Cerebrospinal Fluid Levels of AFP and hCG: Validation of the Analytical Method and Application in the Diagnosis of Central Nervous System Germ Cell Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental
2.1.1. Chemicals and Instrumentation
2.1.2. CSF Samples
2.1.3. Study Population
2.2. Method Validation
2.2.1. Linearity
2.2.2. Analytical Performance
2.2.3. Statistical Analysis
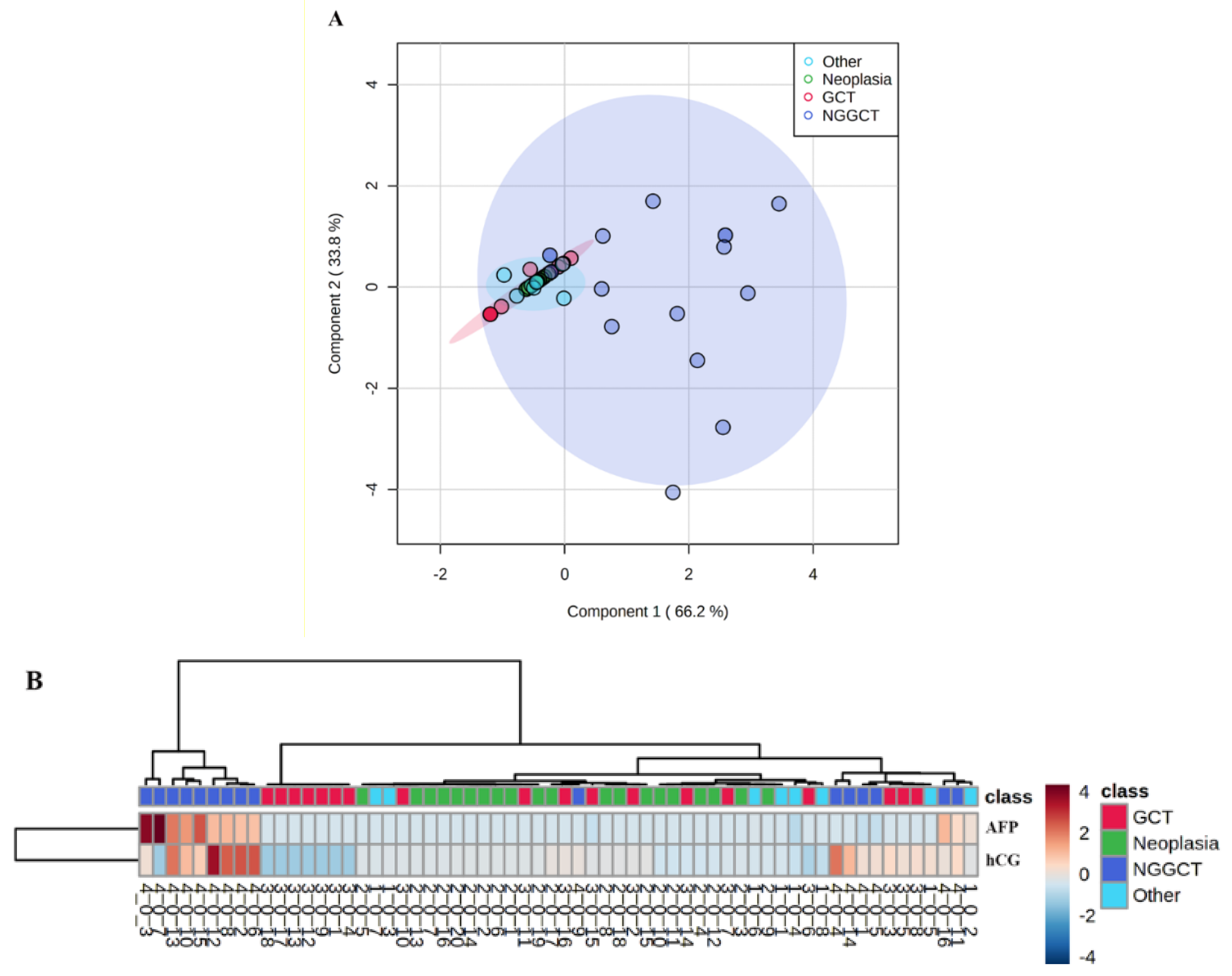
3. Results
3.1. Method Validation
3.2. Clinical Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stenman, U.H.; Alfthan, H.; Hotakainen, K. Human chorionic gonadotropin in cancer. Clin. Biochem. 2004, 37, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Valmu, L.; Alfthan, H.; Hotakainen, K.; Birken, S.; Stenman, U.H. Site-specific glycan analysis of human chorionic gonadotropin beta-subunit from malignancies and pregnancy by liquid chromatography--electrospray mass spectrometry. Glycobiology 2006, 16, 1207–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakkar, J.P.; Chew, L.; Villano, J.L. Primary CNS germ cell tumors: Current epidemiology and update on treatment. Med. Oncol. 2013, 30, 496. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [PubMed] [Green Version]
- Haase, J.; Borgaard-Pedersen, B. alpha-feto-protein (AFP) and human chorionic gonadotropin (HCG) as biochemical markers of intracranial germ-cell tumours. Acta Neurochir. 1979, 50, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.J.; Cohen, B.H.; Cooney, K. Intracranial germ cell tumors. Oncologist 2000, 5, 312–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnusson, B.; Örnemark, U. The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; 2014. Available online: http://www.eurachem.org (accessed on 3 September 2021).
- Danzer, K.; Currie, L.A. Guidelines for calibration in analytical chemistry. Part I. Fundamentals and single component calibration. Pure Appl. Chem. 1998, 70, 993–1014. [Google Scholar] [CrossRef]
- Davidian, M.; Haaland, P.D. Regression and calibration with non-constant error variance. Chemometr. Intell. Lab Syst. 1990, 9, 231–248. [Google Scholar] [CrossRef]
- Almeida, A.M.; Castel-Branco, M.M.; Falcão, A.C. Linear regression for calibration lines revisited: Weighting schemes for bioanalytical methods. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 774, 215–222. [Google Scholar] [CrossRef]
- Funk, W.; Dammann, V.; Donnovert, G. Quality Assurance in Analytical Chemistry: Application in Environmental, Food, and Material Analysis. In Biotechnology and Medical Engineering, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using Metabo-Analyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Mitsios, J.V.; McClellan, A.; Brown, S.; Gronowski, A.M. Human chorionic gonadotropin and α-fetoprotein in cerebral spinal fluid: Method validation and retrospective review. Clin. Biochem. 2014, 47, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Echevarría, M.E.; Fangusaro, J.; Goldman, S. Pediatric central nervous system germ cell tumors: A review. Oncologist 2008, 13, 690–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fetcko, K.; Dey, M. Primary Central Nervous System Germ Cell Tumors: A Review and Update. Med. Res. Arch. 2018, 6, 1719. [Google Scholar] [PubMed]
- Gittleman, H.; Cioffi, G.; Vecchione-Koval, T.; Ostrom, Q.T.; Kruchko, C.; Osorio, D.S.; Finlay, J.L.; Barnholtz-Sloan, J.S. Descriptive epidemiology of germ cell tumors of the central nervous system diagnosed in the United States from 2006 to 2015. J. Neurooncol. 2019, 143, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Susan, M.; Blaney, M.D.; Lee, J.; Helman, M.D.; Peter, C.; Adamson, M.D. Pizzo & Poplack’s Pediatric Oncology, 8th ed.; Kluver, W., Ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2020. [Google Scholar]
- Liang, L.; Korogi, Y.; Sugahara, T.; Ikushima, I.; Shigematsu, Y.; Okuda, T.; Takahashi, M.; Kochi, M.; Ushio, Y. MRI of intracranial germ-cell tumours. Neuroradiology 2002, 44, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Guerrini-Rousseau, L.; Grill, J. Central nervous system germ cell tumors: An update. Curr. Opin. Oncol. 2014, 26, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Bartels, U.; Nishikawa, R.; Fangusaro, J.; Matsutani, M.; Nicholson, J.C. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 2015, 16, e470–e477. [Google Scholar] [CrossRef]
- Fizazi, K.; Pagliaro, L.; Laplanche, A.; Fléchon, A.; Mardiak, J.; Geoffrois, L.; Kerbrat, P.; Chevreau, C.; Delva, R.; Rolland, F.; et al. Personalised chemotherapy based on tumour marker decline in poor-prognosis germ-cell tumours (GETUG 13): A phase III, multicentre, randomised trial. Lancet Oncol. 2014, 15, 1442–1450. [Google Scholar] [CrossRef] [Green Version]


| Calibration Function | Precision | Trueness | Recovery Function | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Linear Range a | Bartlett Test, p b | b0 (CI) c | b1 (CI) c | r2 | Mandel Test, p b | LOD a | LOQ a | Spike Level a | Measured Concentration, Mean a (SD d, RSD% e, n = 6) | R% f (SD d, n = 6) | b0 (CI) c | b1 (CI) c |
| AFP | 4.35–286 | <0.001 | 2110 (197) | 399 (20) | 0.993 | 0.23 | 1 | 2 | 15 | 14.2 (0.5, 3.8) | 95 (4) | 0.2 (1.0) | 0.91 (0.03) |
| 45 | 41 (2, 4) | 91 (4) | |||||||||||
| 125 | 117 (2, 3) | 88 (6) | |||||||||||
| hCG | 4.59–695 | <0.001 | 5471 (366) | 846 (27) | 0.994 | 0.27 | 3 | 4 | 35 | 34.5 (0.8, 2.4) | 99 (2) | −2 (4) | 0.971 (0.007) |
| 125 | 112 (1, 1) | 90 (1) | |||||||||||
| 600 | 570 (15, 3) | 95 (7) | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D'Alessandro, A.; Ciavardelli, D.; Pastore, A.; Giannone, G.; Del Baldo, G.; Carai, A.; Mastronuzzi, A.; Onetti Muda, A.; Porzio, O. Cerebrospinal Fluid Levels of AFP and hCG: Validation of the Analytical Method and Application in the Diagnosis of Central Nervous System Germ Cell Tumors. Diagnostics 2021, 11, 1980. https://doi.org/10.3390/diagnostics11111980
D'Alessandro A, Ciavardelli D, Pastore A, Giannone G, Del Baldo G, Carai A, Mastronuzzi A, Onetti Muda A, Porzio O. Cerebrospinal Fluid Levels of AFP and hCG: Validation of the Analytical Method and Application in the Diagnosis of Central Nervous System Germ Cell Tumors. Diagnostics. 2021; 11(11):1980. https://doi.org/10.3390/diagnostics11111980
Chicago/Turabian StyleD'Alessandro, Annamaria, Domenico Ciavardelli, Anna Pastore, Germana Giannone, Giada Del Baldo, Andrea Carai, Angela Mastronuzzi, Andrea Onetti Muda, and Ottavia Porzio. 2021. "Cerebrospinal Fluid Levels of AFP and hCG: Validation of the Analytical Method and Application in the Diagnosis of Central Nervous System Germ Cell Tumors" Diagnostics 11, no. 11: 1980. https://doi.org/10.3390/diagnostics11111980
APA StyleD'Alessandro, A., Ciavardelli, D., Pastore, A., Giannone, G., Del Baldo, G., Carai, A., Mastronuzzi, A., Onetti Muda, A., & Porzio, O. (2021). Cerebrospinal Fluid Levels of AFP and hCG: Validation of the Analytical Method and Application in the Diagnosis of Central Nervous System Germ Cell Tumors. Diagnostics, 11(11), 1980. https://doi.org/10.3390/diagnostics11111980








