Clinical Relevance of the Serial Measurement of Krebs von den Lungen-6 Levels in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Laboratory Data
2.3. Trends in Serum KL-6 Level Changes
2.4. ILD Progression
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics at Baseline
3.2. Factors Associated with the Variability of Serial KL-6 Levels
3.3. Potential Utility of Short-Term KL-6 Change in Predicting ILD Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volkmann, E.R.; Chung, A.; Tashkin, D.P. Managing Systemic Sclerosis-Related Interstitial Lung Disease in the Modern Treatment Era. J. Scleroderma Relat. Disord. 2017, 2, 72–83. [Google Scholar] [CrossRef]
- Hoffmann-Vold, A.M.; Maher, T.M.; Philpot, E.E.; Ashrafzadeh, A.; Barake, R.; Barsotti, S.; Bruni, C.; Carducci, P.; Carreira, P.E.; Castellví, I.; et al. The Identification and Management of Interstitial Lung Disease in Systemic Sclerosis: Evidence-Based European Consensus Statements. Lancet Rheumatol. 2020, 2, e71–e83. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Kuwana, M. Nintedanib for the Treatment of Systemic Sclerosis-Associated Interstitial Lung Disease. Expert Rev. Clin. Immunol. 2020, 16, 547–560. [Google Scholar] [CrossRef]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Lin, C.J.F.; Furst, D.E.; Goldin, J.; Kim, G.; Kuwana, M.; Allanore, Y.; Matucci-Cerinic, M.; Distler, O.; Shima, Y.; et al. Tocilizumab in Systemic Sclerosis: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Respir. Med. 2020, 8, 963–974. [Google Scholar] [CrossRef]
- Kuwana, M.; Distler, O. Recent Progress and Missing Gaps to Achieve Goal in the Care of Systemic Sclerosis-Associated Interstitial Lung Disease. J. Scleroderma Relat. Disord. 2020, 5, 3–5. [Google Scholar] [CrossRef]
- Hoffmann-Vold, A.M.; Allanore, Y.; Alves, M.; Brunborg, C.; Airó, P.; Ananieva, L.P.; Czirják, L.; Guiducci, S.; Hachulla, E.; Li, M.; et al. Progressive Interstitial Lung Disease in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease in the EUSTAR database. Ann. Rheum. Dis. 2021, 80, 219–227. [Google Scholar] [CrossRef]
- Kuwana, M.; Shirai, Y.; Takeuchi, T. Elevated serum Krebs von den Lungen-6 in Early Disease Predicts Subsequent Deterioration of Pulmonary Function in Patients with Systemic Sclerosis and Interstitial Lung Disease. J. Rheumatol. 2016, 43, 1825–1831. [Google Scholar] [CrossRef]
- Man, A.; Davidyock, T.; Ferguson, L.T.; Ieong, M.; Zhang, Y.; Simms, R.W. Changes in Forced Vital Capacity over Time in Systemic Sclerosis: Application of Group-Based Trajectory Modelling. Rheumatology 2015, 54, 1464–1471. [Google Scholar] [CrossRef] [Green Version]
- Winstone, T.A.; Assayag, D.; Wilcox, P.G.; Dunne, J.V.; Hague, C.J.; Leipsic, J.; Colllard, H.R.; Ryerson, C.J. Predictors of Mortality and Progression in Scleroderma-Associated Interstitial Lung Disease: A Systematic Review. Chest 2014, 146, 422–436. [Google Scholar] [CrossRef]
- Distler, O.; Assassi, S.; Cottin, V.; Cutolo, M.; Danoff, S.K.; Denton, C.P.; Distler, J.H.W.; Hoffmann-Vold, A.M.; Johnson, S.R.; Ladner, U.M.; et al. Predictors of Progression in Systemic Sclerosis Patients with Interstitial Lung Disease. Eur. Respir. J. 2020, 55, 1902026. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, N.; Hattori, N.; Yokoyama, A.; Kohno, N. Utility of KL-6/MUC1 in the Clinical Management of Interstitial Lung Diseases. Respir. Investig. 2012, 50, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonhomme, O.; André, B.; Gester, F.; de Seny, D.; Moermans, C.; Struman, I.; Louis, R.; Malaise, M.; Guiot, J. Biomarkers in Systemic Sclerosis-Associated Interstitial Lung Disease: Review of the Literature. Rheumatology 2019, 58, 1534–1546. [Google Scholar] [CrossRef] [Green Version]
- Inoue, Y.; Kaner, R.J.; Guiot, J.; Maher, T.M.; Tomassetti, S.; Moiseev, S.; Kuwana, M.; Brown, K.K. Diagnostic and Prognostic Biomarkers for Chronic Fibrosing Interstitial Lung Diseases with a Progressive Phenotype. Chest 2020, 158, 646–659. [Google Scholar] [CrossRef]
- Salazar, G.A.; Kuwana, M.; Wu, M.; Estrada-Y-Martin, R.M.; Ying, J.; Charles, J.; Mayes, M.D.; Assassi, S. KL-6 but Not CCL-18 Is a Predictor of Early Progression in Systemic Sclerosis-Related Interstitial Lung Disease. J. Rheumatol. 2018, 45, 1153–1158. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Tashkin, D.P.; Kuwana, M.; Li, N.; Roth, M.D.; Charles, J.; Hant, F.N.; Bogatkevich, G.S.; Akter, T.; Kim, G.; et al. Progression of Interstitial Lung Disease in Systemic Sclerosis: The Importance of Pneumoproteins Krebs von den Lungen 6 and CCL18. Arthritis Rheumatol. 2019, 71, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Kumánovics, G.; Görbe, E.; Minier, T.; Simon, D.; Berki, T.; Czirják, L. Follow-up of Serum KL-6 lung Fibrosis Biomarker Levels in 173 Patients with Systemic Sclerosis. Clin. Exp. Rheumatol. 2014, 32, S138–S144. [Google Scholar]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. ATS/ERS Task Force. Standardisation of Spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef]
- Goh, N.S.L.; Desai, S.R.; Veeraraghavan, S.; Hansell, D.M.; Copley, S.J.; Maher, T.M.; Corte, T.J.; Sander, C.R.; Ratoff, J.; Devaraj, A.; et al. Interstitial Lung Disease in Systemic Sclerosis: A Simple Staging System. Am. J. Respir. Crit. Care Med. 2008, 177, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Hoyles, R.K.; Ellis, R.W.; Wellsbury, J.; Lees, B.; Newlands, P.; Goh, N.S.L.; Roberts, C.; Desai, S.; Herrick, A.L.; McHugh, N.J.; et al. A Multicenter, Prospective, Randomized, Double-Blind, Placebo-Controlled Trial of Corticosteroids and Intravenous Cyclophosphamide Followed by Oral Azathioprine for the Treatment of Pulmonary Fibrosis in Scleroderma. Arthritis Rheum. 2006, 54, 3962–3970. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Elashoff, R.; Clements, P.J.; Goldin, J.; Roth, M.D.; Furst, D.E.; Arriola, E.; Silver, R.; Strange, C.; Bolster, M.; et al. Cyclophosphamide versus Placebo in Scleroderma Lung Disease. N. Engl. J. Med. 2006, 354, 2655–2666. [Google Scholar] [CrossRef] [Green Version]
- Tashkin, D.P.; Roth, M.D.; Clements, P.J.; Furst, D.E.; Khanna, D.; Kleerup, E.C.; Goldin, J.; Arriola, E.; Volkmann, E.R.; Kafaja, S.; et al. Mycophenolate Mofetil versus Oral Cyclophosphamide in Scleroderma-Related Interstitial Lung Disease (SLS II): A Randomised Controlled, Double-Blind, Parallel Group Trial. Lancet Respir. Med. 2016, 4, 708–719. [Google Scholar] [CrossRef] [Green Version]
- Kuwana, M. Circulating Anti-Nuclear Antibodies in Systemic Sclerosis: Utility in Diagnosis and Disease Subsetting. J. Nippon Med. Sch. 2017, 84, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Khanna, D.; Mittoo, S.; Aggarwal, R.; Proudman, S.M.; Dalbeth, N.; Matteson, E.L.; Brown, K.; Flaherty, K.; Wells, A.U.; Seibold, J.R.; et al. Connective Tissue Disease-Associated Interstitial Lung Diseases (CTD-ILD)—Report from OMERACT CTD-ILD Working Group. J. Rheumatol. 2015, 42, 2168–2171. [Google Scholar] [CrossRef] [Green Version]
- Goh, N.S.; Hoyles, R.K.; Denton, C.P.; Hansell, D.M.; Renzoni, E.A.; Maher, T.M.; Nicholson, A.G.; Wells, A.U. Short-Term Pulmonary Function Trends Are Predictive of Mortality in Interstitial Lung Disease Associated with Systemic Sclerosis. Arthritis Rheumatol. 2017, 69, 1670–1678. [Google Scholar] [CrossRef]
- George, P.M.; Spagnolo, P.; Kreuter, M.; Altinisik, G.; Bonifazi, M.; Martinez, F.J.; Molyneaux, P.L.; Renzoni, E.A.; Richeldi, L.; Tomassetti, S.; et al. Progressive Fibrosing Interstitial Lung Disease: Clinical Uncertainties, Consensus Recommendations, and Research Priorities. Lancet Respir. Med. 2020, 8, 925–934. [Google Scholar] [CrossRef]
- Guilford, J.P. Fundamental Statistics in Psychology and Education, McGraw-Hill Series in Psychology; McGraw-Hill Book Company: New York, NY, USA, 1950; p. 156. [Google Scholar]
- Young, D.S. Handbook of Regression Methods; CRC Press: Boca Raton, FL, USA, 2017; pp. 109–136. [Google Scholar]
- Arai, S.; Kurasawa, K.; Maezawa, R.; Owada, T.; Okada, H.; Fukuda, T. Marked Increase in Serum KL-6 and Surfactant Protein D Levels During the First 4 Weeks after Treatment Predicts Poor Prognosis in Patients with Active Interstitial Pneumonia Associated with Polymyositis/Dermatomyositis. Mod. Rheumatol. 2013, 23, 872–883. [Google Scholar] [CrossRef]
- Hanaoka, M.; Katsumata, Y.; Kawasumi, H.; Kawaguchi, Y.; Yamanaka, H. KL-6 is a Long-Term Disease-Activity Biomarker for Interstitial Lung Disease Associated with Polymyositis/Dermatomyositis, but is Not a Short-Term Disease-Activity Biomarker. Mod. Rheumatol. 2019, 29, 625–632. [Google Scholar] [CrossRef]
- Takanashi, S.; Nishina, N.; Nakazawa, M.; Kaneko, Y.; Takeuchi, T. Usefulness of Serum Krebs von den Lungen-6 for the Management of Myositis-Associated Interstitial Lung Disease. Rheumatology 2019, 58, 1034–1039. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Iwamoto, H.; Sakamoto, S.; Horimasu, Y.; Masuda, T.; Miyamoto, S.; Nakashima, T.; Ohshimo, S.; Fujitaka, K.; Hamada, H.; et al. Serial Measurements of KL-6 For Monitoring Activity and Recurrence of Interstitial Pneumonia with Anti-Aminoacyl-Trna Synthetase Antibody: A Retrospective Cohort Study. Medicine 2018, 97, e13542. [Google Scholar] [CrossRef]
- Takei, R.; Yamano, Y.; Kataoka, K.; Yokoyama, T.; Matsuda, T.; Kimura, T.; Johkoh, T.; Takahashi, O.; Kondoh, Y. Predictive Factors for the Recurrence of Anti-Aminoacyl-Trna Synthetase Antibody-Associated Interstitial Lung Disease. Respir. Investig. 2020, 58, 83–90. [Google Scholar] [CrossRef]
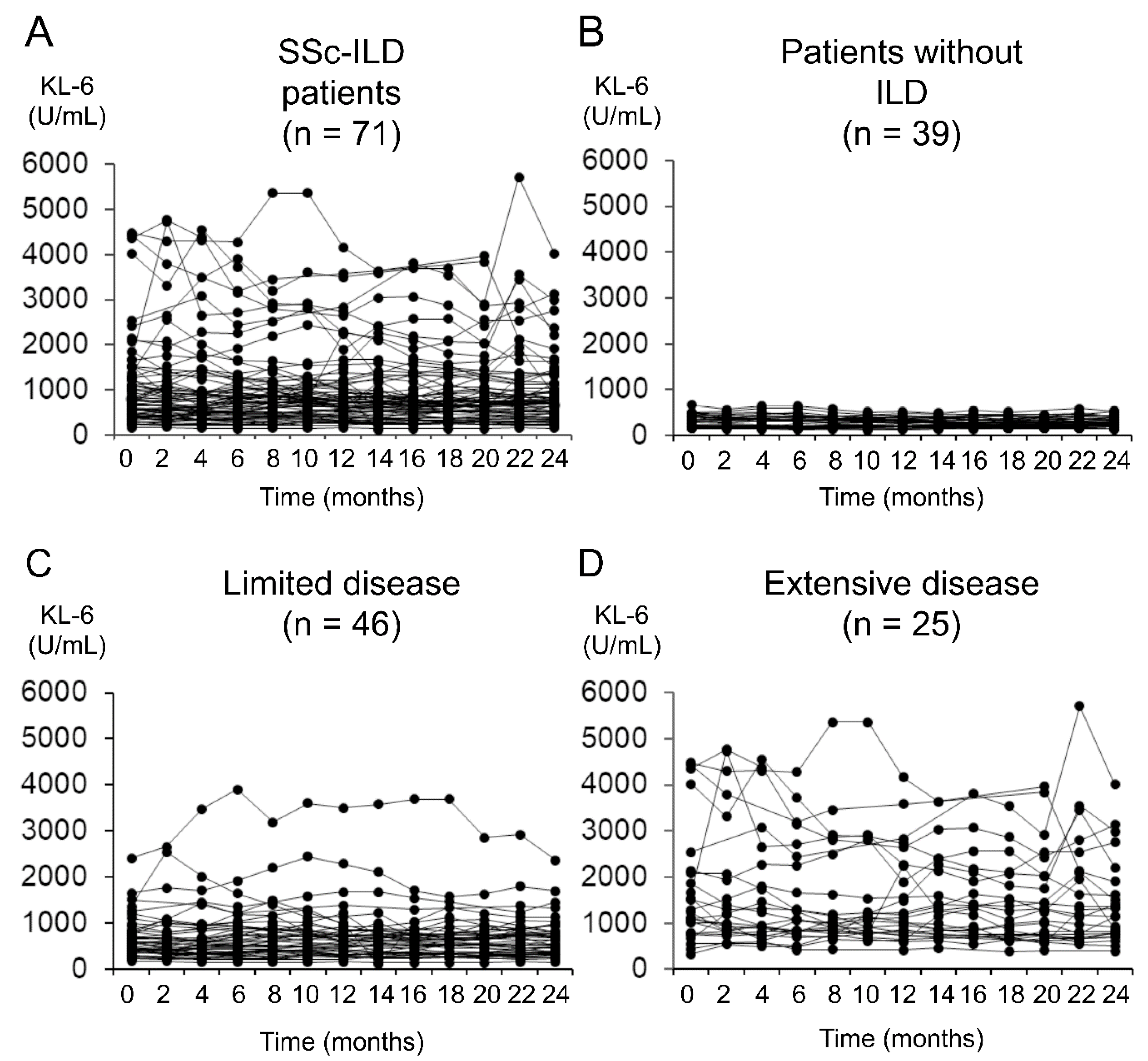
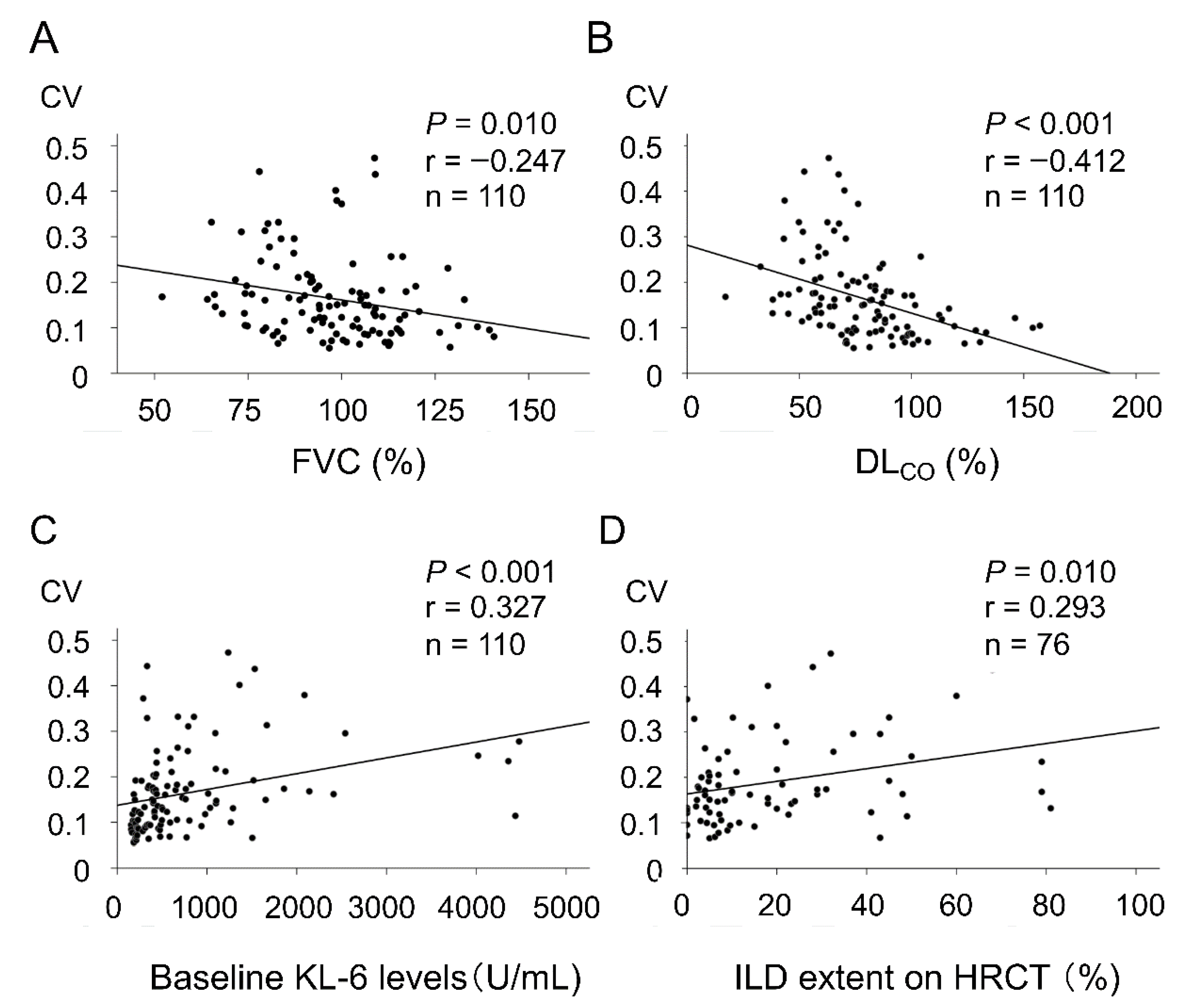
| Clinical Parameters | All SSc Patients (n = 110) | SSc-ILD Patients (n = 64) 1 |
|---|---|---|
| Sex (female) | 96 (87%) | 57 (89%) |
| Age at study entry | 54 ± 13 | 53 ± 13 |
| Disease duration (years) | 2.6 ± 2.0 | 2.4 ± 1.9 |
| dcSSc | 46 (42%) | 33 (52%) |
| SSc-related autoantibodies | ||
| Anti-topoisomerase I | 45 (41%) | 33 (52%) |
| Anticentromere | 31 (28%) | 10 (16%) |
| Anti-U1 RNP | 11 (10%) | 8 (13%) |
| Anti-RNAP III | 3 (5%) | 2 (3%) |
| Anti-U3 RNP | 2 (2%) | 0 |
| Anti-Th/To | 1 (1%) | 1 (2%) |
| Current or past smoker | 31 (28%) | 21 (33%) |
| ILD | 71 (65%) | 64 (100%) |
| ILD extent shown on HRCT (%) | NA | 20.7 ± 20.9 |
| Extensive disease | NA | 23 (36%) |
| FVC (% predicted) 2 | 97.5 ± 17.6 | 89.8 ± 15.3 |
| DLCO (% predicted) 2 | 78.5 ± 25.1 | 69.1 ± 21.8 |
| CRP levels (mg/dL) | 0.14 ± 0.28 | 0.16 ± 0.29 |
| KL-6 levels (U/mL) 2 | 775 ± 857 | 994 ± 899 |
| Any immunomodulatory treatment during the first 2 years after diagnosis | 39 (35%) | 30 (47%) |
| Clinical Parameters | β | p |
|---|---|---|
| Sex (female) | 0.016 | 0.866 |
| Age at study entry | 0.033 | 0.733 |
| Disease duration (years) | −0.186 | 0.052 |
| dcSSc | 0.198 | 0.038 |
| Anti-topoisomerase I | 0.241 | 0.011 |
| Anticentromere | −0.256 | 0.007 |
| Anti-U1 RNP | 0.089 | 0.356 |
| Anti-RNAP III | 0.035 | 0.720 |
| Current or past smoking | −0.065 | 0.611 |
| ILD | 0.462 | <0.001 |
| Disease extent shown on HRCT (%) 1 | 0.293 | 0.01 |
| Extensive disease 1 | 0.358 | 0.001 |
| FVC (% predicted) | −0.252 | 0.008 |
| DLCO (% predicted) | −0.413 | <0.001 |
| CRP levels (mg/dL) | 0.104 | 0.279 |
| KL-6 levels (U/mL) | 0.369 | <0.001 |
| Any immunomodulatory treatment during the first 2 years after diagnosis | 0.292 | 0.002 |
| Clinical Parameters | ILD Progression (n = 12) | No ILD Progression (n = 52) | p |
|---|---|---|---|
| Sex (female) | 11 (92%) | 46 (88%) | 1.00 |
| Age at study entry | 52 ± 14 | 53 ± 13 | 0.77 |
| Disease duration (years) | 1.7 ±1.4 | 2.6 ± 2.0 | 0.17 |
| dcSSc | 8 (67%) | 25 (48%) | 0.34 |
| SSc-related autoantibodies | |||
| Anti-topoisomerase I | 8 (67%) | 25 (48%) | 0.34 |
| Anticentromere | 2 (17%) | 8 (15%) | 1.00 |
| Anti-U1RNP | 2 (17%) | 6 (12%) | 0.64 |
| Current or past smoker | 4 (28%) | 17 (36%) | 1.00 |
| ILD extent shown on HRCT (%) | 29.6 ± 21.2 | 18.7 ± 20.4 | 0.041 |
| Extensive disease | 8 (67%) | 15 (20%) | 0.020 |
| FVC (% predicted) | 91.1 ± 19.6 | 89.5 ± 14.4 | 0.58 |
| DLCO (% predicted) | 67.9 ± 27.0 | 69.4 ± 20.7 | 0.99 |
| CRP levels (mg/dL) | 0.24 ± 0.33 | 0.14 ± 0.29 | 0.14 |
| KL-6 levels (U/mL) | 1124 ± 682 | 964 ± 945 | 0.18 |
| Any immunomodulatory treatment | 5 (42%) | 25 (48%) | 0.76 |
| Progression of ILD | ||
|---|---|---|
| OMERACT Criteria 1 | PF-ILD 2 | |
| KL-6 levels at baseline | 0.58 | 0.32 |
| Regression coefficient over 6 months | 0.83 | 0.78 |
| A ratio of the KL-6 level at 6 months to the baseline | 0.39 | 0.69 |
| AUC above the standard line | 0.52 | 0.59 |
| AUC above the line of baseline KL-6 levels | 0.77 | 0.31 |
| Consecutive rise over 6 months | 1.00 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirai, Y.; Fukue, R.; Kaneko, Y.; Kuwana, M. Clinical Relevance of the Serial Measurement of Krebs von den Lungen-6 Levels in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease. Diagnostics 2021, 11, 2007. https://doi.org/10.3390/diagnostics11112007
Shirai Y, Fukue R, Kaneko Y, Kuwana M. Clinical Relevance of the Serial Measurement of Krebs von den Lungen-6 Levels in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease. Diagnostics. 2021; 11(11):2007. https://doi.org/10.3390/diagnostics11112007
Chicago/Turabian StyleShirai, Yuichiro, Ryosuke Fukue, Yuko Kaneko, and Masataka Kuwana. 2021. "Clinical Relevance of the Serial Measurement of Krebs von den Lungen-6 Levels in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease" Diagnostics 11, no. 11: 2007. https://doi.org/10.3390/diagnostics11112007
APA StyleShirai, Y., Fukue, R., Kaneko, Y., & Kuwana, M. (2021). Clinical Relevance of the Serial Measurement of Krebs von den Lungen-6 Levels in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease. Diagnostics, 11(11), 2007. https://doi.org/10.3390/diagnostics11112007






