From Immune Dysregulations to Therapeutic Perspectives in Myelodysplastic Syndromes: A Review
Abstract
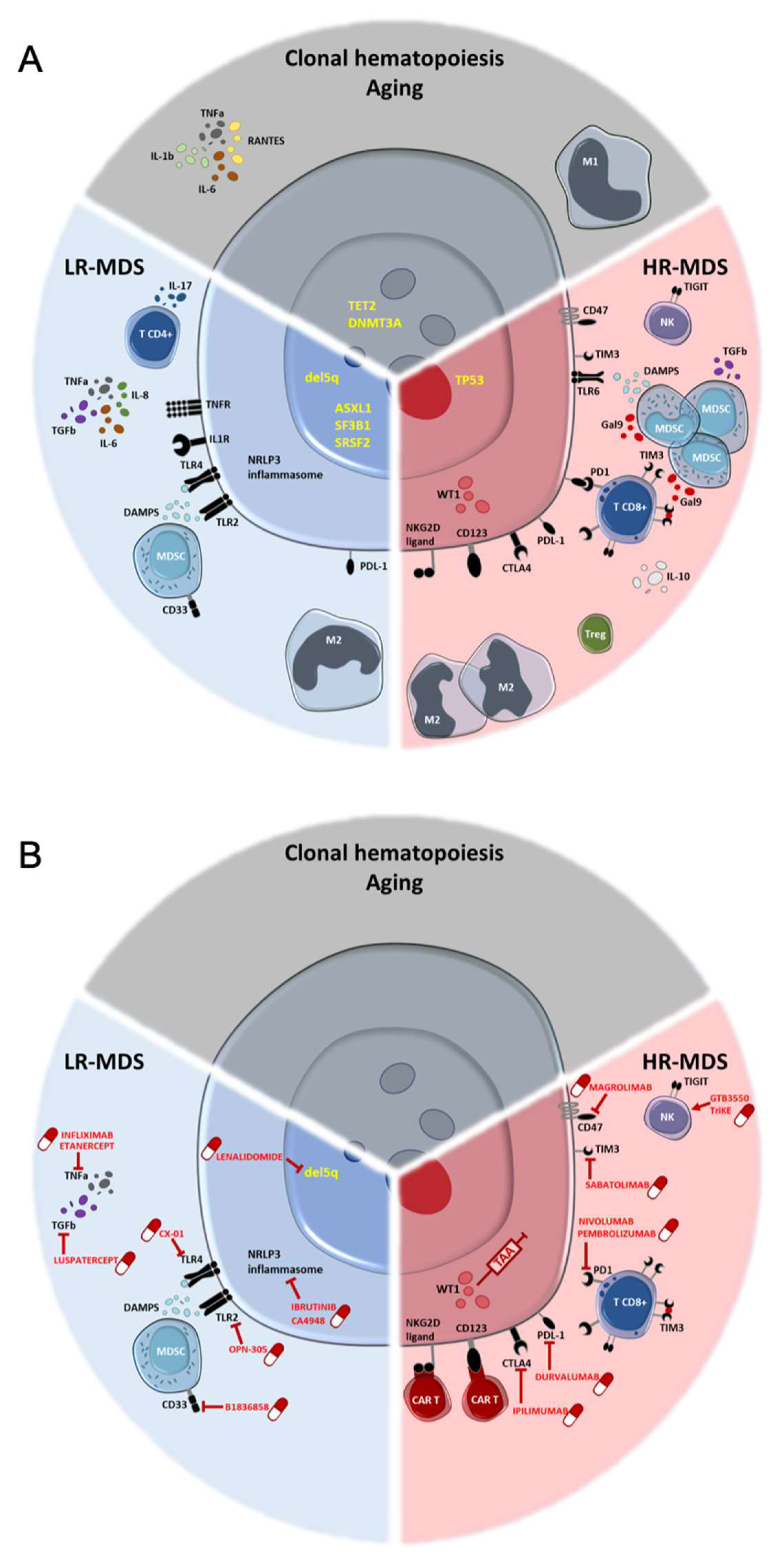
:1. Introduction
2. Inflamm-Aging as a Risk for Clonal Hematopoiesis
3. Smoldering Inflammation Acts Early in MDS Pathophysiology
3.1. Proinflammatory Cytokines
3.2. NLRP3 Inflammasome
3.3. Toll-like-Receptors (TLR)
3.4. Immune Cells
4. Positive and Negative Immune Regulators
4.1. Co-Stimulatory Molecules
- -
- OX40, Inducible T-cell COStimulator (ICOS) and 4-1BB+: Tumor protein 3 (TP53) MDS patients display reduced numbers of OX40+ cytotoxic T-cells and helper T-cells, as well as reduced ICOS+ and 4-1BB+ NK-cells [74].
- -
4.2. Coinhibitory Molecules
5. Opposite Adaptive Dysfunctions in LR and HR-MDS
6. Immune Phenotypes Can Be Associated with Somatic Mutations
7. Immune Strategies in MDS: Past, Present and Future
7.1. “Old” Treatments
7.2. Targeting TGF-β Superfamily in LR-MDS with Anemia
7.3. Targeting the Inflammasomes
7.4. Checkpoint Inhibitors
7.5. Targeting NK-Cells
7.6. Vaccination Strategies
7.7. Adoptive T-Cell Transfer Therapy
| Molecule | Patients | NCT/Phase/Status | Ref. | |
|---|---|---|---|---|
| Cytokines | ||||
| TGF-β | Luspatercept | LR-MDS with anemia | NCT02604433/Phase III/Authorization | [126] |
| TNF-α | Etanercept | HR-MDS (+Aza) | NCT00118287/I-II/Completed | [122] |
| IL-1 | Canakinumab | LR-MDS (+Aza) LR-MDS (+luspa, +TIM3inh) | NCT04239157/II/Recruiting NCT04810611/Ib/Recruiting | |
| Inflammasome | ||||
| IRAK-4 | CA-4948 | HR-MDS (+aza and/or Ven) | NCT04278768/I-II/Recruiting | [128] |
| NLRP3 | ibrutinib | HR-MDS (+Aza) HR-MDS (+Len) | NCT02553941/I/Recruiting NCT03359460/I/Recruiting | [129] |
| TLR | ||||
| TLR-4 | CX-01 | HR-MDS (+Aza) | NCT02995655/I/Completed | [131] |
| TLR-2 | OPN-305 | LR-MDS | NCT02363491/I-II/Completed | [132] |
| NF-kB | Bortezomib | HR-MDS (+Len) | NCT00580242/I/completed | [130] |
| MDSC | ||||
| CD33 inh | BI 836858 | LR-MDS | NCT02240706/II/Terminated | |
| Immune check-points | ||||
| CTLA-4 | ipilimumab | HR-MDS HR-MDS (and/or nivolumab +/−Aza) HR-MDS (+Dec) | NCT01757639/I/Completed NCT02530463/II/recruiting NCT02890329/I/Recruiting | [134,135] |
| PD-1 | Nivolumab Prembrolizumab PDR001 | HR-MDS (and/or ipi +/−Aza) HR-MDS (+chemo) HR-MDS (+Aza) HR-MDS(+Dec) HR-MDS (+Aza+/Tim3 inh) | NCT02530463/II/recruiting NCT02464657/II/completed NCT03094637/II/recruiting NCT03969446/I/recruiting NCT03066648/I/active | [135,137,139] |
| PD-L1 | Durvalumab Atezolizumab | HR-MDS (+Aza) HR-MDS (+Guadecitabine) HR-MDS (+/−Aza) | NCT02775903/II/active NCT02935361/I-II/active NCT02508870/I/completed | [141,143,179] |
| TIM3 | Sabatolimab (MBG453) | HR-MDS (+Aza+/−PD-1 inh) HR-MDS (+Aza) HR-MDS (+Aza+Ven) | NCT03066648/I/active NCT04266301/III/Recruiting NCT04812548/II/recruiting | [145,146] |
| CD70 | Cusatuzumab (ARGX-110) | HR-MDS (+Aza) HR-MDS (+Aza) | NCT04241549/I/Active NCT03030612/I-II/Active | [146] |
| CD47 | AK117 Hu5F9-G4 CC-90002 ALX148 Magrolimab | HR-MDS (+Aza) HR-MDS HR-MDS HR-MDS (+Aza) HR-MDS (+/−Aza) HR-MDS (+Aza) | NCT04900350/I-II/Recruiting NCT02678338/I/completed NCT02641002/I/Terminated NCT04417517/I-II/recruiting NCT03248479/I/completed NCT04313881/III/recruiting | [152,153] |
| NK-cells | ||||
| CD16/IL-15/CD33 | GTB-3550 TriKE™ | HR-MDS | NCT03214666/I-II/recruiting | [156] |
| KIR inh | Lirilumab | HR-MDS (+/−Aza) | NCT02599649/II/terminated | [157] |
| CAR-T-cells | ||||
| Cyad-O1/02 | Cyad-O1/02 | HR-MDS | NCT03466320/I-II/completed, NCT04167696/I/recruiting | [178] |
| NKX101 | NKX101 | HR-MDS | NCT04623944/I/Recruiting | [179] |
| Prgn-3006 | Prgn-3006 | HR-MDS | NCT03927261/I/recruiting | [180] |
| Vaccine | ||||
| DSP-7888 | LR and HR-MDS | NCT02436252/I-II/completed | [158] | |
| K562/GM-CSF/CD40L | HR-MD | NCT00840931/I/Completed | [175] | |
| NPMW | HR-MDS | NCT02750995/I/completed | [173] |
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cazzola, M. Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 383, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, T.P.; Pappa, V.; Papageorgiou, S.G. Comorbidities and frailty predict outcome of patients with myelodysplastic syndromes. Should we integrate them in novel prognostic scoring systems? J. Geriatr. Oncol. 2021, 12, 1122–1129. [Google Scholar] [CrossRef]
- Haferlach, T. The Molecular Pathology of Myelodysplastic Syndrome. Pathobiology 2018, 86, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Shreve, J.; Nazha, A. The Evolving Landscape of Myelodysplastic Syndrome Prognostication. Clin. Hematol. Int. 2020, 2, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Manero, G.; Chien, K.S.; Montalban-Bravo, G. Myelodysplastic syndromes: 2021 update on diagnosis, risk stratification and management. Am. J. Hematol. 2020, 95, 1399–1420. [Google Scholar] [CrossRef]
- Fozza, C. Retuning the immune system in myelodysplastic syndromes: From immunomodulatory approaches to vaccination strategies and non myeloablative hemopoietic cell transplant. Crit. Rev. Oncol. 2019, 133, 112–119. [Google Scholar] [CrossRef]
- Sharma, P.; Wagner, K.; Wolchok, J.D.; Allison, J.P. Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nat. Rev. Cancer 2011, 11, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Vercauteren, S.M.; Starczynowski, D.T.; Sung, S.; McNeil, K.; Salski, C.; Jensen, C.-L.; Bruyere, H.; Lam, W.L.; Karsan, A. T cells of patients with myelodysplastic syndrome are frequently derived from the malignant clone. Br. J. Haematol. 2011, 156, 409–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Yang, Y.; Gao, S.; Chen, J.; Yu, J.; Zhang, H.; Li, M.; Zhan, X.; Li, W. Immune dysregulation in myelodysplastic syndrome: Clinical features, pathogenesis and therapeutic strategies. Crit. Rev. Oncol. 2018, 122, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Rollison, D.E.; Howlader, N.; Smith, M.T.; Strom, S.S.; Merritt, W.D.; Ries, L.A.; Edwards, B.K.; List, A. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood 2008, 112, 45–52. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haferlach, T.; Nagata, Y.; Grossmann, V.; Okuno, Y.; Bacher, U.; Nagae, G.; Schnittger, S.; Sanada, M.; Kon, A.; Alpermann, T.; et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 2013, 28, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Papaemmanuil, E.; Gerstung, M.; Malcovati, L.; Tauro, S.; Gundem, G.; Van Loo, P.; Yoon, C.J.; Ellis, P.; Wedge, D.; Pellagatti, A.; et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 2013, 122, 3616–3627. [Google Scholar] [CrossRef]
- Abdel-Wahab, O.; E Figueroa, M. Interpreting new molecular genetics in myelodysplastic syndromes. Hematology 2012, 2012, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Bejar, R.; Stevenson, K.; Abdel-Wahab, O.; Galili, N.; Nilsson, B.; Garcia-Manero, G.; Kantarjian, H.; Raza, A.; Levine, R.L.; Neuberg, D.; et al. Clinical Effect of Point Mutations in Myelodysplastic Syndromes. N. Engl. J. Med. 2011, 364, 2496–2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Sanada, M.; Shiraishi, Y.; Nowak, D.; Nagata, Y.; Yamamoto, R.; Sato, Y.; Sato-Otsubo, A.; Kon, A.; Nagasaki, M.; et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 2011, 478, 64–69. [Google Scholar] [CrossRef]
- Kon, A.; Shih, L.-Y.; Minamino, M.; Sanada, M.; Shiraishi, Y.; Nagata, Y.; Yoshida, K.; Okuno, Y.; Bando, M.; Nakato, R.; et al. Recurrent mutations in multiple components of the cohesin complex in myeloid neoplasms. Nat. Genet. 2013, 45, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Ptashkin, R.; Bolton, K.L.; Sirenko, M.; Fong, C.; Spitzer, B.; Menghrajani, K.; Ossa, J.E.A.; Zhou, Y.; Bernard, E.; et al. Interplay between chromosomal alterations and gene mutations shapes the evolutionary trajectory of clonal hematopoiesis. Nat. Commun. 2021, 12, 338. [Google Scholar] [CrossRef]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.; Lindsley, C.; Mermel, C.; Burtt, N.; Chavez, A.; et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwok, B.; Hall, J.M.; Witte, J.S.; Xu, Y.; Reddy, P.; Lin, K.; Flamholz, R.; Dabbas, B.; Yung, A.; Al-Hafidh, J.; et al. MDS-associated somatic mutations and clonal hematopoiesis are common in idiopathic cytopenias of undetermined significance. Blood 2015, 126, 2355–2361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuser, M.; Thol, F.; Ganser, A. Clonal Hematopoiesis of Indeterminate Potential. Dtsch. Aerzteblatt Online 2016, 113, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.; Hasserjian, R.P.; Ebert, B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Genovese, G.; Kähler, A.K.; Handsaker, R.; Lindberg, J.; Rose, S.; Bakhoum, S.; Chambert, K.; Mick, E.; Neale, B.M.; Fromer, M.; et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. N. Engl. J. Med. 2014, 371, 2477–2487. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Lu, C.; Wang, J.; McLellan, M.D.; Johnson, K.J.; Wendl, M.C.; McMichael, J.F.; Schmidt, H.K.; Yellapantula, V.; Miller, C.A.; et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 2014, 20, 1472–1478. [Google Scholar] [CrossRef]
- Barreyro, L.; Chlon, T.; Starczynowski, D.T. Chronic immune response dysregulation in MDS pathogenesis. Blood 2018, 132, 1553–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rea, I.M.; Gibson, D.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Bogeska, R.; Kaschutnig, P.; Fawaz, M.; Mikecin, A.-M.; Büchler-Schäff, M.; Paffenholz, S.; Asada, N.; Frauhammer, F.; Buettner, F.; Ball, M.; et al. Hematopoietic Stem Cells Fail to Regenerate Following Inflammatory Challenge. bioRxiv 2020. [Google Scholar]
- Matatall, K.A.; Jeong, M.; Chen, S.; Sun, D.; Chen, F.; Mo, Q.; Kimmel, M.; King, K.Y. Chronic Infection Depletes Hematopoietic Stem Cells through Stress-Induced Terminal Differentiation. Cell Rep. 2016, 17, 2584–2595. [Google Scholar] [CrossRef] [Green Version]
- Esplin, B.L.; Shimazu, T.; Welner, R.S.; Garrett, K.P.; Nie, L.; Zhang, Q.; Humphrey, M.B.; Yang, Q.; Borghesi, L.A.; Kincade, P.W. Chronic Exposure to a TLR Ligand Injures Hematopoietic Stem Cells. J. Immunol. 2011, 186, 5367–5375. [Google Scholar] [CrossRef] [Green Version]
- Mann, M.; Mehta, A.; de Boer, C.; Kowalczyk, M.S.; Lee, K.; Haldeman, P.; Rogel, N.; Knecht, A.R.; Farouq, D.; Regev, A.; et al. Heterogeneous Responses of Hematopoietic Stem Cells to Inflammatory Stimuli Are Altered with Age. Cell Rep. 2018, 25, 2992–3005.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abegunde, S.O.; Buckstein, R.; Wells, R.A.; Rauh, M.J. An inflammatory environment containing TNFα favors Tet2 -mutant clonal hematopoiesis. Exp. Hematol. 2018, 59, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Leoni, C.; Montagner, S.; Rinaldi, A.; Bertoni, F.; Polletti, S.; Balestrieri, C.; Monticelli, S. Dnmt3arestrains mast cell inflammatory responses. Proc. Natl. Acad. Sci. USA 2017, 114, E1490–E1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Zheng, Y.-H.; Xu, L.; Cao, C.; Dong, B.; Chen, X. The inflammatory cytokine profile of myelodysplastic syndromes. Medicine 2019, 98, e15844. [Google Scholar] [CrossRef]
- Molnár, L.; Berki, T.; Hussain, A.; Németh, P.; Losonczy, H. Detection of TNFα expression in the bone marrow and determination of TNFα production of peripheral blood mononuclear cells in myelodysplastic syndrome. Pathol. Oncol. Res. 2000, 6, 18–23. [Google Scholar] [CrossRef]
- Stifter, G.; Heiss, S.; Gastl, G.; Tzankov, A.; Stauder, R. Over-expression of tumor necrosis factor-alpha in bone marrow biopsies from patients with myelodysplastic syndromes: Relationship to anemia and prognosis. Eur. J. Haematol. 2005, 75, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Guo, J.; Xu, F.; He, Q.; Zhao, Y.; Yang, Y.; Gu, S.; Zhang, Y.; Wu, L.; et al. Interleukin-17 enhances the production of interferon-γ and tumour necrosis factor-α by bone marrow T lymphocytes from patients with lower risk myelodysplastic syndromes. Eur. J. Haematol. 2013, 90, 375–384. [Google Scholar] [CrossRef]
- Pardanani, A.; Finke, C.; Lasho, T.L.; Al-Kali, A.; Begna, K.H.; A Hanson, C.; Tefferi, A. IPSS-independent prognostic value of plasma CXCL10, IL-7 and IL-6 levels in myelodysplastic syndromes. Leukemia 2011, 26, 693–699. [Google Scholar] [CrossRef] [PubMed]
- De Matos, A.G.; Junior, H.L.R.; Borges, D.D.P.; Okubo, B.M.; De Sousa, J.C.; Barbosa, M.C.; De Castro, M.F.; Gonçalves, R.P.; Pinheiro, R.F.; Magalhães, S.M.M. Interleukin-8 and nuclear factor kappa B are increased and positively correlated in myelodysplastic syndrome. Med. Oncol. 2017, 34, 168. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Estey, E.; Wen, S.; Pierce, S.; Kantarjian, H.; Albitar, M.; Kurzrock, R. The prognostic significance of cytokine levels in newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndromes. Cancer 2008, 113, 1605–1613. [Google Scholar] [CrossRef]
- Shetty, V.; Mundle, S.; Alvi, S.; Showel, M.; Broady-Robinson, L.; Dar, S.; Borok, R.; Showel, J.; Gregory, S.; Rifkin, S.; et al. Measurement of apoptosis, proliferation and three cytokines in 46 patients with myelodysplastic syndromes. Leuk. Res. 1996, 20, 891–900. [Google Scholar] [CrossRef]
- Zeng, Q.; Shu, J.; Hu, Q.; Zhou, S.-H.; Qian, Y.-M.; Hu, M.-H.; Hu, L.-Y.; Wang, Y.-G.; Zhou, Y.-M.; Lu, J.-H. Apoptosis in human myelodysplastic syndrome CD34+ cells is modulated by the upregulation of TLRs and histone H4 acetylation via a β-arrestin 1 dependent mechanism. Exp. Cell Res. 2016, 340, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kordasti, S.Y.; Afzali, B.; Lim, Z.; Ingram, W.; Hayden, J.; Barber, L.; Matthews, K.; Chelliah, R.; Guinn, B.; Lombardi, G.; et al. IL-17-producing CD4+T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br. J. Haematol. 2009, 145, 64–72. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Bujko, K.; Cymer, M.; Thapa, A.; Adamiak, M.; Ratajczak, J.; Abdel-Latif, A.K.; Kucia, M. The Nlrp3 inflammasome as a “rising star” in studies of normal and malignant hematopoiesis. Leukemia 2020, 34, 1512–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallman, D.A.; List, A. The central role of inflammatory signaling in the pathogenesis of myelodysplastic syndromes. Blood 2019, 133, 1039–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, T.; Calvi, L.M.; Becker, M.W.; Liesveld, J.L. Flaming and fanning: The Spectrum of inflammatory influences in myelodysplastic syndromes. Blood Rev. 2019, 36, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Bergsbaken, T.; Fink, S.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Genet. 2009, 7, 99–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basiorka, A.A.; McGraw, K.L.; Eksioglu, E.A.; Chen, X.; Johnson, J.; Zhang, L.; Zhang, Q.; Irvine, B.A.; Cluzeau, T.; Sallman, D.A.; et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood 2016, 128, 2960–2975. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.-C.; Cesaro, A.; Chapeton-Montes, J.; Tardif, M.; Antoine, F.; Girard, D.; Tessier, P.A. S100A8 and S100A9 Induce Cytokine Expression and Regulate the NLRP3 Inflammasome via ROS-Dependent Activation of NF-κB1. PLoS ONE 2013, 8, e72138. [Google Scholar] [CrossRef] [Green Version]
- Rhyasen, G.W.; Bolanos, L.; Fang, J.; Jerez, A.; Wunderlich, M.; Rigolino, C.; Mathews, L.; Ferrer, M.; Southall, N.; Guha, R.; et al. Targeting IRAK1 as a Therapeutic Approach for Myelodysplastic Syndrome. Cancer Cell 2013, 24, 90–104. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giudice, V.; Wu, Z.; Kajigaya, S.; Ibanez, M.D.P.F.; Rios, O.; Cheung, F.; Ito, S.; Young, N.S. Circulating S100A8 and S100A9 protein levels in plasma of patients with acquired aplastic anemia and myelodysplastic syndromes. Cytokine 2018, 113, 462–465. [Google Scholar] [CrossRef]
- Chen, X.; Eksioglu, E.A.; Zhou, J.; Zhang, L.; Djeu, J.; Fortenbery, N.; Epling-Burnette, P.; Van Bijnen, S.; Dolstra, H.; Cannon, J.; et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J. Clin. Investig. 2013, 123, 4595–4611. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Garrett, K.P.; Ohta, S.; Bahrun, U.; Kouro, T.; Akira, S.; Takatsu, K.; Kincade, P.W. Toll-like Receptors on Hematopoietic Progenitor Cells Stimulate Innate Immune System Replenishment. Immunity 2006, 24, 801–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paracatu, L.C.; Schuettpelz, L.G. Contribution of Aberrant Toll Like Receptor Signaling to the Pathogenesis of Myelodysplastic Syndromes. Front. Immunol. 2020, 11, 1236. [Google Scholar] [CrossRef]
- Wei, Y.; Dimicoli, S.; Bueso-Ramos, C.; Chen, R.; Yang, H.; Neuberg, N.; Pierce, S.; Jia, Y.; Zheng, H.; Wang, H.; et al. Toll-like receptor alterations in myelodysplastic syndrome. Leukemia 2013, 27, 1832–1840. [Google Scholar] [CrossRef] [Green Version]
- Maratheftis, C.I.; Andreakos, E.; Moutsopoulos, H.M.; Voulgarelis, M. Toll-like Receptor-4 Is Up-Regulated in Hematopoietic Progenitor Cells and Contributes to Increased Apoptosis in Myelodysplastic Syndromes. Clin. Cancer Res. 2007, 13, 1154–1160. [Google Scholar] [CrossRef] [Green Version]
- Dimicoli, S.; Wei, Y.; Bueso-Ramos, C.; Yang, H.; Dinardo, C.; Jia, Y.; Zheng, H.; Fang, Z.; Nguyen, M.; Pierce, S.; et al. Overexpression of the Toll-Like Receptor (TLR) Signaling Adaptor MYD88, but Lack of Genetic Mutation, in Myelodysplastic Syndromes. PLoS ONE 2013, 8, e71120. [Google Scholar] [CrossRef]
- Monlish, D.A.; Greenberg, Z.J.; Bhatt, S.T.; Leonard, K.M.; Romine, M.P.; Dong, Q.; Bendesky, L.; Duncavage, E.J.; Magee, J.A.; Schuettpelz, L.G. TLR2/6 signaling promotes the expansion of premalignant hematopoietic stem and progenitor cells in the NUP98–HOXD13 mouse model of MDS. Exp. Hematol. 2020, 88, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Velegraki, M.; Papakonstanti, E.; Mavroudi, I.; Psyllaki, M.; Tsatsanis, C.; Oulas, A.; Iliopoulos, I.; Katonis, P.; Papadaki, H.A. Impaired clearance of apoptotic cells leads to HMGB1 release in the bone marrow of patients with myelodysplastic syndromes and induces TLR4-mediated cytokine production. Haematologica 2013, 98, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Kapor, S.; Santibanez, J. Myeloid-Derived Suppressor Cells and Mesenchymal Stem/Stromal Cells in Myeloid Malignancies. J. Clin. Med. 2021, 10, 2788. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, H.; Shao, Z. Monocyte-Derived Macrophages Are Impaired in Myelodysplastic Syndrome. J. Immunol. Res. 2016, 2016, 5479013. [Google Scholar] [CrossRef]
- Bento, L.C.; Bacal, N.S.; Rocha, F.A.; Severino, P.; Marti, L.C. Bone Marrow Monocytes and Derived Dendritic Cells from Myelodysplastic Patients Have Functional Abnormalities Associated with Defective Response to Bacterial Infection. J. Immunol. 2020, 204, 2098–2109. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, L.; Han, Y.; Niu, H.; Yan, L.; Shao, Z.; Xing, L.; Wang, H. Abnormal Macrophage Polarization in Patients with Myelodysplastic Syndrome. Mediat. Inflamm. 2021, 2021, 9913382. [Google Scholar] [CrossRef]
- Saft, L.; Björklund, E.; Berg, E.; Hellström-Lindberg, E.; Porwit, A. Bone marrow dendritic cells are reduced in patients with high-risk myelodysplastic syndromes. Leuk. Res. 2012, 37, 266–273. [Google Scholar] [CrossRef]
- Ma, L.; Ceuppens, J.; Kasran, A.; Delforge, M.; Boogaerts, M.; Vandenberghe, P. Immature and mature monocyte-derived dendritic cells in myelodysplastic syndromes of subtypes refractory anemia or refractory anemia with ringed sideroblasts display an altered cytokine profile. Leuk. Res. 2007, 31, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Delforge, M.; Van Duppen, V.; Verhoef, G.; Emanuel, B.; Boogaerts, M.; Hagemeijer, A.; Vandenberghe, P. Circulating myeloid and lymphoid precursor dendritic cells are clonally involved in myelodysplastic syndromes. Leukemia 2004, 18, 1451–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mailloux, A.W.; Sugimori, C.; Komrokji, R.S.; Yang, L.; Maciejewski, J.P.; Sekeres, M.A.; Paquette, R.; Loughran, T.P.; List, A.F.; Epling-Burnette, P.K. Expansion of effector memory regulatory T cells represents a novel prognostic factor in lower risk myelodysplastic syndrome. J. Immunol. 2012, 189, 3198–3208. [Google Scholar] [CrossRef] [Green Version]
- Epling-Burnette, P.K.; Bai, F.; Painter, J.S.; Rollison, D.E.; Salih, H.R.; Krusch, M.; Zou, J.; Ku, E.; Zhong, B.; Boulware, D.; et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood 2007, 109, 4816–4824. [Google Scholar] [CrossRef]
- Hejazi, M.; Manser, A.R.; Fröbel, J.; Kündgen, A.; Zhao, X.; Schönberg, K.; Germing, U.; Haas, R.; Gattermann, N.; Uhrberg, M. Impaired cytotoxicity associated with defective natural killer cell differentiation in myelodysplastic syndromes. Haematologica 2015, 100, 643–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringaris, K.; Marin, D.; Barrett, A.J.; Hills, R.; Sobieski, C.; Cao, K.; Saltarrelli, J.G.; Daher, M.; Shaim, H.; Smith, N.; et al. KIR gene haplotype: An independent predictor of clinical outcome in MDS patients. Blood 2016, 128, 2819–2823. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.; Basu, S.; Garcia-Manero, G.; Hourigan, C.S.; Oetjen, K.A.; Cortes, J.E.; Ravandi, F.; Jabbour, E.J.; Al-Hamal, Z.; Konopleva, M.; et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer 2018, 125, 1470–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallman, D.A.; McLemore, A.F.; Aldrich, A.L.; Komrokji, R.S.; McGraw, K.L.; Dhawan, A.; Geyer, S.; Hou, H.-A.; Eksioglu, E.A.; Sullivan, A.; et al. TP53 mutations in myelodysplastic syndromes and secondary AML confer an immunosuppressive phenotype. Blood 2020, 136, 2812–2823. [Google Scholar] [CrossRef]
- Epling-Burnette, P.K.; Painter, J.S.; E Rollison, D.; Ku, E.; Vendron, D.; Widen, R.; Boulware, D.; Zou, J.X.; Bai, F.; List, A. Prevalence and clinical association of clonal T-cell expansions in Myelodysplastic Syndrome. Leukemia 2007, 21, 659–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tcvetkov, N.; Gusak, A.; Morozova, E.; Moiseev, I.; Baykov, V.; Barabanshikova, M.; Lepik, K.; Bakin, E.; Vlasova, J.; Osipova, A.; et al. Immune checkpoints bone marrow expression as the predictor of clinical outcome in myelodysplastic syndrome. Leuk. Res. Rep. 2020, 14, 100215. [Google Scholar] [CrossRef]
- Yang, H.; Bueso-Ramos, C.; Dinardo, C.D.; Estecio, M.; Davanlou, M.; Geng, Q.-R.; Fang, Z.; Nguyen, M.; Pierce, S.; Wei, Y.; et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 2013, 28, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Moskorz, W.; Cosmovici, C.; Jäger, P.S.; Cadeddu, R.P.; Timm, J.; Haas, R. Myelodysplastic syndrome patients display alterations in their immune status reflected by increased PD-L1-expressing stem cells and highly dynamic exhausted T-cell frequencies. Br. J. Haematol. 2021, 193, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Kondo, A.; Yamashita, T.; Tamura, H.; Zhao, W.; Tsuji, T.; Shimizu, M.; Shinya, E.; Takahashi, H.; Tamada, K.; Chen, L.; et al. Interferon-γ and tumor necrosis factor-α induce an immunoinhibitory molecule, B7-H1, via nuclear factor-κB activation in blasts in myelodysplastic syndromes. Blood 2010, 116, 1124–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, P.; Eksioglu, E.A.; Chen, X.; Kandell, W.; Le Trinh, T.; Cen, L.; Qi, J.; Sallman, D.A.; Zhang, Y.; Tu, N.; et al. S100A9-induced overexpression of PD-1/PD-L1 contributes to ineffective hematopoiesis in myelodysplastic syndromes. Leukemia 2019, 33, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.-L.; Li, L.-J.; Fu, R.; Wang, H.-Q.; Jiang, H.-J.; Yue, L.-Z.; Zhang, W.; Liu, H.; Ruan, E.-B.; Qu, W.; et al. Elevated TIM3+ hematopoietic stem cells in untreated myelodysplastic syndrome displayed aberrant differentiation, overproliferation and decreased apoptosis. Leuk. Res. 2014, 38, 714–721. [Google Scholar] [CrossRef]
- Tao, J.; Li, L.; Wang, Y.; Fu, R.; Wang, H.; Shao, Z. Increased TIM3+CD8+T cells in Myelodysplastic Syndrome patients displayed less perforin and granzyme B secretion and higher CD95 expression. Leuk. Res. 2016, 51, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Asayama, T.; Tamura, H.; Ishibashi, M.; Kuribayashi-Hamada, Y.; Onodera-Kondo, A.; Okuyama, N.; Yamada, A.; Shimizu, M.; Moriya, K.; Takahashi, H.; et al. Functional expression of Tim-3 on blasts and clinical impact of its ligand galectin-9 in myelodysplastic syndromes. Oncotarget 2017, 8, 88904–88917. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Han, D.; Gao, S.; Zhang, W.; Yu, H.; Liu, P.; Fu, R.; Li, L.; Shao, Z. CD8 + T cells exhaustion induced by myeloid-derived suppressor cells in myelodysplastic syndromes patients might be through TIM3/Gal-9 pathway. J. Cell. Mol. Med. 2019, 24, 1046–1058. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Li, L.; Lu, F.; Yue, J.; Liu, Z.; Zhang, W.; Fu, R. Overexpression of TIGIT in NK and T Cells Contributes to Tumor Immune Escape in Myelodysplastic Syndromes. Front. Oncol. 2020, 10, 1595. [Google Scholar] [CrossRef]
- Moiseev, I.S.; Tcvetkov, N.Y.; Barkhatov, I.M.; Barabanshikova, M.V.; Bug, D.S.; Petuhova, N.V.; Tishkov, A.V.; Bakin, E.A.; Izmailova, E.A.; Shakirova, A.I.; et al. High mutation burden in the checkpoint and micro-RNA processing genes in myelodysplastic syndrome. PLoS ONE 2021, 16, e0248430. [Google Scholar] [CrossRef]
- Bouchliou, I.; Miltiades, P.; Nakou, E.; Spanoudakis, E.; Goutzouvelidis, A.; Vakalopoulou, S.; Garypidou, V.; Kotoula, V.; Bourikas, G.; Tsatalas, C.; et al. Th17 and Foxp3+ T regulatory cell dynamics and distribution in myelodysplastic syndromes. Clin. Immunol. 2011, 139, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.R.; Traina, F.; Campos, P.D.M.; Pereira, J.K.N.; Machado-Neto, J.A.; Machado, H.D.C.; Gilli, S.C.O.; Saad, S.T.O.; Favaro, P. IL10 inversely correlates with the percentage of CD8+ cells in MDS patients. Leuk. Res. 2013, 37, 541–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordasti, S.Y.; Ingram, W.; Hayden, J.; Darling, D.; Barber, L.; Afzali, B.; Lombardi, G.; Wlodarski, M.; Maciejewski, J.P.; Farzaneh, F.; et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS). Blood 2007, 110, 847–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotsianidis, I.; Bouchliou, I.; Nakou, E.; Spanoudakis, E.; Margaritis, D.; Christophoridou, A.V.; Anastasiades, A.; Tsigalou, C.; Bourikas, G.; Karadimitris, A.; et al. Kinetics, function and bone marrow trafficking of CD4+CD25+FOXP3+ regulatory T cells in myelodysplastic syndromes (MDS). Leukemia 2008, 23, 510–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haroun, F.; Solola, S.A.; Nassereddine, S.; Tabbara, I. PD-1 signaling and inhibition in AML and MDS. Ann. Hematol. 2017, 96, 1441–1448. [Google Scholar] [CrossRef]
- Kikushige, Y.; Shima, T.; Takayanagi, S.-I.; Urata, S.; Miyamoto, T.; Iwasaki, H.; Takenaka, K.; Teshima, T.; Tanaka, T.; Inagaki, Y.; et al. TIM-3 Is a Promising Target to Selectively Kill Acute Myeloid Leukemia Stem Cells. Cell Stem Cell 2010, 7, 708–717. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Fu, R.; Wang, H.; Li, L.; Liu, H.; Shao, Z. CD47 is expressed abnormally on hematopoietic cells in myelodysplastic syndrome. Leuk. Res. 2013, 37, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Han, Y.; Liu, Y.; Yang, L.; Niu, H.; Yan, L.; Liu, C.; Shao, Z.; Xing, L.; Wang, H. Phagocytosis checkpoints on hematopoietic stem cells in patients with myelodysplastic syndromes. Asia-Pac. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Fuster, J.J.; MacLauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.-L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.R.; Nix, D.; Gregory, M.; Ciorba, M.A.; Ostrander, E.L.; Newberry, R.D.; Spencer, D.H.; Challen, G.A. Inflammatory cytokines promote clonal hematopoiesis with specific mutations in ulcerative colitis patients. Exp. Hematol. 2019, 80, 36–41.e3. [Google Scholar] [CrossRef]
- Cook, E.K.; Izukawa, T.; Young, S.; Rosen, G.; Jamali, M.; Zhang, L.; Johnson, D.; Bain, E.; Hilland, J.; Ferrone, C.K.; et al. Comorbid and inflammatory characteristics of genetic subtypes of clonal hematopoiesis. Blood Adv. 2019, 3, 2482–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.-P.; Boy, M.; Azoulay, C.; Clappier, E.; Sébert, M.; Amable, L.; Klibi, J.; Benlagha, K.; Espeli, M.; Balabanian, K.; et al. MDS/CMML with TET2 or IDH mutation Are Associated with Systemic Inflammatory and Autoimmune Diseases (SIAD) and T Cell Dysregulation. Blood 2020, 136, 31–32. [Google Scholar] [CrossRef]
- Smith, M.A.; Choudhary, G.S.; Pellagatti, A.; Choi, K.; Bolanos, L.C.; Bhagat, T.D.; Gordon-Mitchell, S.; Von Ahrens, D.; Pradhan, K.; Steeples, V.; et al. U2AF1 mutations induce oncogenic IRAK4 isoforms and activate innate immune pathways in myeloid malignancies. Nature 2019, 21, 640–650. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, B.P.; Danhorn, T.; De Arras, L.; Flatley, B.R.; Marcus, R.A.; Farias-Hesson, E.; Leach, S.M.; Alper, S. Regulation of Toll-like Receptor Signaling by the SF3a mRNA Splicing Complex. PLoS Genet. 2015, 11, e1004932. [Google Scholar] [CrossRef] [Green Version]
- Pollyea, D.A.; Harris, C.; Rabe, J.L.; Hedin, B.R.; De Arras, L.; Katz, S.; Wheeler, E.; Bejar, R.; Walter, M.J.; Jordan, C.T.; et al. Myelodysplastic syndrome-associated spliceosome gene mutations enhance innate immune signaling. Haematologica 2019, 104, e388–e392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, D.B.; Ferrada, M.A.; Sikora, K.A.; Ombrello, A.K.; Collins, J.C.; Pei, W.; Balanda, N.; Ross, D.L.; Cardona, D.O.; Wu, Z.; et al. Somatic Mutations in UBA1 and Severe Adult-Onset Autoinflammatory Disease. N. Engl. J. Med. 2020, 383, 2628–2638. [Google Scholar] [CrossRef] [PubMed]
- Georgin-Lavialle, S.; Terrier, B.; Guedon, A.; Heiblig, M.; Comont, T.; Lazaro, E.; Lacombe, V.; Terriou, L.; Ardois, S.; Bouaziz, J.; et al. Further characterization of clinical and laboratory features occurring in VEXAS syndrome in a large-scale analysis of multicenter case-series of 116 French patients. Br. J. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Poulter, J.A.; Collins, J.C.; Cargo, C.; De Tute, R.M.; Evans, P.; Cardona, D.O.; Bowen, D.T.; Cunnington, J.R.; Baguley, E.; Quinn, M.; et al. Novel somatic mutations in UBA1 as a cause of VEXAS syndrome. Blood 2021, 137, 3676–3681. [Google Scholar] [CrossRef] [PubMed]
- Bourbon, E.; Heiblig, M.; Valentin, M.G.; Barba, T.; Durel, C.-A.; Lega, J.C.; Barraco, F.; Sève, P.; Jamilloux, Y.; Sujobert, P. Therapeutic options in VEXAS syndrome: Insights from a retrospective series. Blood 2021, 137, 3682–3684. [Google Scholar] [CrossRef] [PubMed]
- Obiorah, I.E.; Patel, B.A.; Groarke, E.M.; Wang, W.; Trick, M.; Ombrello, A.K.; Ferrada, M.A.; Wu, Z.; Gutierrez-Rodrigues, F.; Lotter, J.; et al. Benign and malignant hematologic manifestations in patients with VEXAS syndrome due to somatic mutations in UBA1. Blood Adv. 2021, 5, 3203–3215. [Google Scholar] [CrossRef]
- Comont, T.; Heiblig, M.; Rivière, E.; Terriou, L.; Rossignol, J.; Bouscary, D.; Rieu, V.; Le Guenno, G.; Mathian, A.; Aouba, A.; et al. Azacitidine for patients with Vacuoles, E1 Enzyme, X-linked, Autoinflammatory, Somatic syndrome (VEXAS) and myelodysplastic syndrome: Data from the French VEXAS registry. Br. J. Haematol. 2021. [Google Scholar] [CrossRef]
- Thol, F.; Klesse, S.; Köhler, L.; Gabdoulline, R.; Kloos, A.; Liebich, A.; Wichmann, M.; Chaturvedi, A.; Fabisch, J.; I Gaidzik, V.; et al. Acute myeloid leukemia derived from lympho-myeloid clonal hematopoiesis. Leukemia 2016, 31, 1286–1295. [Google Scholar] [CrossRef]
- Dickinson, A.M.; Norden, J.; Li, S.; Hromadnikova, I.; Schmid, C.; Schmetzer, H.; Jochem-Kolb, H. Graft-versus-Leukemia Effect Following Hematopoietic Stem Cell Transplantation for Leukemia. Front. Immunol. 2017, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, B.; Da, W.; Gong, M.; Guan, M. Treatment of Myelodysplastic Syndrome with Cyclosporin A. Int. J. Hematol. 2007, 85, 11–17. [Google Scholar] [CrossRef]
- Passweg, J.R.; Giagounidis, A.A.; Simcock, M.; Aul, C.; Dobbelstein, C.; Stadler, M.; Ossenkoppele, G.; Hofmann, W.-K.; Schilling, K.; Tichelli, A.; et al. Immunosuppressive Therapy for Patients With Myelodysplastic Syndrome: A Prospective Randomized Multicenter Phase III Trial Comparing Antithymocyte Globulin Plus Cyclosporine with Best Supportive Care—SAKK 33/99. J. Clin. Oncol. 2011, 29, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Komrokji, R.S.; Mailloux, A.W.; Chen, D.-T.; Sekeres, M.A.; Paquette, R.; Fulp, W.J.; Sugimori, C.; Paleveda-Pena, J.; Maciejewski, J.P.; List, A.F.; et al. A phase II multicenter rabbit anti-thymocyte globulin trial in patients with myelodysplastic syndromes identifying a novel model for response prediction. Haematologica 2014, 99, 1176–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochenderfer, J.N.; Kobayashi, S.; Wieder, E.D.; Su, C.; Molldrem, J.J. Loss of T-lymphocyte clonal dominance in patients with myelodysplastic syndrome responsive to immunosuppression. Blood 2002, 100, 3639–3645. [Google Scholar] [CrossRef] [Green Version]
- Lim, Z.Y.; Killick, S.; Germing, U.; Cavenagh, J.; Culligan, D.; Bacigalupo, A.; Marsh, J.; Mufti, G.J. Low IPSS score and bone marrow hypocellularity in MDS patients predict hematological responses to antithymocyte globulin. Leukemia 2007, 21, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Deveaux, M.; De Witte, T.; Neukirchen, J.; Sekeres, M.A.; Brunner, A.M.; Roboz, G.J.; Steensma, D.P.; Bhatt, V.R.; Platzbecker, U.; et al. The use of immunosuppressive therapy in MDS: Clinical outcomes and their predictors in a large international patient cohort. Blood Adv. 2018, 2, 1765–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenaux, P.; Giagounidis, A.; Selleslag, D.; Beyne-Rauzy, O.; Mufti, G.; Mittelman, M.; Muus, P.; Boekhorst, P.T.; Sanz, G.; Del Cañizo, C.; et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood 2011, 118, 3765–3776. [Google Scholar] [CrossRef] [PubMed]
- Fozza, C.; Crobu, V.; Isoni, M.A.; Dore, F. The immune landscape of myelodysplastic syndromes. Crit. Rev. Oncol. 2016, 107, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Krönke, J.; Fink, E.; Hollenbach, P.W.; Macbeth, K.J.; Hurst, S.N.; Udeshi, N.D.; Chamberlain, P.P.; Mani, D.R.; Man, H.W.; Gandhi, A.K.; et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nat. Cell Biol. 2015, 523, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Giagounidis, A.; Mufti, G.J.; Mittelman, M.; Sanz, G.; Platzbecker, U.; Muus, P.; Selleslag, D.; Beyne-Rauzy, O.; Boekhorst, P.T.; Del Cañizo, C.; et al. Outcomes in RBC transfusion-dependent patients with L ow-/ I ntermediate-1-risk myelodysplastic syndromes with isolated deletion 5q treated with lenalidomide: A subset analysis from the MDS -004 study. Eur. J. Haematol. 2014, 93, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.; Ranpura, V.; Wu, C.; Olnes, M.J.; Parikh, A.R.; Shenoy, A.; Thompson, J.; Weinstein, B.; Scheinberg, P.; Barrett, A.J.; et al. Long-term outcomes in myelodysplastic syndrome patients treated with alemtuzumab. Blood Adv. 2019, 3, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Baron, F.; Suciu, S.; Amadori, S.; Muus, P.; Zwierzina, H.; Denzlinger, C.; Delforge, M.; Thyss, A.; Selleslag, D.; Indrak, K.; et al. Value of infliximab (Remicade(R)) in patients with low-risk myelodysplastic syndrome: Final results of a randomized phase II trial (EORTC trial 06023) of the EORTC Leukemia Group. Haematologica 2011, 97, 529–533. [Google Scholar] [CrossRef]
- Scott, B.L.; Ramakrishnan, A.; Storer, B.; Becker, P.S.; Petersdorf, S.; Estey, E.H.; Deeg, H. Prolonged responses in patients with MDS and CMML treated with azacitidine and etanercept. Br. J. Haematol. 2010, 148, 944–947. [Google Scholar] [CrossRef] [Green Version]
- Mekinian, A.; Dervin, G.; Lapidus, N.; Kahn, J.E.; Terriou, L.; Liozon, E.; Grignano, E.; Piette, J.-C.; Rauzy, O.B.; Grobost, V.; et al. Biologics in myelodysplastic syndrome-related systemic inflammatory and autoimmune diseases: French multicenter retrospective study of 29 patients. Autoimmun. Rev. 2017, 16, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Malik, J.; Kim, A.R.; Tyre, K.A.; Cherukuri, A.R.; Palis, J. Erythropoietin critically regulates the terminal maturation of murine and human primitive erythroblasts. Haematologica 2013, 98, 1778–1787. [Google Scholar] [CrossRef]
- Suragani, R.N.V.S.; Cadena, S.M.; Cawley, S.M.; Sako, D.; Mitchell, D.; Li, R.; Davies, M.V.; Alexander, M.J.; Devine, M.; Loveday, K.S.; et al. Transforming growth factor-β superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis. Nat. Med. 2014, 20, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Platzbecker, U.; Mufti, G.J.; Garcia-Manero, G.; Buckstein, R.; Santini, V.; Díez-Campelo, M.; Finelli, C.; Cazzola, M.; Ilhan, O.; et al. Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 382, 140–151. [Google Scholar] [CrossRef]
- Kubasch, A.S.; Fenaux, P.; Platzbecker, U. Development of luspatercept to treat ineffective erythropoiesis. Blood Adv. 2021, 5, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; Platzbecker, U.; Tarantolo, S.R.; Gropper, S.; Talati, C.; Götze, K.S.; Dugan, J.; Winer, E.S.; Martinez, D.E.; Lieberman, C.; et al. A Phase 1, Open Label Dose Escalation Trial Evaluating the Safety, Pharmacokinetics, Pharmacodynamics, and Clinical Activity of Orally Administered CA-4948 in Patients with Acute Myelogenous Leukemia or Myelodysplastic Syndrome. Blood 2020, 136, 16. [Google Scholar] [CrossRef]
- Jonas, B.A.; Curtin, P.T.; Schiller, G.J.; Jeyakumar, D.; Wieduwilt, M.J.; Abedi, M.; Bejar, R.; Chow, H.; Oesterich, L.G.; Logan, A.C. A Phase 1 Trial of Ibrutinib (IBR) and Azacitidine (AZA) for the Treatment of Higher-Risk Myelodysplastic Syndromes (HR-MDS): Updated Results of University of California Hematologic Malignancies Consortium (UCHMC) Study 1503. Blood 2018, 132, 3088. [Google Scholar] [CrossRef]
- Attar, E.C.; Amrein, P.C.; Fraser, J.W.; Fathi, A.T.; McAfee, S.; Wadleigh, M.; DeAngelo, D.J.; Steensma, D.; Stone, R.M.; Foster, J.; et al. Phase I dose escalation study of bortezomib in combination with lenalidomide in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). Leuk. Res. 2013, 37, 1016–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huselton, E.; Cashen, A.F.; DiPersio, J.F.; Jacoby, M.; Pusic, I.; Romee, R.; Schroeder, M.A.; Uy, G.L.; Westervelt, P. Updated Study Results of CX-01, an Inhibitor of CXCL12/CXCR4, and Azacitidine for the Treatment of Hypomethylating Agent Refractory AML and MDS. Blood 2019, 134, 3915. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Jabbour, E.J.; Konopleva, M.Y.; Daver, N.G.; Borthakur, G.; Dinardo, C.D.; Bose, P.; Patel, P.; Komrokji, R.S.; Shastri, A.; et al. A Clinical Study of Tomaralimab (OPN-305), a Toll-like Receptor 2 (TLR-2) Antibody, in Heavily Pre-Treated Transfusion Dependent Patients with Lower Risk Myelodysplastic Syndromes (MDS) That Have Received and Failed on Prior Hypomethylating Agent (HMA) Therapy. Blood 2018, 132, 798. [Google Scholar] [CrossRef]
- Linder, K.; Lulla, P. Myelodysplastic syndrome and immunotherapy novel to next in-line treatments. Hum. Vaccines Immunother. 2021, 17, 2602–2616. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Knaus, H.A.; Robinson, T.M.; Towlerton, A.M.; Warren, E.; Zeidner, J.; Blackford, A.L.; Duffield, A.S.; Rizzieri, D.; Frattini, M.G.; et al. A Multi-center Phase I Trial of Ipilimumab in Patients with Myelodysplastic Syndromes following Hypomethylating Agent Failure. Clin. Cancer Res. 2018, 24, 3519–3527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, K.; Kantarjian, H.M.; Bravo, G.M.; Sasaki, K.; Daver, N.; Jabbour, E.; Alvarado, Y.; Chien, K.S.; Dinardo, C.D.; Ravandi, F.; et al. A Phase II Study of Double Immune Checkpoint Inhibitor Blockade with Nivolumab and Ipilimumab with or without Azacitidine in Patients with Myelodysplastic Syndrome (MDS). Blood 2020, 136, 7–9. [Google Scholar] [CrossRef]
- Boddu, P.; Kantarjian, H.; Garcia-Manero, G.; Allison, J.; Sharma, P.; Daver, N. The emerging role of immune checkpoint based approaches in AML and MDS. Leuk. Lymphoma 2017, 59, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Assi, R.; Daver, N.; Benton, C.B.; Kadia, T.; A Thompson, P.; Borthakur, G.; Alvarado, Y.; Jabbour, E.J.; Konopleva, M.; et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: A single-arm, phase 2 study. Lancet Haematol. 2019, 6, e480–e488. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Sasaki, K.; Montalban-Bravo, G.; Daver, N.G.; Jabbour, E.J.; Alvarado, Y.; Dinardo, C.D.; Ravandi, F.; Borthakur, G.; Bose, P.; et al. A Phase II Study of Nivolumab or Ipilimumab with or without Azacitidine for Patients with Myelodysplastic Syndrome (MDS). Blood 2018, 132, 465. [Google Scholar] [CrossRef]
- Chien, K.S.; Kim, K.; Nogueras-Gonzalez, G.M.; Borthakur, G.; Naqvi, K.; Daver, N.G.; Montalban-Bravo, G.; Cortes, J.E.; DiNardo, C.D.; Jabbour, E.; et al. Phase II study of azacitidine with pembrolizumab in patients with intermediate-1 or higher-risk myelodysplastic syndrome. Br. J. Haematol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; Tallman, M.S.; Martinelli, G.; Ribrag, V.; Yang, H.; Balakumaran, P.S.A.; Chlosta, S.; Zhang, Y.; Smith, B.D. Pembrolizumab, a PD-1 Inhibitor, in Patients with Myelodysplastic Syndrome (MDS) after Failure of Hypomethylating Agent Treatment. Blood 2016, 128, 345. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Cavenagh, J.; Voso, M.T.; Taussig, D.; Tormo, M.; Boss, I.; Copeland, W.B.; Gray, V.E.; Previtali, A.; O’Connor, T.; et al. Efficacy and Safety of Azacitidine (AZA) in Combination with the Anti-PD-L1 Durvalumab (durva) for the Front-Line Treatment of Older Patients (pts) with Acute Myeloid Leukemia (AML) Who Are Unfit for Intensive Chemotherapy (IC) and Pts with Higher-Risk Myelodysplastic Syndromes (HR-MDS): Results from a Large, International, Randomized Phase 2 Study. Blood 2019, 134, 829. [Google Scholar] [CrossRef]
- Gerds, M.A.T.; Scott, B.L.; Greenberg, P.L.; Khaled, S.K.; Lin, T.L.; A Pollyea, D.; Verma, A.; Dail, M.; Green, C.; Ma, C.; et al. PD-L1 Blockade with Atezolizumab in Higher-Risk Myelodysplastic Syndrome: An Initial Safety and Efficacy Analysis. Blood 2018, 132, 466. [Google Scholar] [CrossRef]
- O’Connell, C.L.; Kropf, P.L.; Punwani, N.; Rogers, D.; Sposto, R.; Grønbæk, K. Phase I Results of a Multicenter Clinical Trial Combining Guadecitabine, a DNA Methyltransferase Inhibitor, with Atezolizumab, an Immune Checkpoint Inhibitor, in Patients with Relapsed or Refractory Myelodysplastic Syndrome or Chronic Myelomonocytic Leukemia. Blood 2018, 132, 1811. [Google Scholar] [CrossRef]
- Brunner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Vey, N.; Scholl, S.; Garcia-Manero, G.; Wermke, M.; Janssen, J.; Traer, E.; et al. Efficacy and Safety of Sabatolimab (MBG453) in Combination with Hypomethylating Agents (HMAs) in Patients with Acute Myeloid Leukemia (AML) and High-Risk Myelodysplastic Syndrome (HR-MDS): Updated Results from a Phase 1b Study. Blood 2020, 136, 1–2. [Google Scholar] [CrossRef]
- Zeidan, M.A.M.; Esteve, J.; Giagounidis, A.; Kim, H.-J.; Miyazaki, Y.; Platzbecker, U.; Schuh, A.C.; Sekeres, M.A.; Westermann, J.; Xiao, Z.; et al. The STIMULUS Program: Clinical Trials Evaluating Sabatolimab (MBG453) Combination Therapy in Patients (Pts) with Higher-Risk Myelodysplastic Syndromes (HR-MDS) or Acute Myeloid Leukemia (AML). Blood 2020, 136, 45–46. [Google Scholar] [CrossRef]
- Riether, C.; Pabst, T.; Höpner, S.; Bacher, U.; Hinterbrandner, M.; Banz, Y.; Müller, R.; Manz, M.G.; Gharib, W.H.; Francisco, D.; et al. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat. Med. 2020, 26, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Sick, E.; Jeanne, A.; Schneider, C.; Dedieu, S.; Takeda, K.; Martiny, L. CD47 update: A multifaceted actor in the tumour microenvironment of potential therapeutic interest. Br. J. Pharmacol. 2012, 167, 1415–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, M.P.; Takimoto, C.H.; Feng, D.D.; McKenna, K.; Gip, P.; Liu, J.; Volkmer, J.-P.; Weissman, I.L.; Majeti, R. Therapeutic Targeting of the Macrophage Immune Checkpoint CD47 in Myeloid Malignancies. Front. Oncol. 2020, 9, 1380. [Google Scholar] [CrossRef] [PubMed]
- Matlung, H.L.; Szilagyi, K.; Barclay, N.A.; Berg, T.K.V.D. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017, 276, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.; Van Rooijen, N.; Weissman, I.L. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeidan, M.A.M.; DeAngelo, D.J.; Palmer, J.M.; Seet, C.S.; Tallman, M.S.; Wei, X.; Li, Y.F.; Hock, R.N.; Burgess, M.R.; Hege, K.; et al. A Phase I Study of CC-90002, a Monoclonal Antibody Targeting CD47, in Patients with Relapsed and/or Refractory (R/R) Acute Myeloid Leukemia (AML) and High-Risk Myelodysplastic Syndromes (MDS): Final Results. Blood 2019, 134, 1320. [Google Scholar] [CrossRef]
- A Sallman, D.; Asch, A.S.; Al Malki, M.M.; Lee, D.J.; Donnellan, W.B.; Marcucci, G.; Kambhampati, S.; Daver, N.G.; Garcia-Manero, G.; Komrokji, R.S.; et al. The First-in-Class Anti-CD47 Antibody Magrolimab (5F9) in Combination with Azacitidine Is Effective in MDS and AML Patients: Ongoing Phase 1b Results. Blood 2019, 134, 569. [Google Scholar] [CrossRef]
- Aggarwal, N.; Swerdlow, S.H.; TenEyck, S.P.; Boyiadzis, M.; Felgar, R.E. Natural killer cell (NK) subsets and NK-like T-cell populations in acute myeloid leukemias and myelodysplastic syndromes. Cytom. Part B Clin. Cytom. 2016, 90, 349–357. [Google Scholar] [CrossRef]
- Cianga, V.A.; Catafal, L.C.; Cianga, P.; Tanasa, M.P.; Cherry, M.; Collet, P.; Tavernier, E.; Guyotat, D.; Rusu, C.; Aanei, C.M. Natural Killer Cell Subpopulations and Inhibitory Receptor Dynamics in Myelodysplastic Syndromes and Acute Myeloid Leukemia. Front. Immunol. 2021, 12, 665541. [Google Scholar] [CrossRef]
- Sarhan, D.; Brandt, L.; Felices, M.; Guldevall, K.; Lenvik, T.; Hinderlie, P.; Curtsinger, J.; Warlick, E.; Spellman, S.R.; Blazar, B.R.; et al. 161533 TriKE stimulates NK-cell function to overcome myeloid-derived suppressor cells in MDS. Blood Adv. 2018, 2, 1459–1469. [Google Scholar] [CrossRef] [Green Version]
- Warlick, E.D.; Weisdorf, D.J.; Vallera, D.A.; Wangen, R.; Lewis, R.D.; Knox, J.; Schroeder, M.; Felices, M.; Miller, J.S. GTB-3550 TriKE™ for the Treatment of High-Risk Myelodysplastic Syndromes (MDS) and Refractory/Relapsed Acute Myeloid Leukemia (AML) Safely Drives Natural Killer (NK) Cell Proliferation At Initial Dose Cohorts. Blood 2020, 136, 7–8. [Google Scholar] [CrossRef]
- Yalniz, F.; Daver, N.; Rezvani, K.; Kornblau, S.; Ohanian, M.; Borthakur, G.; Dinardo, C.D.; Konopleva, M.; Burger, J.; Gasior, Y.; et al. A Pilot Trial of Lirilumab With or Without Azacitidine for Patients With Myelodysplastic Syndrome. Clin. Lymphoma Myeloma Leuk. 2018, 18, 658–663.e2. [Google Scholar] [CrossRef]
- Miyakoshi, S.; Usuki, K.; Matsumura, I.; Ueda, Y.; Iwasaki, H.; Miyamoto, T.; Origuchi, M.; Tagashira, S.; Naoi, I.; Naoe, T.; et al. Preliminary Results from a Phase 1/2 Study of DSP-7888, a Novel WT1 Peptide-Based Vaccine, in Patients with Myelodysplastic Syndrome (MDS). Blood 2016, 128, 4335. [Google Scholar] [CrossRef]
- Brayer, J.; Lancet, J.E.; Powers, J.; List, A.; Balducci, L.; Komrokji, R.; Pinilla-Ibarz, J. WT1 vaccination in AML and MDS: A pilot trial with synthetic analog peptides. Am. J. Hematol. 2015, 90, 602–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qazilbash, M.H.; Wieder, E.; Thall, P.F.; Wang, X.; Rios, R.; Lu, S.; Kanodia, S.; Ruisaard, K.E.; Giralt, S.A.; Estey, E.H.; et al. PR1 peptide vaccine induces specific immunity with clinical responses in myeloid malignancies. Leukemia 2016, 31, 697–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, L.; Maurer, U.; Weidmann, E. Wilms Tumor Gene Expression in Acute Myeloid Leukemias. Leuk. Lymphoma 1997, 25, 435–443. [Google Scholar] [CrossRef]
- Cilloni, D.; Gottardi, E.; Messa, F.; Fava, M.; Scaravaglio, P.; Bertini, M.; Girotto, M.; Marinone, C.; Ferrero, D.; Gallamini, A.; et al. Significant Correlation Between the Degree of WT1 Expression and the International Prognostic Scoring System Score in Patients With Myelodysplastic Syndromes. J. Clin. Oncol. 2003, 21, 1988–1995. [Google Scholar] [CrossRef]
- Rautenberg, C.; Germing, U.; Pechtel, S.; Lamers, M.; Fischermanns, C.; Jäger, P.; Geyh, S.; Haas, R.; Kobbe, G.; Schroeder, T. Prognostic impact of peripheral blood WT1-mRNA expression in patients with MDS. Blood Cancer J. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jo, T.; Sakai, K.; Muranushi, H.; Okamoto, Y.; Tsukamoto, T.; Sugiura, H.; Matsui, H.; Ueda, T.; Okada, K.; Maeda, T.; et al. Pre-Treatment WT1 mRNA Expression Level In Peripheral Blood Predicts Response and Overall Survival Of Myelodysplastic Syndrome Patients In The Azacitidine Era. Blood 2013, 122, 1528. [Google Scholar] [CrossRef]
- Rezvani, K.; Yong, A.S.; Mielke, S.; Jafarpour, B.; Savani, B.N.; Le, R.Q.; Eniafe, R.; Musse, L.; Boss, C.; Kurlander, R.; et al. Repeated PR1 and WT1 peptide vaccination in Montanide-adjuvant fails to induce sustained high-avidity, epitope-specific CD8+ T cells in myeloid malignancies. Haematologica 2010, 96, 432–440. [Google Scholar] [CrossRef]
- Rezvani, K.; Yong, A.S.M.; Mielke, S.; Savani, B.N.; Musse, L.; Superata, J.; Jafarpour, B.; Boss, C.; Barrett, A.J. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood 2008, 111, 236–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Ueda, Y.; Ogura, M.; Uchida, T.; Ozawa, K.; Miyakoshi, M.S.; Naoe, T.; Murata, M.; Kizaki, M.; Uike, N.; et al. A Phase 1/2 Study of WT1 Peptide Cancer Vaccine WT4869 in Patients with Myelodysplastic Syndromes (MDS). Blood 2015, 126, 2868. [Google Scholar] [CrossRef]
- Keilholz, U.; Letsch, A.; Busse, A.; Asemissen, A.M.; Bauer, S.; Blau, I.W.; Hofmann, W.-K.; Uharek, L.; Thiel, E.; Scheibenbogen, C. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood 2009, 113, 6541–6548. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Al-Khadairi, G.; Roelands, J.; Hendrickx, W.; Dermime, S.; Bedognetti, D.; Decock, J. NY-ESO-1 Based Immunotherapy of Cancer: Current Perspectives. Front. Immunol. 2018, 9, 947. [Google Scholar] [CrossRef]
- Atanackovic, D.; Luetkens, T.; Kloth, B.; Fuchs, G.; Cao, Y.; Hildebrandt, Y.; Meyer, S.; Bartels, K.; Reinhard, H.; Lajmi, N.; et al. Cancer-testis antigen expression and its epigenetic modulation in acute myeloid leukemia. Am. J. Hematol. 2011, 86, 918–922. [Google Scholar] [CrossRef]
- Srivastava, P.; Matsuzaki, J.; Paluch, B.E.; Kaufman, S.; Karpf, A.R.; Odunsi, K.; Miller, A.; Kocent, J.; Wang, E.S.; Nemeth, M.J.; et al. Vaccination with NY-ESO-1 in Combination with Decitabine for Patients with MDS. Blood 2016, 128, 4326. [Google Scholar] [CrossRef]
- Griffiths, E.A.; Srivastava, P.; Matsuzaki, J.; Brumberger, Z.; Wang, E.S.; Kocent, J.; Miller, A.; Roloff, G.; Wong, H.Y.; Paluch, B.E.; et al. NY-ESO-1 Vaccination in Combination with Decitabine Induces Antigen-Specific T-lymphocyte Responses in Patients with Myelodysplastic Syndrome. Clin. Cancer Res. 2017, 24, 1019–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmberg-Thydén, S.; Dufva, M.I.H.; Gang, M.A.O.; Breinholt, M.F.; Schejbel, L.; Andersen, D.M.K.; Kadivar, M.; Svane, I.M.; Grønbæk, K.; Hadrup, S.R.; et al. Therapeutic Cancer Vaccination Targeting Shared Tumor Associated Antigens in Combination with Azacitidine for High Risk Myelodysplastic Syndrome—A Phase I Clinical Trial. Blood 2020, 136, 23–24. [Google Scholar] [CrossRef]
- Anguille, S.; Van de Velde, A.L.; Smits, E.L.; Van Tendeloo, V.F.; Juliusson, G.; Cools, N.; Nijs, G.; Stein, B.; Lion, E.; Van Driessche, A.; et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 2017, 130, 1713–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, J.; Padron, E.; Dubovsky, J.; Berchmans, E.; Farnum, T.; Bai, F.; Sahakian, E.; Ahmed, J.; Komrokji, R.S.; Lancet, J.E.; et al. Enhanced Immunological Responses Following K562/GM-CSF/CD40L Vaccine Plus Lenalidomide in High-Risk Myelodysplastic Syndrome. Blood 2011, 118, 1725. [Google Scholar] [CrossRef]
- Spear, P.; Wu, M.-R.; Sentman, M.-L.; Sentman, C.L. NKG2D ligands as therapeutic targets. Cancer Immun. 2013, 13, 8. [Google Scholar] [PubMed]
- Le Bert, N.; Gasser, S. Advances in NKG2D ligand recognition and responses by NK cells. Immunol. Cell Biol. 2014, 92, 230–236. [Google Scholar] [CrossRef]
- Sallman, D.A.; Al-Homsi, A.S.; Davila, M.L.; Kerre, T.; Moors, I.; Poire, X.; Havelange, V.; Lewalle, P.; Pollyea, D.A.; Wang, E.S.; et al. Results from the Phase I Clinical Studies Evaluating Cyad-01, a First-Generation NKG2D CAR T-Cell Product in Relapsed or Refractory Acute Myeloid Leukemia and Myelodysplastic Syndrome Patients. Blood 2020, 136, 40–41. [Google Scholar] [CrossRef]
- Bachier, C.; Borthakur, G.; Hosing, C.; Blum, W.; Rotta, M.; Ojeras, P.; Barnett, B.; Rajangam, K.; Majhail, M.N.S.; Nikiforow, S. A Phase 1 Study of NKX101, an Allogeneic CAR Natural Killer (NK) Cell Therapy, in Subjects with Relapsed/Refractory (R/R) Acute Myeloid Leukemia (AML) or Higher-Risk Myelodysplastic Syndrome (MDS). Blood 2020, 136, 42–43. [Google Scholar] [CrossRef]
- Sallman, D.A.; Elmariah, M.H.; Sweet, M.K.L.; Talati, C.; Mishra, A.; Kelley, L.L.; Lankford, A.; Chan, T.; Shah, R.R.; Padron, E.; et al. A Phase 1/1b Safety Study of Prgn-3006 Ultracar-T™ in Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia and Higher Risk Myelodysplastic Syndrome. Blood 2020, 136, 17. [Google Scholar] [CrossRef]
- Stevens, B.M.; Zhang, W.; Pollyea, D.A.; Winters, A.; Gutman, J.; Smith, C.; Budde, E.; Forman, S.J.; Jordan, C.T.; Purev, E. CD123 CAR T cells for the treatment of myelodysplastic syndrome. Exp. Hematol. 2019, 74, 52–63.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claiborne, J.; Bandyopathyay, D.; Roberts, C.; Hawks, K.; Aziz, M.; Simmons, G.; Wiedl, C.; Chung, H.; Clark, W.; Mccarty, J.; et al. Managing post allograft relapse of myeloid neoplasms: Azacitidine and donor lymphocyte infusions as salvage therapy. Leuk. Lymphoma 2019, 60, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, P.; Potter, V.T.; Barber, L.D.; Kulasekararaj, A.G.; Lim, Z.Y.; Pearce, R.M.; De Lavallade, H.; Kenyon, M.; Ireland, R.M.; Marsh, J.C.; et al. Outcome of Donor Lymphocyte Infusion after T Cell–depleted Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndromes. Biol. Blood Marrow Transplant. 2013, 19, 562–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comont, T.; Treiner, E.; Vergez, F. From Immune Dysregulations to Therapeutic Perspectives in Myelodysplastic Syndromes: A Review. Diagnostics 2021, 11, 1982. https://doi.org/10.3390/diagnostics11111982
Comont T, Treiner E, Vergez F. From Immune Dysregulations to Therapeutic Perspectives in Myelodysplastic Syndromes: A Review. Diagnostics. 2021; 11(11):1982. https://doi.org/10.3390/diagnostics11111982
Chicago/Turabian StyleComont, Thibault, Emmanuel Treiner, and François Vergez. 2021. "From Immune Dysregulations to Therapeutic Perspectives in Myelodysplastic Syndromes: A Review" Diagnostics 11, no. 11: 1982. https://doi.org/10.3390/diagnostics11111982
APA StyleComont, T., Treiner, E., & Vergez, F. (2021). From Immune Dysregulations to Therapeutic Perspectives in Myelodysplastic Syndromes: A Review. Diagnostics, 11(11), 1982. https://doi.org/10.3390/diagnostics11111982






