A Prospective Comparative Study of Using Ultrasonography, 4D-CT and Parathyroid Dual-Phase Scintigraphy with SPECT in Patients with Primary Hyperparathyroidism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Characteristics
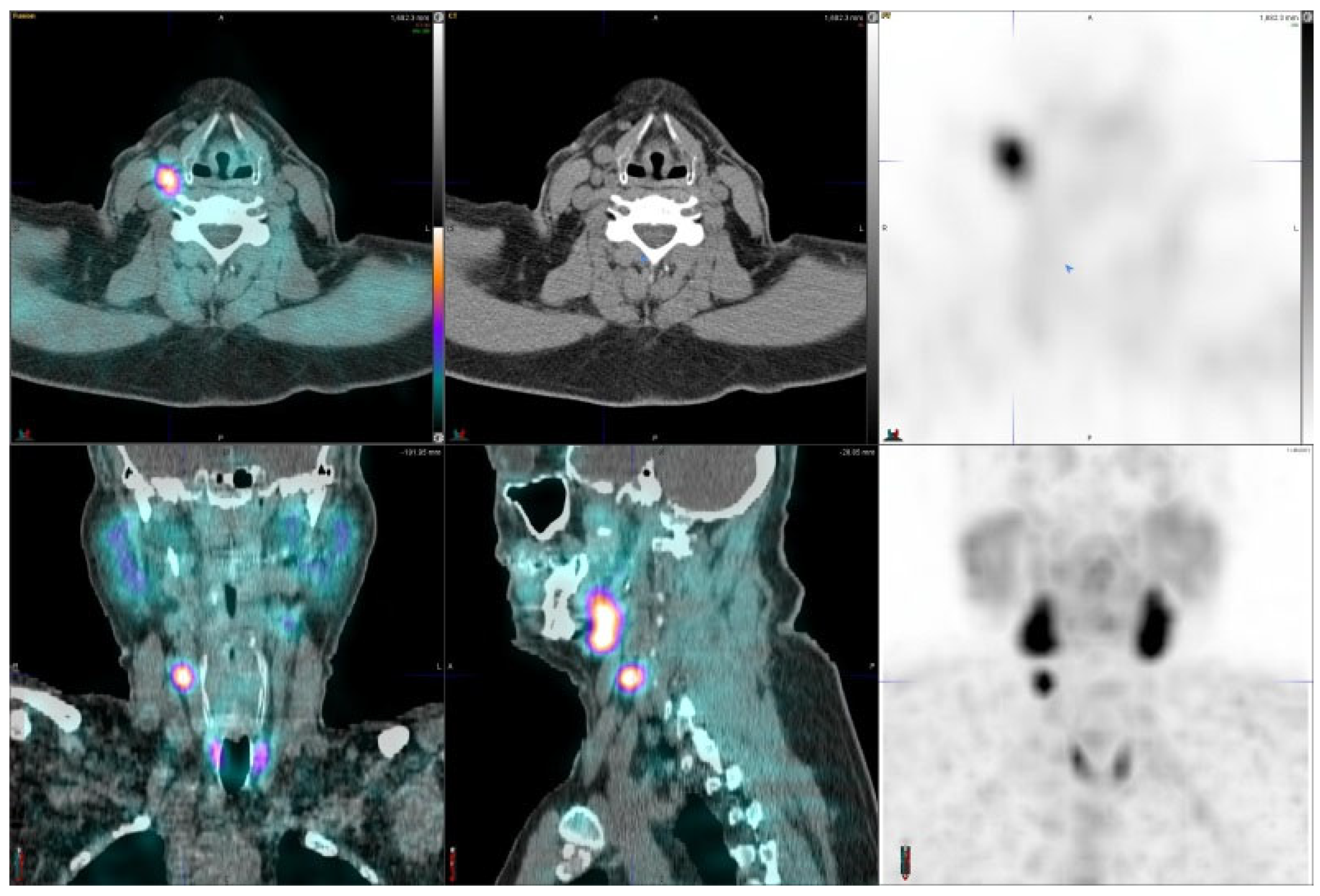
2.2. Imaging Examinations
2.3. Image Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, W.J.; Shen, C.T.; Song, Z.L.; Luo, Q.Y. Comparison of SPECT/CT, SPET and planar imaging using 99mTc-MIBI as independent techniques to support minimally invasive parathyroidectomy in primary hyperparathyroidism: A meta-analysis. Hell. J. Nucl. Med. 2015, 18, 127–135. [Google Scholar]
- Krakauer, M.; Wieslander, B.; Myschetzky, P.S.; Lundstrøm, A.; Bacher, T.; Sørensen, C.H.; Trolle, W.; Nygaard, B.; Bennedbæk, F.N. A prospective comparative study of parathyroid dual-phase scintigraphy, dual-isotope subtraction scintigraphy, 4D-CT and ultrasonography in primary hyperparathyroidism. Clin. Nucl. Med. 2016, 41, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Cachovan, M.; Kaul, F.; Caobelli, F.; Bäumer, M.; Vija, A.H.; Pagenstert, G.; Wild, D.; Kretzschmar, M. Accuracy comparison of various quantitative [99mTc]Tc-DPD SPECT/CT reconstruction techniques in patients with symptomatic hip and knee joint prostheses. EJNMMI Res. 2021, 11, 60. [Google Scholar] [CrossRef]
- Vija, A.H.; Bartenstein, P.A.; Froelich, J.W.; Kuwert, T.; Macapinlac, H.; Daignault, C.P.; Gowda, N.; Hadjiev, O.; Hephzibah, J.; Huang, P.; et al. ROC study and SUV threshold using quantitative multi-modal SPECT for bone imaging. Eur. J. Hybrid Imaging 2019, 3, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vija, A.H. Introduction to the xSPECT Technology: Evolving Multi Modal SPECT to Become Context Based and Quantitative; Molecular Imaging White Paper; Vija, A.H., Ed.; Siemens Medical Solutions USA, Inc.: Malvern, PA, USA, 2013. [Google Scholar]
- Vija, A.H. Characteristics of the xSPECT Reconstruction Method; Siemens Medical Solutions USA, Inc.: Malvern, PA, USA, 2017. [Google Scholar]
- Mighell, K.J. Parameter Estimation in Astronomy with Poisson-distributed Data. I. The χγ2 Statistic. Astrophys. J. 1999, 518, 380–393. [Google Scholar] [CrossRef]
- Sandström, I. Om en ny körtel hos menniskan och åtskilliga däggdjur. In Upsala Läkareförenings Förhandlingar; Band XV: Upsala, Sweden, 1879. [Google Scholar]
- Shah, S.; Win, Z.; Al-Nahhas, A. Multimodality imaging of the parathyroid glands in primary hyperparathyroidism. Minerva Endocrinol. 2008, 33, 193–202. [Google Scholar] [PubMed]
- Lavely, W.C.; Goetze, S.; Friedman, K.P.; Leal, J.P.; Zhang, Z.; Garret-Mayer, E.; Dackiw, A.P.; Tufano, R.P.; Zeiger, M.A.; Ziessman, H.A. Comparison of SPECT/CT, SPET, and planar imaging with single- and dual-phase 99mTc-MIBI parathyroid scintigraphy. J. Nucl. Med. 2007, 48, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Neumann, D.R.; Obuchowski, N.A.; Difilippo, F.P. Preoperative 123I/99mTc-MIBI subtraction SPET and SPET/CT in PHPT. J. Nucl. Med. 2008, 49, 2012–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu, T.; Schellingerhout, D.; Guha-Thakurta, N.; Sun, J.; Wei, W.; Kappadth, S.; Perrier, N.; Kim, E.; Rohren, E.; Chuang, H.; et al. Solitary Parathyroid Adenoma Localization in Technetium Tc99m Sestamibi SPECT and Multiphase Multidetector 4D CT. Am. J. Neuroradiol. 2019, 40, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiromatsu, Y.; Ishibashi, M.; Nishida, H.; Okuda, S.; Miyake, I. Technetium-99m Tetrofosmin Parathyroid Imaging in Patients with Primary Hyperparathyroidism. Intern. Med. 2000, 39, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.R.; Oates, M.E. Radionuclide imaging of the parathyroid glands: Patterns, pearls, and pitfalls. Radiographics 2004, 24, 1101–1115. [Google Scholar] [CrossRef]
- Treglia, G.; Sadeghi, R.; Schalin-Jäntti, C.; Caldarella, C.; Ceriani, L.; Giovanella, L.; Eisele, D.W. Detection rate of 99m Tc-MIBI single photon emission computed tomography (SPECT)/CT in preoperative planning for patients with primary hyperparathyroidism: A meta-analysis. Head Neck 2016, 38, E2159–E2172. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.-L.; Wu, B.; Shen, C.; Zhu, R.-S.; Luo, Q.-Y. Dual-phase 99mTc-MIBI scintigraphy with delayed neck and thorax SPECT/CT and bone scintigraphy in patients with primary hyperparathyroidism: Correlation with clinical or pathological variables. Ann. Nucl. Med. 2014, 28, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, G.; Profanter, C.; Kovacs, P.; Sieb, M.; Gabriel, M.; Putzer, D.; Bale, R.; Margreiter, R.; Prommegger, R. CT-MIBI-SPECT image fusion predicts multiglandular disease in hyperparathyroidism. Langenbeck’s Arch. Surg. 2009, 395, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kedarisetty, S.; Fundakowski, C.; Ramakrishnan, K.; Dadparvar, S. Clinical Value of Tc99m-MIBI SPECT/CT Versus 4D-CT or US in Management of Patients With Hyperparathyroidism. Ear Nose Throat J. 2019, 98, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandqvist, P.; Nilsson, I.-L.; Grybäck, P.; Sanchez-Crespo, A.; Sundin, A. Multiphase Iodine Contrast-Enhanced SPECT/CT Outperforms Nonenhanced SPECT/CT for Preoperative Localization of Small Parathyroid Adenomas. Clin. Nucl. Med. 2019, 44, 929–935. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, W.; Yin, L.; Guo, R.; Wei, B.; Jin, M.; Zhou, X.; Zhang, C.; Lv, X. Efficacy of ultrasonography and Tc-99m MIBI SPECT/CT in preoperative localization of parathyroid adenomas causing primary hyperthyroidism. BMC Med. Imaging 2021, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Naples, R.; Thomas, J.D.; Monteiro, R.; Zolin, S.J.; Timmerman, C.K.; Crawford, K.; Jin, J.; Shin, J.J.; Krishnamurthy, V.D.; Berber, E.; et al. Preoperative Calcium and Parathyroid Hormone Values Are Poor Predictors of Gland Volume and Multigland Disease in Primary Hyperparathyroidism: A Review of 2000 Consecutive Patients. Endocr. Pract. 2021, 1530. [Google Scholar] [CrossRef]
- Han, C.H.; Fry, C.H.; Sharma, P.; Han, T.S. A clinical perspective of parathyroid hormone related hypercalcaemia. Rev. Endocr. Metab. Disord. 2020, 21, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Kandil, E.; Tufaro, A.P.; Carson, K.A.; Lin, F.; Somervell, H.; Farrag, T.; Dackiw, A.; Zeiger, M.; Tufano, R.P. Correlation of Plasma 25-Hydroxyvitamin D Levels With Severity of Primary Hyperparathyroidism and Likelihood of Parathyroid Adenoma Localization on Sestamibi Scan. Arch. Otolaryngol.-Head Neck Surg. 2008, 134, 1071–1075. [Google Scholar] [CrossRef] [Green Version]
- Michaud, L.; Burgess, A.; Huchet, V.; Lefèvre, M.; Tassart, M.; Ohnona, J.; Kerrou, K.; Balogova, S.; Talbot, J.-N.; Perie, S. Is 18F-Fluorocholine-Positron Emission Tomography/Computerized Tomography a New Imaging Tool for Detecting Hyperfunctioning Parathyroid Glands in Primary or Secondary Hyperparathyroidism? J. Clin. Endocrinol. Metab. 2014, 99, 4531–4536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beheshti, M.; Hehenwarter, L.; Paymani, Z.; Rendl, G.; Imamovic, L.; Rettenbacher, R.; Tsybrovskyy, O.; Langsteger, W.; Pirich, C. 18F-Fluorocholine PET/CT in the assessment of primary hyperparathyroidism compared with 99mTc-MIBI or 99mTc-tetrofosmin SPECT/CT: A prospective dual-centre study in 100 patients. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1762–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovčariček, P.P.; Giovanella, L.; Gasset, I.C.; Hindié, E.; Huellner, M.W.; Luster, M.; Piccardo, A.; Weber, T.; Talbot, J.-N.; Verburg, F.A. The EANM practice guidelines for parathyroid imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, M.; Kjaer, A.; Bennedbæk, F.N. 18F-FET-PET in Primary Hyperparathyroidism: A Pilot Study. Diagnostics 2016, 6, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bioletto, F.; Barale, M.; Parasiliti-Caprino, M.; Prencipe, N.; Berton, A.M.; Procopio, M.; Deandreis, D.; Ghigo, E. Comparison of the diagnostic accuracy of 18F-Fluorocholine PET and 11C-Methionine PET for parathyroid localization in primary hyperparathyroidism: A systematic review and meta-analysis. Eur. J. Endocrinol. 2021, 185, 109–120. [Google Scholar] [CrossRef] [PubMed]





| Age/Gender | Dgn/SPECT | Thyroid/US Volume | Lesion/ US Volume | Lesion/ CT Size | Lesion wt /Surgery | Lesion Size/Surgery | S-PTH | S-Ca | CGZAS kBq/mL | CGZAS SUVmax | CGAS kBq/mL | CGAS SUVmax |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 66/f | adenoma L | 6.8 | 0.084 | 7 mm L | 123 mg | 10 mm | 154 | 10.9 | 58.5 | 5.1 | 35.3 | 3.1 |
| 53/m | adenoma L | 6.5 | 1.3 | 12 × 14 L | 1854 mg | 30 mm | 83 | 10.9 | 161 | 18.9 | 83.1 | 9.8 |
| 36/f | adenoma L | 11.2 | 0.43 | 0 | nd | nd | 39 | 10.5 | 88.7 | 6.6 | 65.8 | 4.9 |
| 65/f | adenoma R | 9.1 | 0.36 | 13 × 14 R | 585 mg | nm | 118 | 10.2 | 13.3 | 1.2 | 25.7 | 2.3 |
| 55/m | adenoma R | 12.7 | 1.6 | 18 mm R | 2400 mg | 30 mm | 162 | 10.9 | 195 | 21.8 | 149 | 16.7 |
| 81/f | no adenoma | 3.7 | 0.05 | 5 mm R | 244 mg | 27 mm | 129 | 9.1 | ||||
| 62/f | adenoma R | 8.8 | 2.4 | 12 mm R | 2400 mg | 34 mm | 92 | 11.3 | 36.2 | 5.6 | 26.9 | 4.1 |
| 53/f | adenoma L | 10.0 | 0 | 4 mm L | nd | nd | 44 | 10.9 | 51.3 | 3.1 | 41.3 | 2.5 |
| 63/m | no adenoma | 15.1 | 0 | 7 mm R | nd | nd | 59 | 10.7 | ||||
| 59/f | no adenoma | nm | 0 | 0 | nd | nd | 93 | 10.4 | ||||
| 60/f | double adenoma L | 0.784 | 11 mm L | 117 mg | nm | 115 | 10.7 | 22.5 | 3.4 | 20.8 | 3.2 | |
| 60/f | double adenoma L | 0.216 | 11 mm L | 155 mg | nm | 115 | 10.7 | 45.6 | 7 | 36.2 | 5.5 | |
| 72/f | adenoma L | 10.1 | 0 | 10 × 17 L | nm | 15 × 10 | 89 | 10.4 | 64.6 | 7.5 | 53.5 | 6.2 |
| 68/f | adenoma R | 4.8 | 0.1 | 8 mm R | nd | nd | 90 | 10.6 | 24.2 | 4 | 15.7 | 2.6 |
| 64/f | adenoma R | 4.0 | 0.9 | 7 mm R | nd | nd | 258 | 12.7 | 147 | 13.9 | 102 | 9.7 |
| 31/m | adenoma R | 11.0 | 0.084 | 9 × 12 R | nd | nd | 110 | 10.8 | 53.4 | 5.9 | 32.2 | 3.6 |
| 68/f | no adenoma | nm | 0 | 0 | nd | nd | 44 | 10.1 | ||||
| 61/m | adenoma R | nm | nm | 8 mm R | 37 | 11.4 | 63.5 | 8.5 | 33.4 | 4.5 | ||
| 73/m | no adenoma | 12.0 | 0 | 0 | nd | nd | 72 | 11.2 | ||||
| 58/f | no adenoma | 17.4 | 0 | 5 × 8 R | nd | nd | 95 | 10.6 | ||||
| 56/f | adenoma R | nm | nm | 6 mm R | nd | nd | 143 | 9.0 | 20.5 | 2.4 | 14.3 | 1.7 |
| 70/m | adenoma L | 11.6 | 0.158 | 32.18.26 R | 3680 mg | 23 × 21 | 142 | 11.4 | 190 | 27.4 | 143 | 20.7 |
| 68/m | adenoma R | 19.2 | 0 | 13 × 13 R | 736 mg | 10 × 7 | 124 | 11.1 | 27.5 | 6.1 | 29.5 | 4.9 |
| 73/f | adenoma L | 7.5 | 0 | 8 × 18 L | 478 mg | 22 × 9 | 146 | 11.3 | 80.9 | 14.7 | 62 | 3.6 |
| 55/m | adenoma R | nm | nd | 21 × 24 R | nd | nd | 478 | 11.1 | 83.3 | 6.3 | 49.6 | 3.8 |
| 55/m | carcinoma L | nm | nd | 25 × 30 L | nd | nd | 478 | 11.1 | 109 | 8.3 | 96.2 | 7.3 |
| 69/f | adenoma L | 18.8 | 1.52 | 17 × 38 R | nd | nd | 113 | 11.0 | 94.5 | 12 | 73.9 | 9.3 |
| 69/f | adenoma R | 18.8 | 0.972 | 11 × 24 L | nd | nd | 113 | 11.0 | 82.3 | 10.4 | 61.4 | 7.8 |
| 85/f | no adenoma | 6.2 | 0.113 | 6 × 12 L | nd | nd | 97 | 10.6 | ||||
| 71/f | no adenoma | nm | 0 | nm R | nd | nd | 109 | 10.6 | ||||
| 77/f | adenomaL | 5.5 | 0 | 8 mmL | 173 mg | 10 × 6 | 125 | 11.4 | 76.8 | 9.2 | 43.5 | 5.2 |
| 68/m | no adenoma | 22.8 | 1.9 diam | 12 × 14 L | 1130 mg | 20 × 12 | 49 | 9.9 | ||||
| 53/m | adenoma L | nm | 0 | 9 × 21 R | 1540 mg | 20 × 13 | 125 | 11.0 | 68.4 | 7.8 | 40.8 | 4.7 |
| 37/m | adenoma L | 8.9 | 0 | 0 | 200 mg | 12 mm | 5 | 11.3 | 34.3 | 4 | 25.1 | 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kairemo, K.; Jessop, A.C.; Vija, A.H.; Ding, X.; Spence, D.; Kappadath, S.C.; Macapinlac, H.A. A Prospective Comparative Study of Using Ultrasonography, 4D-CT and Parathyroid Dual-Phase Scintigraphy with SPECT in Patients with Primary Hyperparathyroidism. Diagnostics 2021, 11, 2006. https://doi.org/10.3390/diagnostics11112006
Kairemo K, Jessop AC, Vija AH, Ding X, Spence D, Kappadath SC, Macapinlac HA. A Prospective Comparative Study of Using Ultrasonography, 4D-CT and Parathyroid Dual-Phase Scintigraphy with SPECT in Patients with Primary Hyperparathyroidism. Diagnostics. 2021; 11(11):2006. https://doi.org/10.3390/diagnostics11112006
Chicago/Turabian StyleKairemo, Kalevi, Aaron C. Jessop, A. Hans Vija, Xinhong Ding, Don Spence, S. Cheenu Kappadath, and Homer A. Macapinlac. 2021. "A Prospective Comparative Study of Using Ultrasonography, 4D-CT and Parathyroid Dual-Phase Scintigraphy with SPECT in Patients with Primary Hyperparathyroidism" Diagnostics 11, no. 11: 2006. https://doi.org/10.3390/diagnostics11112006
APA StyleKairemo, K., Jessop, A. C., Vija, A. H., Ding, X., Spence, D., Kappadath, S. C., & Macapinlac, H. A. (2021). A Prospective Comparative Study of Using Ultrasonography, 4D-CT and Parathyroid Dual-Phase Scintigraphy with SPECT in Patients with Primary Hyperparathyroidism. Diagnostics, 11(11), 2006. https://doi.org/10.3390/diagnostics11112006






