Deep Learning-Based Artificial Intelligence System for Automatic Assessment of Glomerular Pathological Findings in Lupus Nephritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Datasets/Specimen Preparation
2.2. Annotation Procedure
2.3. Image Preparation and Module Design
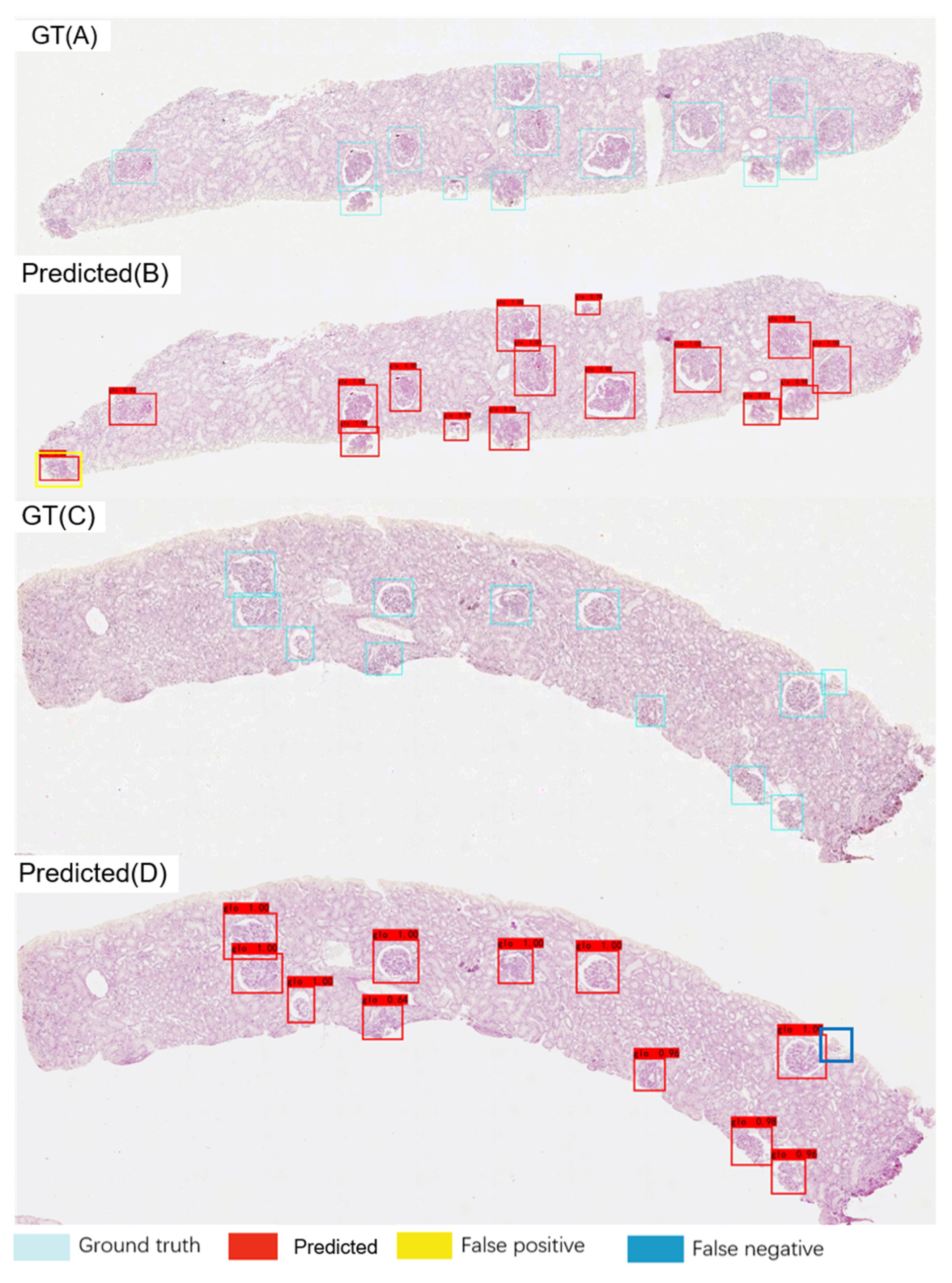
2.4. Glomerular Detection and Localisation
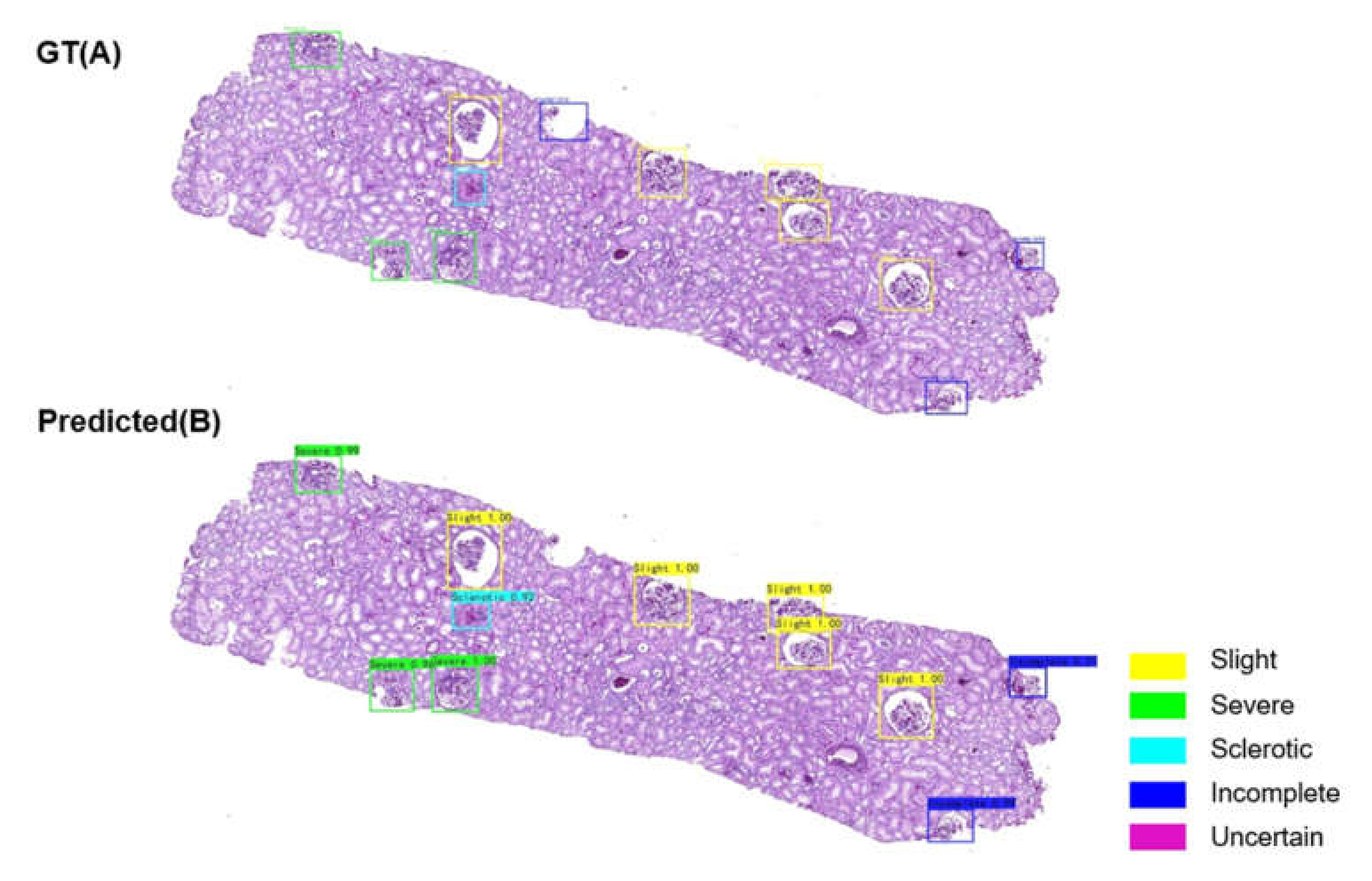
2.5. Classification of Glomerular Findings
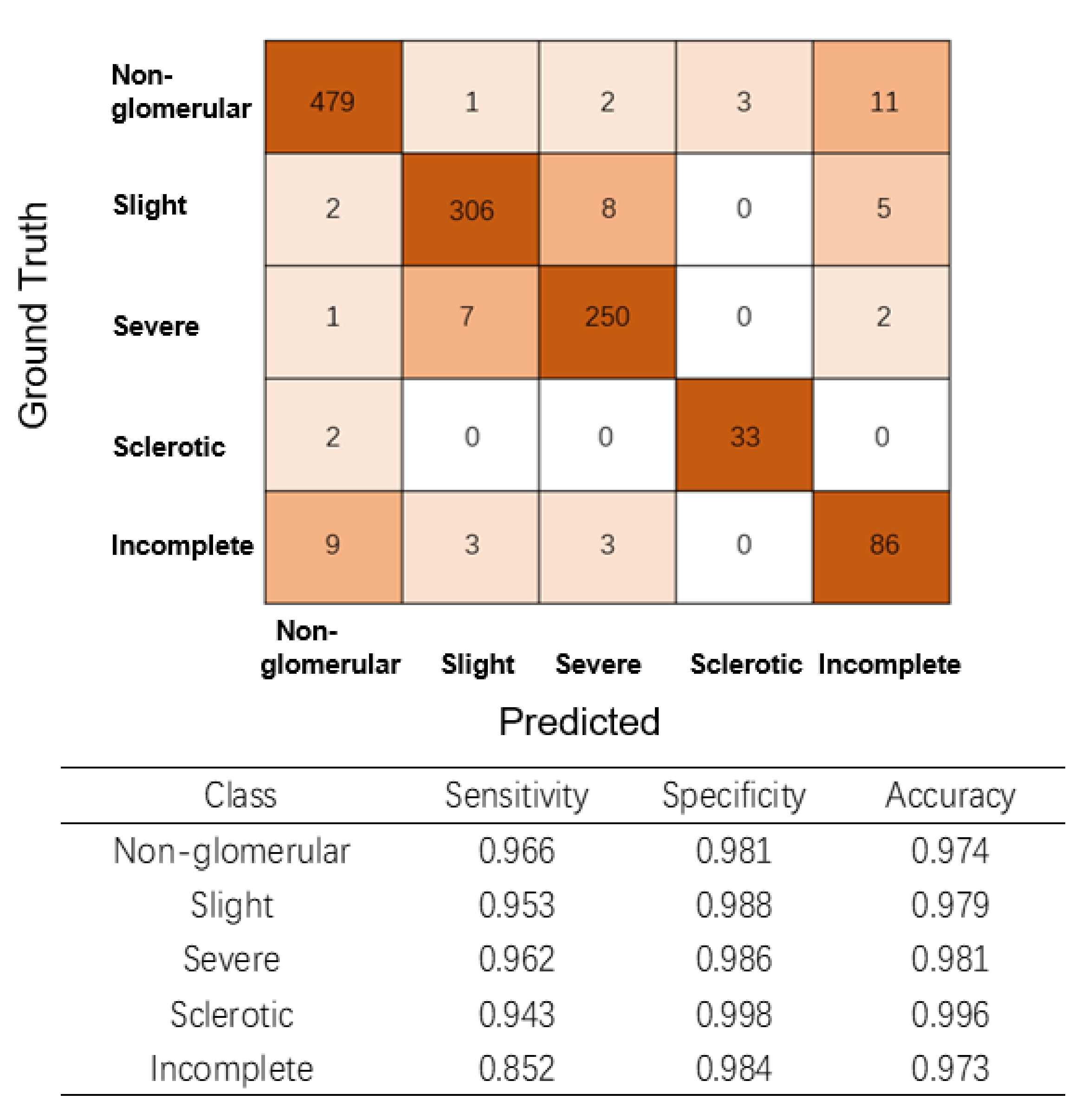
2.6. Multiclass Glomerular Performance Evaluation at the Glomerular Level
2.7. Performance Evaluation of LN Classification among Nephrologists and the AI Model at the Renal Level
2.8. Evaluation Metrics and Statistical Analysis
3. Results
3.1. Patients and Image Annotations
3.2. Glomerular Localisation Performance
3.3. AI model Performance for Glomerular Classification at the Glomerular Level
3.4. Glomerulus Multiclass Detection Performance
3.5. AI Model Performance in Glomerular Classification at the Renal Level
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maria, N.I.; Davidson, A. Protecting the kidney in systemic lupus erythematosus: From diagnosis to therapy. Nat. Rev. Rheumatol. 2020, 16, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, W.; Leng, X.; Li, Z.; Ye, Z.; Li, C.; Li, X.; Zhu, P.; Wang, Z.; Zheng, Y.; et al. Chinese SLE Treatment and Research group (CSTAR) registry: I. Major clinical characteristics of Chinese patients with systemic lupus erythematosus. Lupus 2013, 22, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Liu, Z.H. Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int. 2004, 66, 920–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertsias, G.K.; Tektonidou, M.; Amoura, Z.; Aringer, M.; Bajema, I.; Berden, J.H.; Boletis, J.; Cervera, R.; Dörner, T.; Doria, A.; et al. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann. Rheum. Dis. 2012, 71, 1771–1782. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Cheema, K.; Anders, H.J.; Aringer, M.; Bajema, I.; Boletis, J.; Frangou, E.; Houssiau, F.A.; Hollis, J.; et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann. Rheum. Dis. 2020, 79, 713–723. [Google Scholar] [CrossRef] [Green Version]
- Bajema, I.M.; Wilhelmus, S.; Alpers, C.E.; Bruijn, J.A.; Colvin, R.B.; Cook, H.T.; D’Agati, V.D.; Ferrario, F.; Haas, M.; Jennette, J.C.; et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: Clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018, 93, 789–796. [Google Scholar] [CrossRef]
- Weening, J.J.; D’agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.N.A.; Cook, T.; Ferrario, F.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004, 65, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Gasparotto, M.; Gatto, M.; Binda, V.; Doria, A.; Moroni, G. Lupus nephritis: Clinical presentations and outcomes in the 21st century. Rheumatology 2020, 59, v39–v51. [Google Scholar] [CrossRef]
- Moroni, G.; Vercelloni, P.G.; Quaglini, S.; Gatto, M.; Gianfreda, D.; Sacchi, L.; Raffiotta, F.; Zen, M.; Costantini, G.; Urban, M.L.; et al. Changing patterns in clinical-histological presentation and renal outcome over the last five decades in a cohort of 499 patients with lupus nephritis. Ann. Rheum. Dis. 2018, 77, 1318–1325. [Google Scholar] [CrossRef] [Green Version]
- Wilhelmus, S.; Cook, H.T.; Noël, L.H.; Ferrario, F.; Wolterbeek, R.; Bruijn, J.A.; Bajema, I.M. Interobserver agreement on histopathological lesions in class III or IV lupus nephritis. Clin. J. Am. Soc. Nephrol. 2015, 10, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Dasari, S.; Chakraborty, A.; Truong, L.; Mohan, C. A systematic review of interpathologist agreement in histologic classification of lupus nephritis. Kidney Int. Rep. 2019, 4, 1420–1425. [Google Scholar] [CrossRef] [Green Version]
- Bera, K.; Schalper, K.A.; Rimm, D.L.; Velcheti, V.; Madabhushi, A. Artificial intelligence in digital pathology—New tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 2019, 16, 703–715. [Google Scholar] [CrossRef]
- van der Laak, J.; Litjens, G.; Ciompi, F. Deep learning in histopathology: The path to the clinic. Nat. Med. 2021, 27, 775–784. [Google Scholar] [CrossRef]
- Niazi, M.K.K.; Parwani, A.V.; Gurcan, M.N. Digital pathology and artificial intelligence. Lancet Oncol. 2019, 20, e253–e261. [Google Scholar] [CrossRef]
- Bejnordi, B.E.; Veta, M.; Van Diest, P.J.; Van Ginneken, B.; Karssemeijer, N.; Litjens, G.; Van Der Laak, J.A.; Hermsen, M.; Manson, Q.F.; Balkenhol, M.; et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA 2017, 318, 2199–2210. [Google Scholar] [CrossRef]
- Bulten, W.; Pinckaers, H.; van Boven, H.; Vink, R.; de Bel, T.; van Ginneken, B.; van der Laak, J.; Hulsbergen-van de Kaa, C.; Litjens, G. Automated deep-learning system for Gleason grading of prostate cancer using biopsies: A diagnostic study. Lancet Oncol. 2020, 21, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Campanella, G.; Hanna, M.G.; Geneslaw, L.; Miraflor, A.; Silva, V.W.K.; Busam, K.J.; Brogi, E.; Reuter, V.E.; Klimstra, D.S.; Fuchs, T.J. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat. Med. 2019, 25, 1301–1309. [Google Scholar] [CrossRef]
- Hermsen, M.; de Bel, T.; Den Boer, M.; Steenbergen, E.J.; Kers, J.; Florquin, S.; Roelofs, J.J.; Stegall, M.D.; Alexander, M.P.; Smith, B.H.; et al. Deep learning-based histopathologic assessment of kidney tissue. J. Am. Soc. Nephrol. 2019, 30, 1968–1979. [Google Scholar] [CrossRef]
- Jayapandian, C.P.; Chen, Y.; Janowczyk, A.R.; Palmer, M.B.; Cassol, C.A.; Sekulic, M.; Hodgin, J.B.; Zee, J.; Hewitt, S.M.; O’Toole, J.; et al. Development and evaluation of deep learning-based segmentation of histologic structures in the kidney cortex with multiple histologic stains. Kidney Int. 2021, 99, 86–101. [Google Scholar] [CrossRef]
- Tzutalin, D. LabelImg. 2015. Available online: https://github.com/tzutalin/labelImg (accessed on 29 September 2020).
- Shapira, D.; Avidan, S.; Hel-Or, Y. Multiple histogram matching. In Proceedings of the IEEE International Conference on Image Processing, Melbourne, VIC, Australia, 15–18 September 2013; pp. 2269–2273. [Google Scholar]
- Bochkovskiy, A.; Wang, C.Y.; Liao, H. YOLOv4: Optimal speed and accuracy of object detection. arXiv 2020, arXiv:2004.10934. [Google Scholar]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. In Proceedings of the International Conference on Learning Representations (ICLR), San Diego, CA, USA, 7–9 May 2015; pp. 1–14. [Google Scholar]
- Ginley, B.; Lutnick, B.; Jen, K.-Y.; Fogo, A.B.; Jain, S.; Rosenberg, A.; Walavalkar, V.; Wilding, G.; Tomaszewski, J.E.; Yacoub, R.; et al. Computational segmentation and classification of diabetic glomerulosclerosis. J. Am. Soc. Nephrol. 2019, 30, 1953–1967. [Google Scholar] [CrossRef]
- Marsh, J.N.; Liu, T.-C.; Wilson, P.C.; Swamidass, S.J.; Gaut, J.P. Development and validation of a deep learning model to quantify glomerulosclerosis in kidney biopsy specimens. JAMA Netw. Open 2021, 4, e2030939. [Google Scholar] [CrossRef]
- Marsh, J.N.; Matlock, M.K.; Kudose, S.; Liu, T.-C.; Stappenbeck, T.S.; Gaut, J.P.; Swamidass, S.J. Deep learning global glomerulosclerosis in transplant kidney frozen sections. IEEE Trans. Med. Imaging 2018, 37, 2718–2728. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Nan, Y.; Xu, F.; Lei, Q.; Li, F.; Chen, T.; Liang, S.; Hou, X.; Lv, B.; Liang, D.; et al. Identification of glomerular lesions and intrinsic glomerular cell types in kidney diseases via deep learning. J. Pathol. 2020, 252, 53–64. [Google Scholar] [CrossRef]
- Chagas, P.; Souza, L.; Araújo, I.; Aldeman, N.; Duarte, A.; Angelo, M.; Dos-Santos, W.L.; Oliveira, L. Classification of glomerular hypercellularity using convolutional features and support vector machine. Artif. Intell. Med. 2020, 103, 101808. [Google Scholar] [CrossRef] [Green Version]
- Cicalese, P.A.; Mobiny, A.; Shahmoradi, Z.; Yi, X.; Mohan, C.; Van Nguyen, H. Kidney level lupus nephritis classification using uncertainty guided Bayesian convolutional neural networks. IEEE J. Biomed. Health Inform. 2021, 25, 315–324. [Google Scholar] [CrossRef]
- Huo, Y.; Deng, R.; Liu, Q.; Fogo, A.B.; Yang, H. AI applications in renal pathology. Kidney Int. 2021, 99, 1309–1320. [Google Scholar] [CrossRef]
- Hou, J.; Nast, C.C. Artificial intelligence: The next frontier in kidney biopsy evaluation. Clin. J. Am. Soc. Nephrol. 2020, 15, 1389–1391. [Google Scholar] [CrossRef]
- Kawazoe, Y.; Shimamoto, K.; Yamaguchi, R.; Shintani-Domoto, Y.; Uozaki, H.; Fukayama, M.; Ohe, K. Faster R-CNN-based glomerular detection in multistained human whole slide images. J. Imaging 2018, 4, 91. [Google Scholar] [CrossRef] [Green Version]
- Heckenauer, R.; Weber, J.; Wemmert, C.; Feuerhake, F.; Hassenforder, M.; Muller, P.A.; Forestier, G. Real-time detection of glomeruli in renal pathology. In Proceedings of the 2020 IEEE 33rd International Symposium on Computer-Based Medical Systems (CBMS), Rochester, MN, USA, 28–30 July 2020. [Google Scholar]
- Uchino, E.; Suzuki, K.; Sato, N.; Kojima, R.; Tamada, Y.; Hiragi, S.; Yokoi, H.; Yugami, N.; Minamiguchi, S.; Haga, H.; et al. Classification of glomerular pathological findings using deep learning and nephrologist-AI collective intelligence approach. Int. J. Med. Inform. 2020, 141, 104231. [Google Scholar] [CrossRef]
- Bueno, G.; Fernandez-Carrobles, M.M.; Gonzalez-Lopez, L.; Deniz, O. Glomerulosclerosis identification in whole slide images using semantic segmentation. Comput. Methods Programs Biomed. 2020, 184, 105273. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Morgan, L.A.; Liang, B.; Cheung, M.G.; Lin, C.Q.; Mun, D.; Nader, R.G.; Belghasem, M.E.; Henderson, J.M.; Francis, J.M.; et al. Segmentation of glomeruli within trichrome images using deep learning. Kidney Int. Rep. 2019, 4, 955–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strufaldi, F.L.; Neves, P.D.M.; Dias, C.B.; Yu, L.; Woronik, V.; Cavalcante, L.B.; Malheiros, D.M.A.C.; Jorge, L.B. Renal thrombotic microangiopathy associated to worse renal prognosis in lupus nephritis. J. Nephrol. 2021, 34, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Woniak, M.; Sika, J.; Wieczorek, M. Deep neural network correlation learning mechanism for CT brain tumor detection. Neural Comput. Appl. 2021, 6. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Song, L.; Woniak, M.; Liu, S. Self-attention negative feedback network for real-time image super-resolution. J. King Saud. Univ. Comput. Inf. Sci. 2021, 4. [Google Scholar] [CrossRef]
- Ligabue, G.; Pollastri, F.; Fontana, F.; Leonelli, M.; Furci, L.; Giovanella, S.; Alfano, G.; Cappelli, G.; Testa, F.; Bolelli, F.; et al. Evaluation of the classification accuracy of the kidney biopsy direct immunofluorescence through convolutional neural networks. Clin. J. Am. Soc. Nephrol. 2020, 15, 1445–1454. [Google Scholar] [CrossRef]


| Nephropathologist | AI Model | Total | Accuracy (%) | ||
|---|---|---|---|---|---|
| II | III | IV | |||
| Class II | 33 | 11 | 0 | 44 | 75.0 |
| Class III | 6 | 34 | 2 | 42 | 81.0 |
| Class IV | 0 | 0 | 61 | 61 | 100.0 |
| Total | 39 | 45 | 63 | 147 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Zhang, X.; Ding, J.; Zhang, D.; Cui, J.; Fu, X.; Han, J.; Zhu, P. Deep Learning-Based Artificial Intelligence System for Automatic Assessment of Glomerular Pathological Findings in Lupus Nephritis. Diagnostics 2021, 11, 1983. https://doi.org/10.3390/diagnostics11111983
Zheng Z, Zhang X, Ding J, Zhang D, Cui J, Fu X, Han J, Zhu P. Deep Learning-Based Artificial Intelligence System for Automatic Assessment of Glomerular Pathological Findings in Lupus Nephritis. Diagnostics. 2021; 11(11):1983. https://doi.org/10.3390/diagnostics11111983
Chicago/Turabian StyleZheng, Zhaohui, Xiangsen Zhang, Jin Ding, Dingwen Zhang, Jihong Cui, Xianghui Fu, Junwei Han, and Ping Zhu. 2021. "Deep Learning-Based Artificial Intelligence System for Automatic Assessment of Glomerular Pathological Findings in Lupus Nephritis" Diagnostics 11, no. 11: 1983. https://doi.org/10.3390/diagnostics11111983
APA StyleZheng, Z., Zhang, X., Ding, J., Zhang, D., Cui, J., Fu, X., Han, J., & Zhu, P. (2021). Deep Learning-Based Artificial Intelligence System for Automatic Assessment of Glomerular Pathological Findings in Lupus Nephritis. Diagnostics, 11(11), 1983. https://doi.org/10.3390/diagnostics11111983






