Changes in Plasma Phospholipid Metabolism Are Associated with Clinical Manifestations of Systemic Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Plasma Samples
2.2. Sample Preparation
2.3. HPLC-IM-QTOF Measurement
2.4. Data Processing and Statistical Analysis
3. Results
3.1. Study Subjects
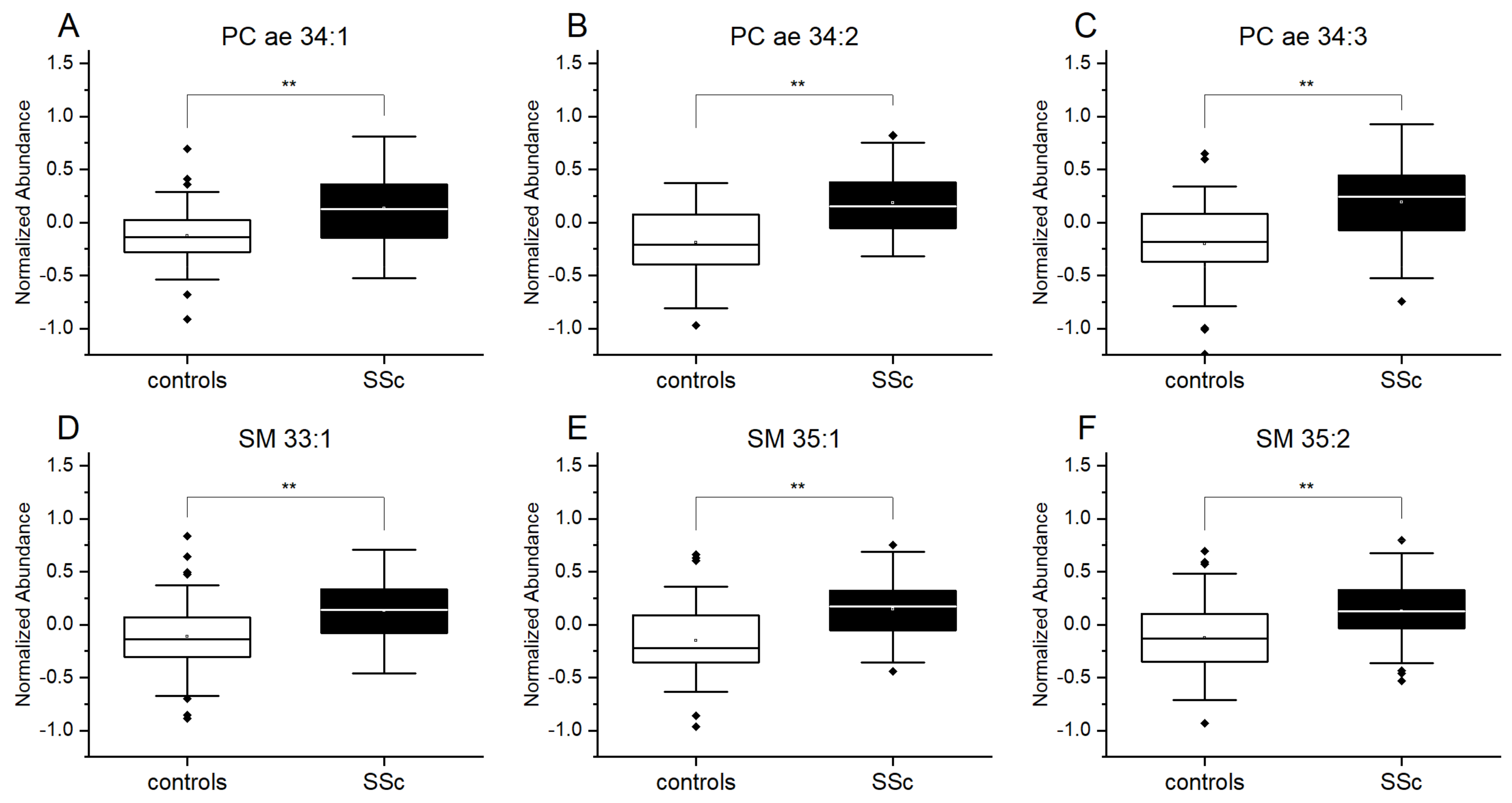
3.2. Plasmalogens and Sphingolipids from Plasma Are Increased in Patients with SSc
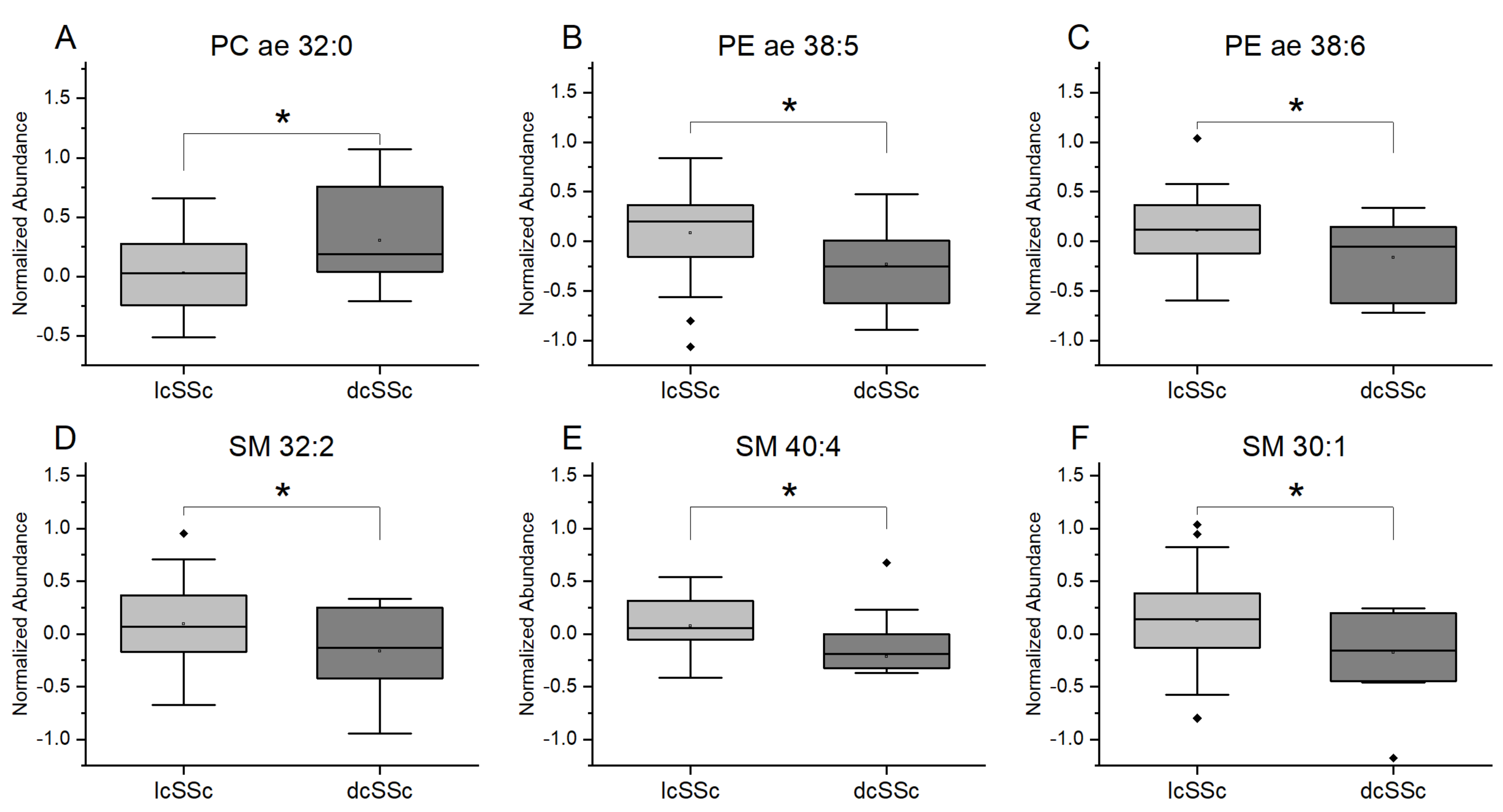
3.3. Differences in Plasmalogens and Sphingolipids from Plasma in Patients with lcSSc and dcSSc
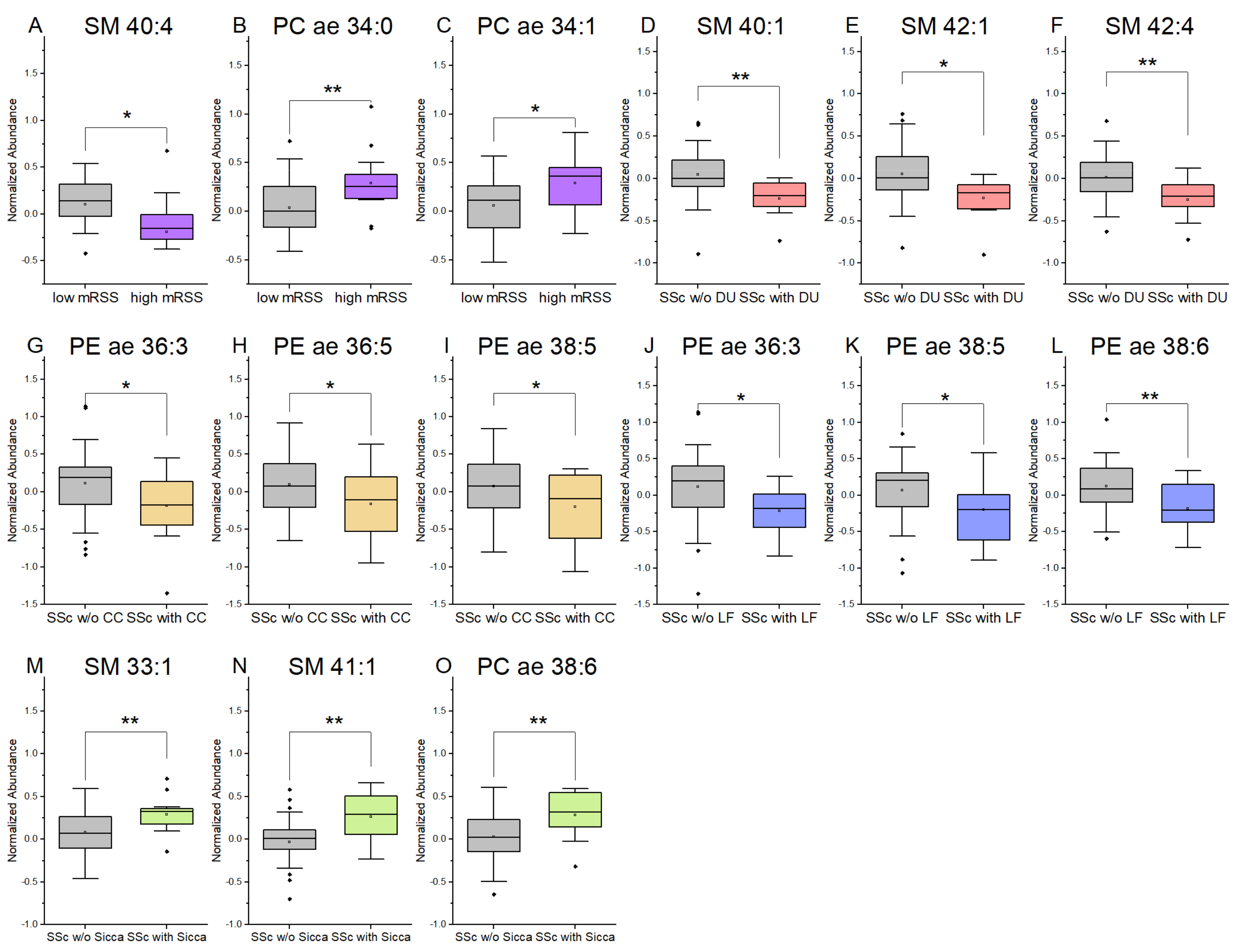
3.4. Plasmalogens and Sphingolipids from Plasma Are Associated with Clinical Symptoms in SSc Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Kowal-Bielecka, O.; Fransen, J.; Avouac, J.; Becker, M.; Kulak, A.; Allanore, Y.; Distler, O.; Clements, P.; Cutolo, M.; Czirjak, L.; et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1327–1339. [Google Scholar] [CrossRef]
- Distler, O.; Gahlemann, M.; Maher, T.M. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. Reply. N. Engl. J. Med. 2019, 381, 1596–1597. [Google Scholar] [CrossRef]
- Ferreira, H.B.; Pereira, A.M.; Melo, T.; Paiva, A.; Domingues, M.R. Lipidomics in autoimmune diseases with main focus on systemic lupus erythematosus. J. Pharm. Biomed. Anal. 2019, 174, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Del Boccio, P.; Pieragostino, D.; Di Ioia, M.; Petrucci, F.; Lugaresi, A.; De Luca, G.; Gambi, D.; Onofrj, M.; Di Ilio, C.; Sacchetta, P.; et al. Lipidomic investigations for the characterization of circulating serum lipids in multiple sclerosis. J. Proteom. 2011, 74, 2826–2836. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; D’Alessandro, M.; Rizzello, A.; De Riccardis, L.; Lunetti, P.; Del Boccio, P.; De Robertis, F.; Trianni, G.; Maffia, M.; Giudetti, A.M. A lipidomic approach to the study of human CD4(+) T lymphocytes in multiple sclerosis. BMC Neurosci. 2015, 16, 46. [Google Scholar] [CrossRef][Green Version]
- Ferreira, H.B.; Melo, T.; Monteiro, A.; Paiva, A.; Domingues, P.; Domingues, M.R. Serum phospholipidomics reveals altered lipid profile and promising biomarkers in multiple sclerosis. Arch. Biochem. Biophys. 2021, 697, 108672. [Google Scholar] [CrossRef]
- Łuczaj, W.; Moniuszko-Malinowska, A.; Domingues, P.; Domingues, M.R.; Gindzienska-Sieskiewicz, E.; Skrzydlewska, E. Plasma lipidomic profile signature of rheumatoid arthritis versus Lyme arthritis patients. Arch. Biochem. Biophys. 2018, 654, 105–114. [Google Scholar] [CrossRef]
- Raouf, J.; Idborg, H.; Englund, P.; Alexanderson, H.; Dastmalchi, M.; Jakobsson, P.-J.; Lundberg, I.E.; Korotkova, M. Targeted lipidomics analysis identified altered serum lipid profiles in patients with polymyositis and dermatomyositis. Arthritis Res. Ther. 2018, 20, 83. [Google Scholar] [CrossRef]
- Borba, E.F.; Borges, C.T.L.; Bonfá, E. Lipoprotein profile in limited systemic sclerosis. Rheumatol. Int. 2005, 25, 379–383. [Google Scholar] [CrossRef]
- Tokumura, A.; Carbone, L.D.; Yoshioka, Y.; Morishige, J.; Kikuchi, M.; Postlethwaite, A.; Watsky, M.A. Elevated serum levels of arachidonoyl-lysophosphatidic acid and sphingosine 1-phosphate in systemic sclerosis. Int. J. Med. Sci. 2009, 6, 168–176. [Google Scholar] [CrossRef]
- Higuchi, T.; Takagi, K.; Tochimoto, A.; Ichimura, Y.; Norose, T.; Katsumata, Y.; Masuda, I.; Yamanaka, H.; Morohoshi, T.; Kawaguchi, Y. Antifibrotic effects of 2-carba cyclic phosphatidic acid (2ccPA) in systemic sclerosis: Contribution to the novel treatment. Arthritis Res. Ther. 2019, 21, 103. [Google Scholar] [CrossRef]
- Stoddard, N.C.; Chun, J. Promising pharmacological directions in the world of lysophosphatidic Acid signaling. Biomol. Ther. (Seoul) 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Ohashi, T.; Yamamoto, T. Antifibrotic effect of lysophosphatidic acid receptors LPA1 and LPA3 antagonist on experimental murine scleroderma induced by bleomycin. Exp. Dermatol. 2015, 24, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Ottria, A.; Hoekstra, A.T.; Zimmermann, M.; van der Kroef, M.; Vazirpanah, N.; Cossu, M.; Chouri, E.; Rossato, M.; Beretta, L.; Tieland, R.G.; et al. Fatty Acid and Carnitine Metabolism Are Dysregulated in Systemic Sclerosis Patients. Front. Immunol. 2020, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.P.; Weiss, S.B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J. Biol. Chem. 1956, 222, 193–214. [Google Scholar] [CrossRef]
- O’Donnell, V.B.; Rossjohn, J.; Wakelam, M.J. Phospholipid signaling in innate immune cells. J. Clin. Investig. 2018, 128, 2670–2679. [Google Scholar] [CrossRef] [PubMed]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta 2012, 1822, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Wallner, S.; Orsó, E.; Grandl, M.; Konovalova, T.; Liebisch, G.; Schmitz, G. Phosphatidylcholine and phosphatidylethanolamine plasmalogens in lipid loaded human macrophages. PLoS ONE 2018, 13, e0205706. [Google Scholar] [CrossRef] [PubMed]
- Leidl, K.; Liebisch, G.; Richter, D.; Schmitz, G. Mass spectrometric analysis of lipid species of human circulating blood cells. Biochim. Biophys. Acta 2008, 1781, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Wallner, S.; Schmitz, G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem. Phys. Lipids 2011, 164, 573–589. [Google Scholar] [CrossRef]
- Ishay, Y.; Nachman, D.; Khoury, T.; Ilan, Y. The role of the sphingolipid pathway in liver fibrosis: An emerging new potential target for novel therapies. Am. J. Physiol. Cell Physiol. 2020, 318, C1055–C1064. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Hussain, M.M. Sphingolipids and Lipoproteins in Health and Metabolic Disorders. Trends Endocrinol. Metab. 2017, 28, 506–518. [Google Scholar] [CrossRef]
- Gabandé-Rodríguez, E.; Boya, P.; Labrador, V.; Dotti, C.G.; Ledesma, M.D. High sphingomyelin levels induce lysosomal damage and autophagy dysfunction in Niemann Pick disease type A. Cell Death Differ. 2014, 21, 864–875. [Google Scholar] [CrossRef]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Lundberg, A.C.; Akesson, A.; Akesson, B. Dietary intake and nutritional status in patients with systemic sclerosis. Ann. Rheum. Dis. 1992, 51, 1143–1148. [Google Scholar] [CrossRef]
- Schmidt, K.G.; Herrero San Juan, M.; Trautmann, S.; Berninger, L.; Schwiebs, A.; Ottenlinger, F.M.; Thomas, D.; Zaucke, F.; Pfeilschifter, J.M.; Radeke, H.H. Sphingosine-1-Phosphate Receptor 5 Modulates Early-Stage Processes during Fibrogenesis in a Mouse Model of Systemic Sclerosis: A Pilot Study. Front. Immunol. 2017, 8, 1242. [Google Scholar] [CrossRef]
- Hu, C.; Zhou, J.; Yang, S.; Li, H.; Wang, C.; Fang, X.; Fan, Y.; Zhang, J.; Han, X.; Wen, C. Oxidative stress leads to reduction of plasmalogen serving as a novel biomarker for systemic lupus erythematosus. Free Radic. Biol. Med. 2016, 101, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, H.; Zhou, P.; Zhang, J.; Dai, Y.; Yang, D.; Fan, X.; Pan, H. Oral Phosphatidylcholine Improves Intestinal Barrier Function in Drug-Induced Liver Injury in Rats. Gastroenterol. Res. Pract. 2019, 2019, 8723460. [Google Scholar] [CrossRef] [PubMed]
- Treede, I.; Braun, A.; Sparla, R.; Kühnel, M.; Giese, T.; Turner, J.R.; Anes, E.; Kulaksiz, H.; Füllekrug, J.; Stremmel, W.; et al. Anti-inflammatory effects of phosphatidylcholine. J. Biol. Chem. 2007, 282, 27155–27164. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wen, B.; Hou, G.; Lei, L.; Mei, Z.; Jia, X.; Chen, X.; Zhu, W.; Li, J.; Kuang, Y.; et al. Lipidomics profiling reveals the role of glycerophospholipid metabolism in psoriasis. Gigascience 2017, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, L.; Lindgärde, F.; Manthorpe, R.; Akesson, B. Correlation of fatty acid composition of adipose tissue lipids and serum phosphatidylcholine and serum concentrations of micronutrients with disease duration in rheumatoid arthritis. Ann. Rheum. Dis. 1990, 49, 901–905. [Google Scholar] [CrossRef]
- Bălănescu, P.; Lădaru, A.; Bălănescu, E.; Băicuş, C.; Dan, G.A. Association of anti phosphatidyl ethanolamine antibodies and low complement levels in systemic sclerosis patients—results of a cross-sectional study. Scand. J. Clin. Lab. Investig. 2015, 75, 476–481. [Google Scholar] [CrossRef]
- Vazquez-de-Lara, L.G.; Tlatelpa-Romero, B.; Romero, Y.; Fernández-Tamayo, N.; Vazquez-de-Lara, F.; Justo-Janeiro, J.M.; Garcia-Carrasco, M.; de-la-Rosa Paredes, R.; Cisneros-Lira, J.G.; Mendoza-Milla, C.; et al. Phosphatidylethanolamine Induces an Antifibrotic Phenotype in Normal Human Lung Fibroblasts and Ameliorates Bleomycin-Induced Lung Fibrosis in Mice. Int. J. Mol. Sci. 2018, 19, 2758. [Google Scholar] [CrossRef]
- Shea, B.S.; Tager, A.M. Sphingolipid regulation of tissue fibrosis. Open Rheumatol. J. 2012, 6, 123–129. [Google Scholar] [CrossRef]
- Huwiler, A.; Pfeilschifter, J. Sphingolipid signaling in renal fibrosis. Matrix Biol. 2018, 68–69, 230–247. [Google Scholar] [CrossRef]
- Myśliwiec, H.; Baran, A.; Harasim-Symbor, E.; Choromańska, B.; Myśliwiec, P.; Milewska, A.J.; Chabowski, A.; Flisiak, I. Increase in circulating sphingosine-1-phosphate and decrease in ceramide levels in psoriatic patients. Arch. Dermatol. Res. 2017, 309, 79–86. [Google Scholar] [CrossRef]
- Cui, L.; Houston, D.A.; Farquharson, C.; MacRae, V.E. Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone 2016, 87, 147–158. [Google Scholar] [CrossRef]
- Shanahan, C.M. Autophagy and matrix vesicles: New partners in vascular calcification. Kidney Int. 2013, 83, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C.; Garimella, R.; Tague, S.E. The role of matrix vesicles in growth plate development and biomineralization. Front. Biosci. 2005, 10, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Shine, W.E.; McCulley, J.P. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch. Ophthalmol. 1998, 116, 849–852. [Google Scholar] [CrossRef]
- Parnetti, L.; Mignini, F.; Tomassoni, D.; Traini, E.; Amenta, F. Cholinergic precursors in the treatment of cognitive impairment of vascular origin: Ineffective approaches or need for re-evaluation? J. Neurol. Sci. 2007, 257, 264–269. [Google Scholar] [CrossRef]
- Choi, J.J.; Hwang, J.S.; Shin, Y.J. Effect of Oral Choline Alfoscerate on Patients with Keratoconjunctivitis Sicca. Nutrients 2020, 12, 1526. [Google Scholar] [CrossRef]
- Messias, M.C.F.; Mecatti, G.C.; Priolli, D.G.; de Oliveira Carvalho, P. Plasmalogen lipids: Functional mechanism and their involvement in gastrointestinal cancer. Lipids Health Dis. 2018, 17, 1–12. [Google Scholar] [CrossRef]
- Perrotti, F.; Rosa, C.; Cicalini, I.; Sacchetta, P.; Del Boccio, P.; Genovesi, D.; Pieragostino, D. Advances in Lipidomics for Cancer Biomarkers Discovery. Int. J. Mol. Sci. 2016, 17, 1992. [Google Scholar] [CrossRef]
- Gerbig, S.; Golf, O.; Balog, J.; Denes, J.; Baranyai, Z.; Zarand, A.; Raso, E.; Timar, J.; Takats, Z. Analysis of colorectal adenocarcinoma tissue by desorption electrospray ionization mass spectrometric imaging. Anal. Bioanal. Chem. 2012, 403, 2315–2325. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Akita, H.; Takemasa, I.; Eguchi, H.; Pastural, E.; Nagano, H.; Monden, M.; Doki, Y.; Mori, M.; Jin, W.; et al. Metabolic system alterations in pancreatic cancer patient serum: Potential for early detection. BMC Cancer 2013, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E.; Lespi, P.; Di Luca, M.; Bustos, C.; Marra, F.A.; de Alaniz, M.J.T.; Marra, C.A. A reliable biomarker derived from plasmalogens to evaluate malignancy and metastatic capacity of human cancers. Lipids 2008, 43, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, H.; Dai, M.; Ai, J.; Li, Y.; Mahon, B.; Dai, S.; Deng, Y. Plasma lipidomics profiling identified lipid biomarkers in distinguishing early-stage breast cancer from benign lesions. Oncotarget 2016, 7, 36622–36631. [Google Scholar] [CrossRef]
- Kostadinova, A.; Topouzova-Hristova, T.; Momchilova, A.; Tzoneva, R.; Berger, M.R. Antitumor Lipids—Structure, Functions, and Medical Applications. Adv. Protein Chem. Struct. Biol. 2015, 101, 27–66. [Google Scholar] [CrossRef] [PubMed]
- Van Blitterswijk, W.J.; Verheij, M. Anticancer mechanisms and clinical application of alkylphospholipids. Biochim. Biophys. Acta 2013, 1831, 663–674. [Google Scholar] [CrossRef]
- Maria, A.T.J.; Partouche, L.; Goulabchand, R.; Rivière, S.; Rozier, P.; Bourgier, C.; Le Quellec, A.; Morel, J.; Noël, D.; Guilpain, P. Intriguing Relationships Between Cancer and Systemic Sclerosis: Role of the Immune System and Other Contributors. Front. Immunol. 2018, 9, 3112. [Google Scholar] [CrossRef]
- Tyndall, A.J.; Bannert, B.; Vonk, M.; Airò, P.; Cozzi, F.; Carreira, P.E.; Bancel, D.F.; Allanore, Y.; Müller-Ladner, U.; Distler, O.; et al. Causes and risk factors for death in systemic sclerosis: A study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann. Rheum. Dis. 2010, 69, 1809–1815. [Google Scholar] [CrossRef]
| Characteristics | SSc (n = 52) | Controls (n = 48) |
|---|---|---|
| Female/Male | 44/8 | 37/11 |
| Age (range) | 60 ± 12 | 51 ± 16 |
| Anti-Scl70/ACA, n (%) | 17 (32.7%)/18 (34.6%) | - |
| ANA only/other Abs, n (%) | 13 (25%)/4 (7.7%) | |
| dcSSc/lcSSc, n (%) | 11 (21.2%)/39 (75%) | - |
| Lung fibrosis (LF), n (%) | 14 (26.7%) | |
| Digital ulcers (DU), n (%) | 10 (19.2%) | - |
| Calcinosis cutis (CC), n (%) | 15 (28.8%) | - |
| Sicca symptoms, n (%) | 14 (26.9%) | - |
| Raynaud symptoms, n (%) | 44 (84.7%) | - |
| Pulmonal arterial hypertension, n (%) | 20 (38.5%) | - |
| Mycophenolate mofetil, n (%) | 5 (9.6%) | - |
| Methotrexate, n (%) | 14 (26.9%) | - |
| Bosentan, n (%) | 8 (15.4%) | - |
| Ambrisentan/Macitentan, n (%) | 2 (3.8%)/10 (19.2%) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geroldinger-Simić, M.; Bögl, T.; Himmelsbach, M.; Sepp, N.; Buchberger, W. Changes in Plasma Phospholipid Metabolism Are Associated with Clinical Manifestations of Systemic Sclerosis. Diagnostics 2021, 11, 2116. https://doi.org/10.3390/diagnostics11112116
Geroldinger-Simić M, Bögl T, Himmelsbach M, Sepp N, Buchberger W. Changes in Plasma Phospholipid Metabolism Are Associated with Clinical Manifestations of Systemic Sclerosis. Diagnostics. 2021; 11(11):2116. https://doi.org/10.3390/diagnostics11112116
Chicago/Turabian StyleGeroldinger-Simić, Marija, Thomas Bögl, Markus Himmelsbach, Norbert Sepp, and Wolfgang Buchberger. 2021. "Changes in Plasma Phospholipid Metabolism Are Associated with Clinical Manifestations of Systemic Sclerosis" Diagnostics 11, no. 11: 2116. https://doi.org/10.3390/diagnostics11112116
APA StyleGeroldinger-Simić, M., Bögl, T., Himmelsbach, M., Sepp, N., & Buchberger, W. (2021). Changes in Plasma Phospholipid Metabolism Are Associated with Clinical Manifestations of Systemic Sclerosis. Diagnostics, 11(11), 2116. https://doi.org/10.3390/diagnostics11112116






