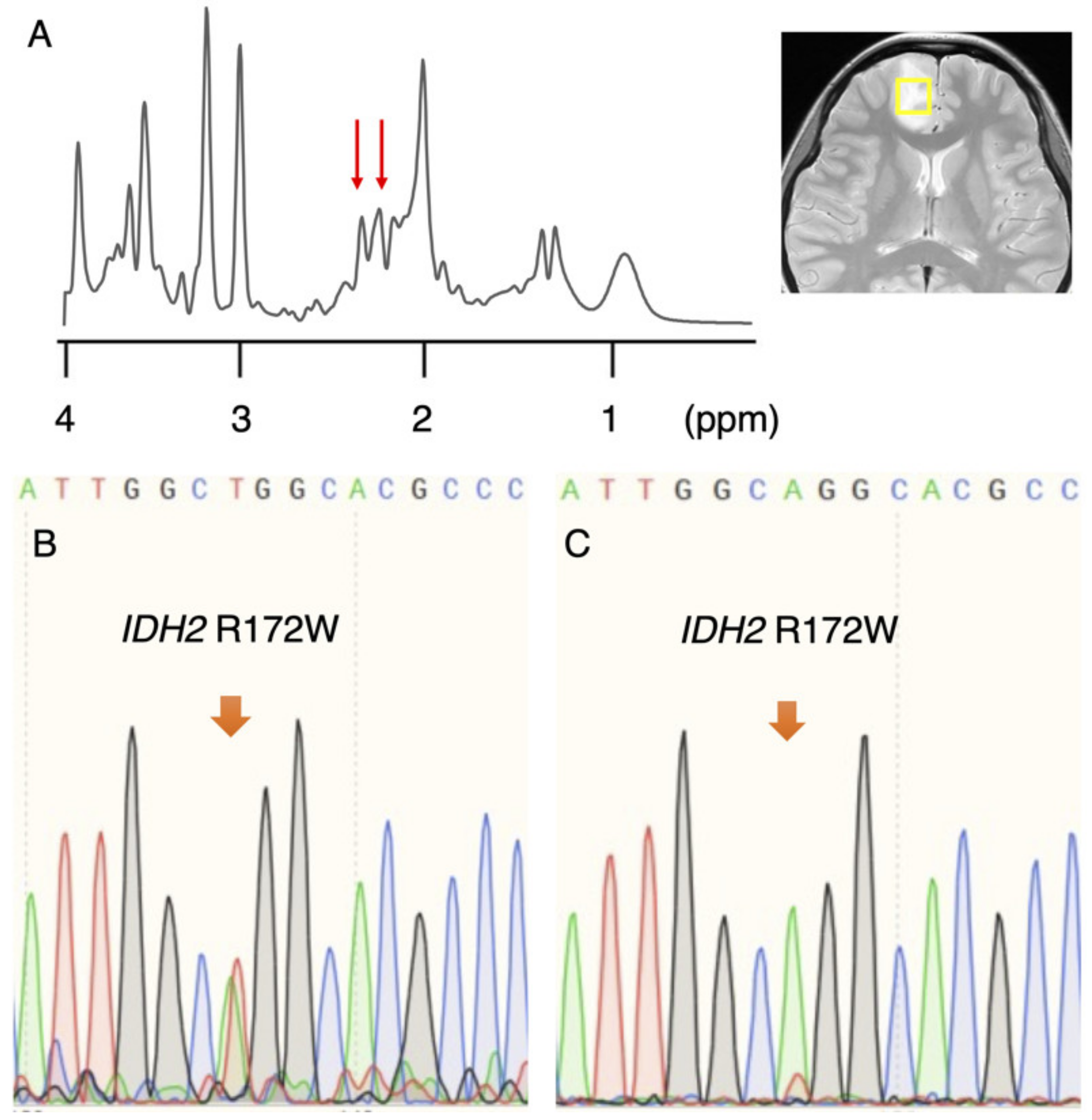
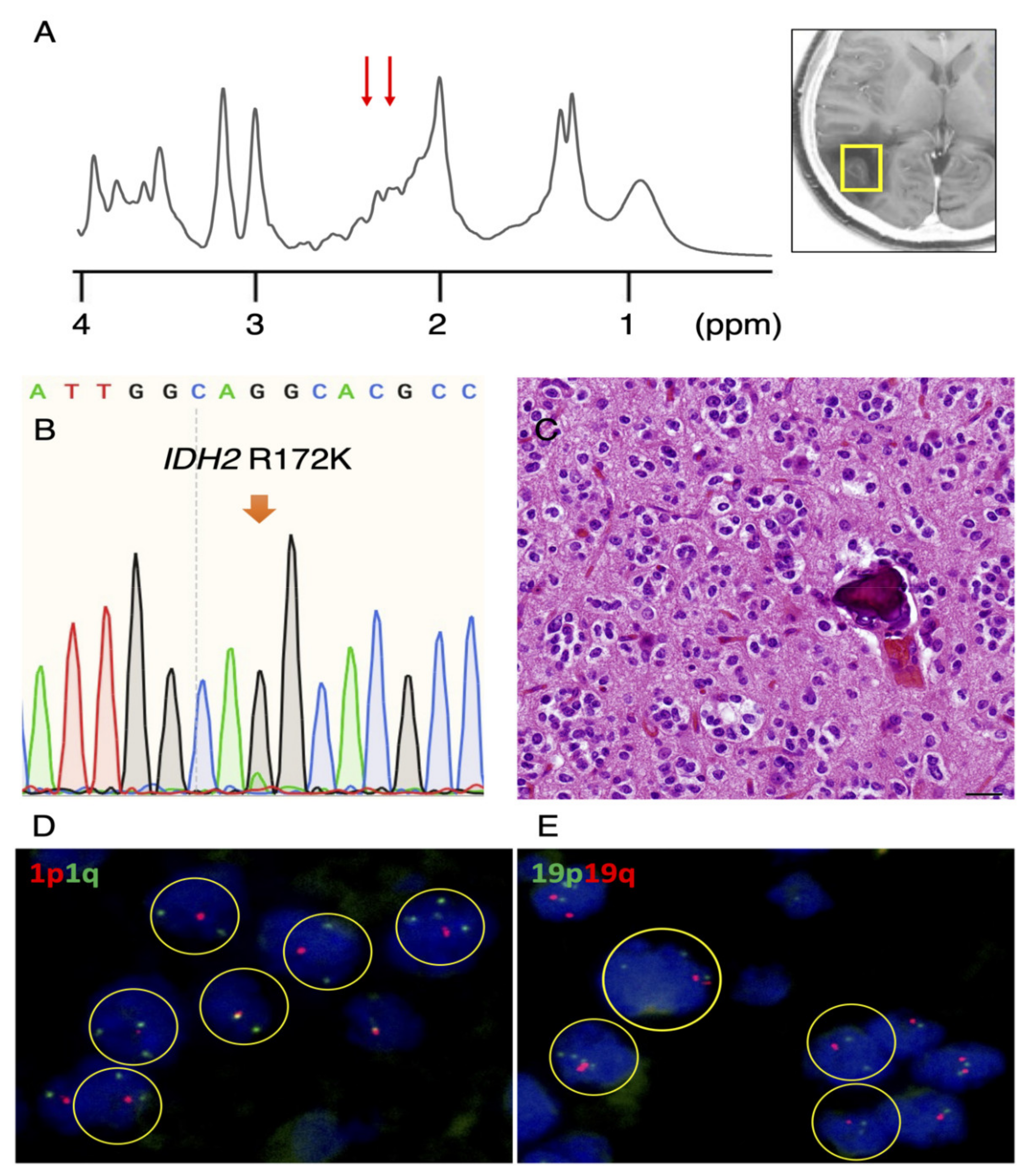
3.1. Rare IDH2 Mutation Detected in a Recurrent “False-Positive”Case
3.2. Re-Evaluation of IDH1 and IDH2 Mutations in Remaining Cases
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Natsumeda, M.; Igarashi, H.; Nomura, T.; Ogura, R.; Tsukamoto, Y.; Kobayashi, T.; Aoki, H.; Okamoto, K.; Kakita, A.; Takahashi, H.; et al. Accumulation of 2-hydroxyglutarate in gliomas correlates with survival: A study by 3.0-tesla magnetic resonance spectroscopy. Acta Neuropathol. Commun. 2014, 2, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provencher, S. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Meyer, J.; Balss, J.; Capper, D.; Mueller, W.; Christians, A.; Felsberg, J.; Wolter, M.; Mawrin, C.; Wick, W.; et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: A study of 1,010 diffuse gliomas. Acta Neuropathol. 2009, 118, 469–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambros, P.F.; Ambros, I.M. Pathology and biology guidelines for resectable and unresectable neuroblastic tumors and bone marrow examination guidelines. Med. Pediatr. Oncol. 2001, 37, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Ellison, D.W.; Figarella-Branger, D.; Perry, A.; Reifenberger, G.; von Deimling, A. WHO Classification of Tumours of the Central Nervous System; IARC: Lyon, France, 2016. [Google Scholar]
- Natsumeda, M.; Motohashi, K.; Igarashi, H.; Nozawa, T.; Abe, H.; Tsukamoto, Y.; Ogura, R.; Okada, M.; Kobayashi, T.; Aoki, H.; et al. Reliable diagnosis of IDH-mutant glioblastoma by 2-hydroxyglutarate detection: A study by 3-T magnetic resonance spectroscopy. Neurosurg. Rev. 2018, 41, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Tanaka, K.; Sasayama, T.; Irino, Y.; Sato, N.; Takeuchi, Y.; Kyotani, K.; Mukasa, A.; Mizukawa, K.; Sakata, J.; et al. Diagnostic value of glutamate with 2-hydroxyglutarate in magnetic resonance spectroscopy for IDH1 mutant glioma. Neuro-Oncology 2016, 18, 1559–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, C.H.; Kim, H.S.; Park, J.E.; Jung, S.C.; Choi, C.G.; Woo, D.C.; Lee, H.B.; Kim, S.J. Comparative Value of 2-Hydroxyglutarate-to-Lipid and Lactate Ratio versus 2-Hydroxyglutarate Concentration on MR Spectroscopic Images for Predicting Isocitrate Dehydrogenase Mutation Status in Gliomas. Radiol. Imaging Cancer 2020, 2, e190083. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Ganji, S.; Hulsey, K.; Madan, A.; Kovacs, Z.; Dimitrov, I.; Zhang, S.; Pichumani, K.; Mendelsohn, D.; Mickey, B.; et al. A comparative study of short- and long-TE (1)H MRS at 3 T for in vivo detection of 2-hydroxyglutarate in brain tumors. NMR Biomed. 2013, 26, 1242–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindaraju, V.Y.K.; Maudsley, A.A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000, 13, 129–153. [Google Scholar] [CrossRef]
- Pope, W.B.; Prins, R.M.; Albert Thomas, M.; Nagarajan, R.; Yen, K.E.; Bittinger, M.A.; Salamon, N.; Chou, A.P.; Yong, W.H.; Soto, H.; et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J. Neurooncol. 2012, 107, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, C.; Ganji, S.K.; DeBerardinis, R.J.; Hatanpaa, K.J.; Rakheja, D.; Kovacs, Z.; Yang, X.L.; Mashimo, T.; Raisanen, J.M.; Marin-Valencia, I.; et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 2012, 18, 624–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barritault, M.; Picart, T.; Poncet, D.; Fenouil, T.; d’Hombres, A.; Gabut, M.; Guyotat, J.; Jouanneau, E.; Ameli, R.; Joubert, B.; et al. Avoiding New Biopsies by Identification of IDH1 and TERT Promoter Mutation in Nondiagnostic Biopsies from Glioma Patients. Neurosurgery 2020, 87, E513–E519. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.; Cairns, R.A.; Minden, M.D.; Driggers, E.M.; Bittinger, M.A.; Jang, H.G.; Sasaki, M.; Jin, S.; Schenkein, D.P.; Su, S.M.; et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J. Exp. Med. 2010, 207, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH 1 and IDH 2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Capper, D.; Weissert, S.; Balss, J.; Habel, A.; Meyer, J.; Jager, D.; Ackermann, U.; Tessmer, C.; Korshunov, A.; Zentgraf, H.; et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010, 20, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Jin, G.; Kuan, C.T.; McLendon, R.E.; Yan, H.; Bigner, D.D. A monoclonal antibody IMab-1 specifically recognizes IDH1R132H, the most common glioma-derived mutation. Biochem. Biophys. Res. Commun. 2009, 390, 547–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, Y. Specific monoclonal antibodies against IDH1/2 mutations as diagnostic tools for gliomas. Brain Tumor Pathol. 2015, 32, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Tesileanu, C.M.S.; Vallentgoed, W.R.; Sanson, M.; Taal, W.; Clement, P.M.; Wick, W.; Brandes, A.A.; Baurain, J.F.; Chinot, O.L.; Wheeler, H.; et al. Non-IDH1-R132H IDH1/2 mutations are associated with increased DNA methylation and improved survival in astrocytomas, compared to IDH1-R132H mutations. Acta Neuropathol. 2021, 141, 945–957. [Google Scholar] [CrossRef] [PubMed]
| Case No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age | 30 | 51 | 59 | 72 | 67 |
| Sex | F | F | M | F | F |
| Location | Rt Frontal | Rt Parietal | Rt Frontal | Rt Temporal | Lt Thalamus |
| 2HG (mM) | 6.820 | 2.763 | 5.589 | 4.477 | 5.448 |
| Lactate (mM) | 1.912 | 4.824 | 0.189 | 0.000 | 6.874 |
| IDH1 R132H IHC | Negative | Negative | Negative | Negative | Negative |
| IDH 1/2 sequence | IDH2 R172W | IDH2 R172K | WT 6 | WT 6 | N/A 4 |
| Mutant peak | Small | Small | None | None | N/A 4 |
| 1p/19q FISH | 1p/19q codel 1 | 1p/19q codel 1 | N/A 4 | N/A 4 | N/A 4 |
| Pathological diagnosis | OD 5 | OD 5 | DA 2 | GBM 3 | DA 2 |
| Metabolite | Median Concentration (mM) True-Positive Cases (n = 2) | Median Concentration (mM) False Positive Cases (n = 3) | p-Value |
|---|---|---|---|
| GSH 1 | 1.020 | 1.839 | 0.40 |
| 2HG 2 | 4.792 | 5.448 | >0.99 |
| mI 3 | 5.561 | 3.862 | 0.40 |
| Lac 4 | 4.393 | 0.189 | 0.40 |
| tCh 5 | 1.563 | 1.860 | 0.80 |
| tNAA 6 | 3.165 | 4.085 | 0.40 |
| tCr 7 | 3.803 | 5.890 | 0.80 |
| Glu+Gln 8 | 5.055 | 12.35 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natsumeda, M.; Igarashi, H.; Gabdulkhaev, R.; Takahashi, H.; Motohashi, K.; Ogura, R.; Watanabe, J.; Tsukamoto, Y.; Okamoto, K.; Kakita, A.; et al. Detection of 2-Hydroxyglutarate by 3.0-Tesla Magnetic Resonance Spectroscopy in Gliomas with Rare IDH Mutations: Making Sense of “False-Positive” Cases. Diagnostics 2021, 11, 2129. https://doi.org/10.3390/diagnostics11112129
Natsumeda M, Igarashi H, Gabdulkhaev R, Takahashi H, Motohashi K, Ogura R, Watanabe J, Tsukamoto Y, Okamoto K, Kakita A, et al. Detection of 2-Hydroxyglutarate by 3.0-Tesla Magnetic Resonance Spectroscopy in Gliomas with Rare IDH Mutations: Making Sense of “False-Positive” Cases. Diagnostics. 2021; 11(11):2129. https://doi.org/10.3390/diagnostics11112129
Chicago/Turabian StyleNatsumeda, Manabu, Hironaka Igarashi, Ramil Gabdulkhaev, Haruhiko Takahashi, Kunio Motohashi, Ryosuke Ogura, Jun Watanabe, Yoshihiro Tsukamoto, Kouichirou Okamoto, Akiyoshi Kakita, and et al. 2021. "Detection of 2-Hydroxyglutarate by 3.0-Tesla Magnetic Resonance Spectroscopy in Gliomas with Rare IDH Mutations: Making Sense of “False-Positive” Cases" Diagnostics 11, no. 11: 2129. https://doi.org/10.3390/diagnostics11112129
APA StyleNatsumeda, M., Igarashi, H., Gabdulkhaev, R., Takahashi, H., Motohashi, K., Ogura, R., Watanabe, J., Tsukamoto, Y., Okamoto, K., Kakita, A., Nakada, T., & Fujii, Y. (2021). Detection of 2-Hydroxyglutarate by 3.0-Tesla Magnetic Resonance Spectroscopy in Gliomas with Rare IDH Mutations: Making Sense of “False-Positive” Cases. Diagnostics, 11(11), 2129. https://doi.org/10.3390/diagnostics11112129







