Tracking SARS-CoV-2: Novel Trends and Diagnostic Strategies
Abstract
:1. Introduction
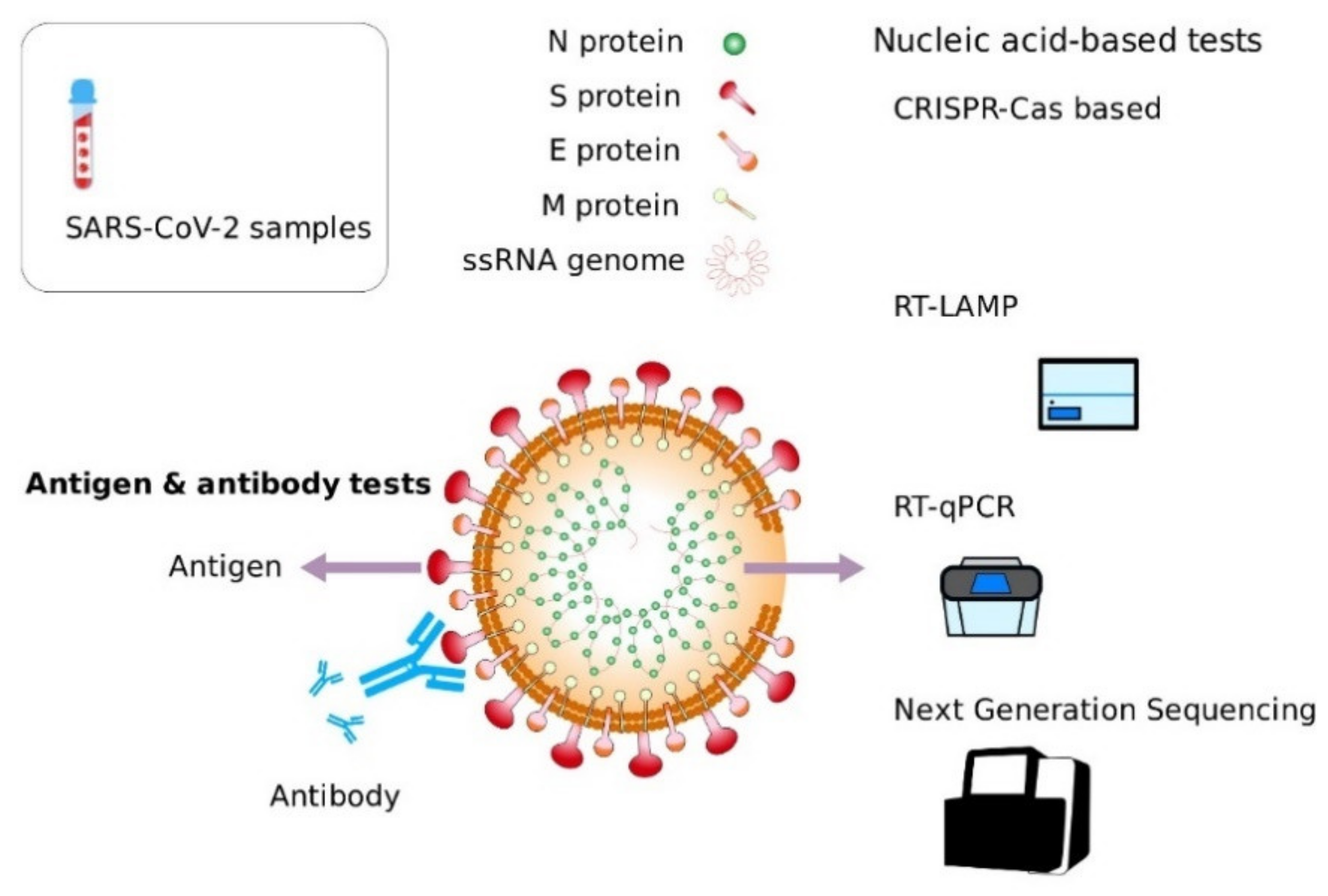
2. Diagnosis
3. Nucleic Acid Amplification-Based Tests (NAAT) for Detection of SARS-CoV-2
3.1. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
3.2. Assays Based on Nucleic Acid Isothermal Amplification
3.3. Nucleic Acid Sequence-Based Amplification (NASBA)
3.4. Loop-Mediated Isothermal Amplification (LAMP)
3.5. Rolling Circle Amplification (RCA)
3.6. Transcription-Mediated Amplification (TMA)
3.7. Recombinase Polymerase Amplification (RPA)
4. Next Generation Sequencing (NGS) for Detection of SARS-CoV-2
5. CRISPR-Based Tests
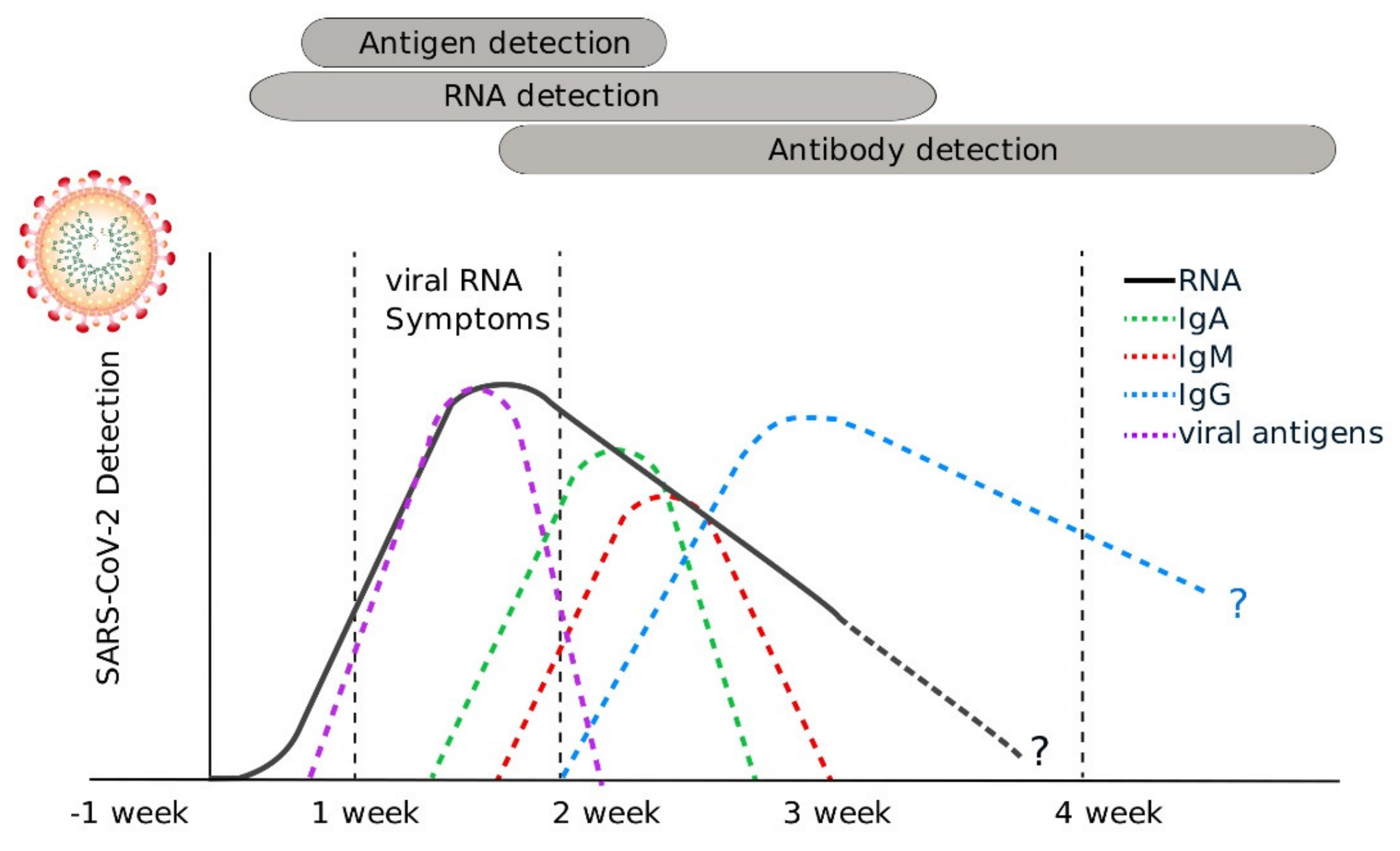
6. Serology-Based Tests
Antigen Testing
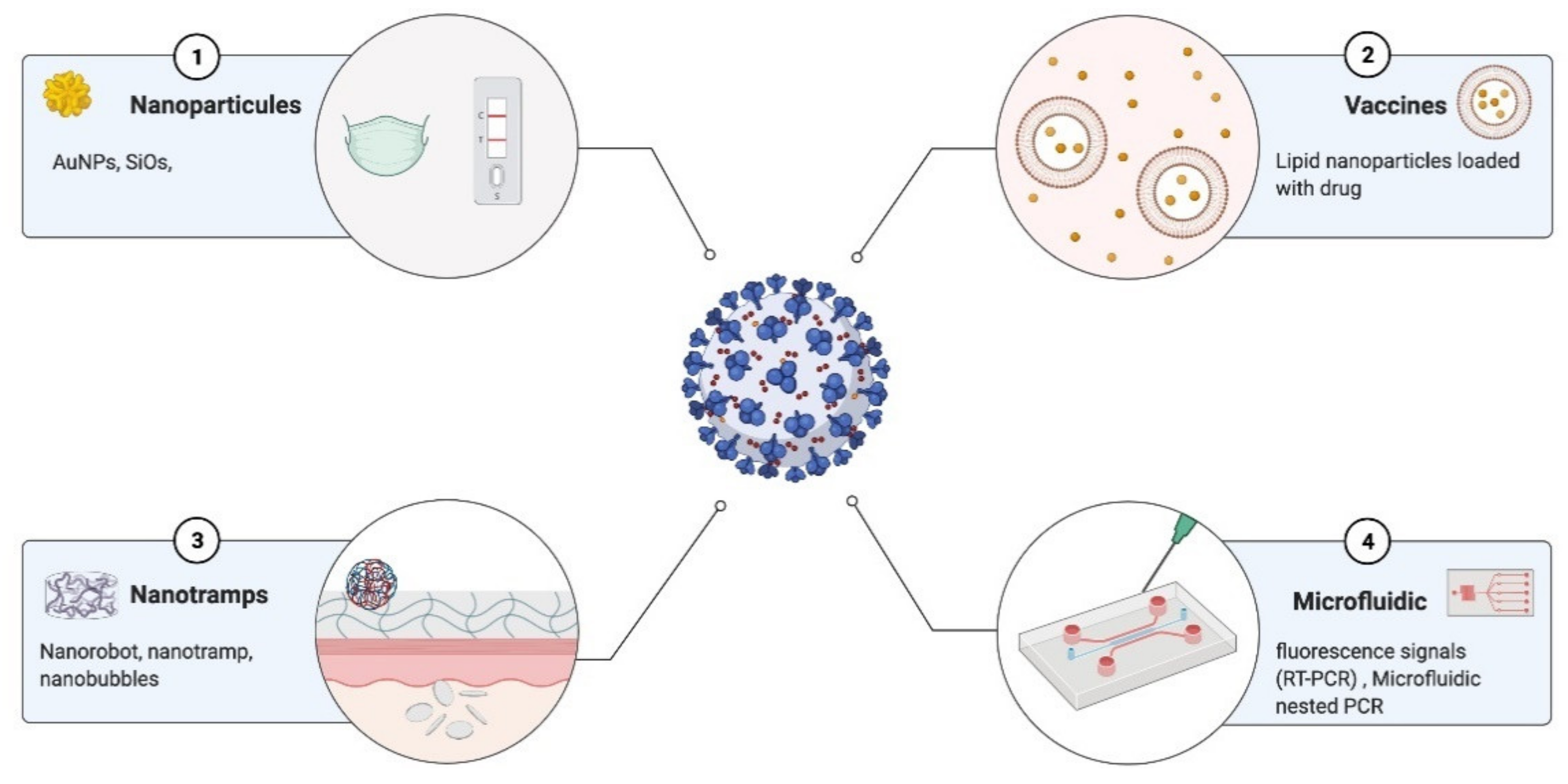
7. Nanotechnology for COVID-19 Diagnostics
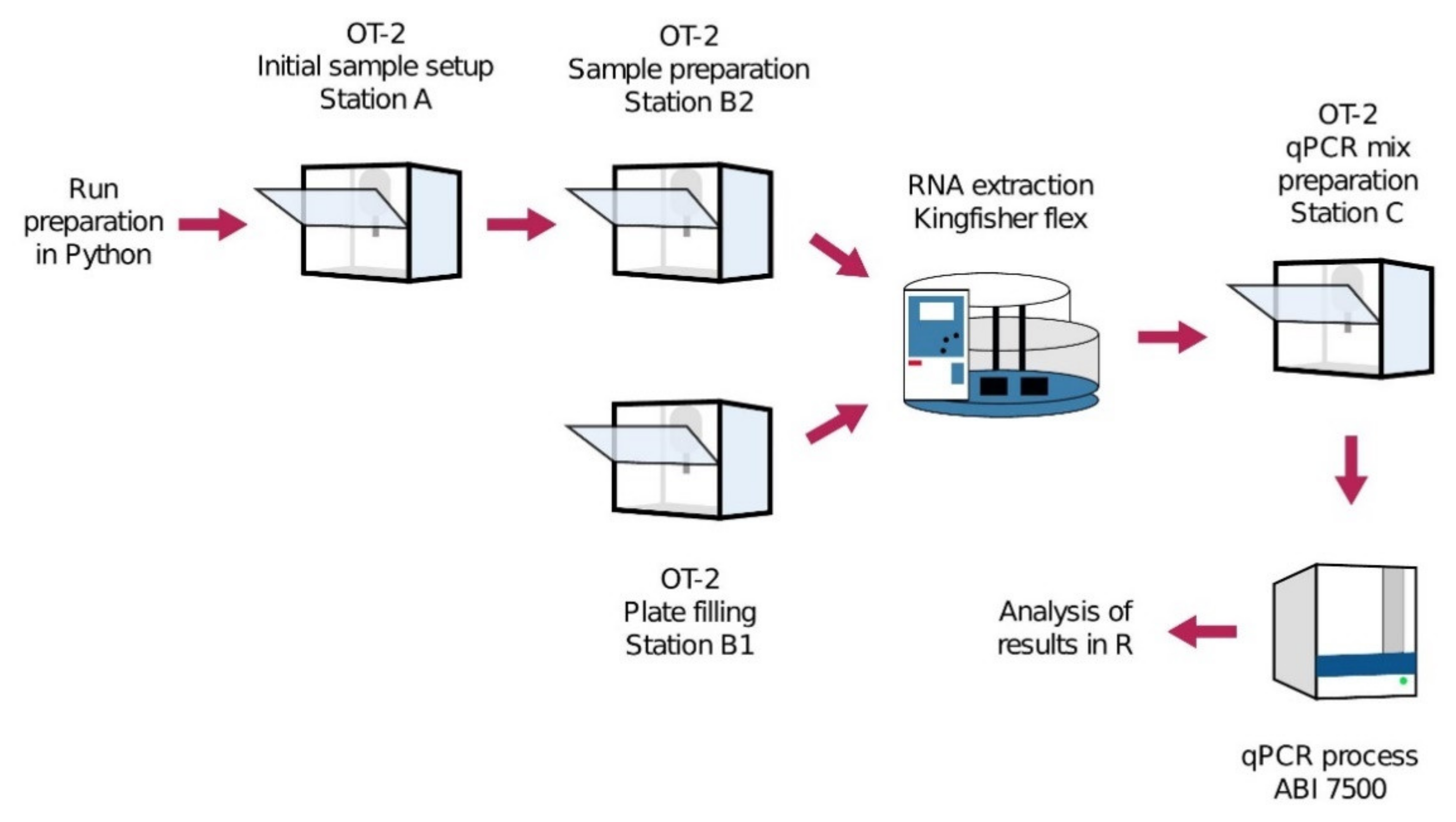
8. High-Throughput and Automated Screening for COVID-19
8.1. Automation Platforms for SARS-CoV-2 Testing
8.2. Biofoundries Role against COVID-19
8.3. Free and Open Source Scientific and Medical Hardware (FOSH)
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and Evolution of Pathogenic Coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, S.R.; Navas-Martin, S. Coronavirus Pathogenesis and the Emerging Pathogen Severe Acute Respiratory Syndrome Coronavirus. Microbiol. Mol. Biol. Rev. 2005, 69, 635–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanat, F.; Krammer, F. SARS-CoV-2 Vaccines: Status Report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef]
- World Health Organization. WHO Consensus Document on Theepidemiology of Severe Acuterespiratory Syndrome (SARS); Department of Communicable Disease Surveillance and Response: Geneva, Switzerland, 2003. [Google Scholar]
- CDC Fact Sheet: Basic Information about SARS. 2004. Available online: https://www.cdc.gov/sars/about/fs-SARS.pdf (accessed on 1 October 2020).
- De Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent Insights into Emerging Coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 7 November 2020).
- Baud, D.; Qi, X.; Nielsen-Saines, K.; Musso, D.; Pomar, L.; Favre, G. Real Estimates of Mortality Following COVID-19 Infection. Lancet Infect. Dis. 2020, 20, 773. [Google Scholar] [CrossRef] [Green Version]
- Wilson, N.; Kvalsvig, A.; Barnard, L.T.; Baker, M.G. Case-Fatality Risk Estimates for COVID-19 Calculated by Using a Lag Time for Fatality. Emerging Infect. Dis. 2020, 26, 1339–1441. [Google Scholar] [CrossRef]
- Rajgor, D.D.; Lee, M.H.; Archuleta, S.; Bagdasarian, N.; Quek, S.C. The Many Estimates of the COVID-19 Case Fatality Rate. Lancet Infect. Dis. 2020, 20, 776–777. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.D.; Goel, A. Estimating Case Fatality Rates of COVID-19. Lancet Infect. Dis. 2020, 20, 773–774. [Google Scholar] [CrossRef]
- Spychalski, P.; Błażyńska-Spychalska, A.; Kobiela, J. Estimating Case Fatality Rates of COVID-19. Lancet Infect. Dis. 2020, 20, 774–775. [Google Scholar] [CrossRef]
- Lipsitch, M. Estimating Case Fatality Rates of COVID-19. Lancet Infect. Dis. 2020, 20, 775. [Google Scholar] [CrossRef]
- Un Describing COVID-19 Pandemic as Wake-Up Call, Dress Rehearsal for Future Challenges, Secretary-General Opens Annual General Assembly Debate with Vision for Solidarity. Available online: https://www.un.org/press/en/2020/ga12268.doc.htm (accessed on 1 November 2020).
- Chan, J.F.-W.; Kok, K.-H.; Zhu, Z.; Chu, H.; To, K.K.-W.; Yuan, S.; Yuen, K.-Y. Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [Green Version]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus Biology and Replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2020, 19, 155–170. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Chen, Z.; Huang, X.; Xu, M.; He, T.; Zhang, Z. The Establishment of Reference Sequence for SARS-CoV-2 and Variation Analysis. J. Med. Virol. 2020, 92, 667–674. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The Proximal Origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, F.K. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2 or n-COV19), the Cause of COVID-19. Protein J. 2020, 39, 198–216. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Miyazawa, T. Genome Evolution of SARS-CoV-2 and Its Virological Characteristics. Inflamm. Regen. 2020, 40, 17. [Google Scholar] [CrossRef] [PubMed]
- Calligari, P.; Bobone, S.; Ricci, G.; Bocedi, A. Molecular Investigation of SARS-CoV-2 Proteins and Their Interactions with Antiviral Drugs. Viruses 2020, 12, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prajapat, M.; Sarma, P.; Shekhar, N.; Avti, P.; Sinha, S.; Kaur, H.; Kumar, S.; Bhattacharyya, A.; Kumar, H.; Bansal, S.; et al. Drug Targets for Corona Virus: A Systematic Review. Indian J. Pharmacol. 2020, 52, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-H.; Zhang, X.; Lu, Z.-H.; Zhu, Y.-S.; Wang, T. Potential Molecular Targets of Nonstructural Proteins for the Development of Antiviral Drugs against SARS-CoV-2 Infection. Biomed. Pharmacother. 2020, 133, 111035. [Google Scholar] [CrossRef] [PubMed]
- Wondmkun, Y.T.; Mohammed, O.A. A Review on Novel Drug Targets and Future Directions for COVID-19 Treatment. Biologics 2020, 14, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.X.; Fung, T.S.; Chong, K.K.-L.; Shukla, A.; Hilgenfeld, R. Accessory Proteins of SARS-CoV and Other Coronaviruses. Antiviral Res. 2014, 109, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Shu, T.; Wu, D.; Mu, J.; Wang, C.; Huang, M.; Han, Y.; Zhang, X.-Y.; Zhou, W.; Qiu, Y.; et al. The ORF3a Protein of SARS-CoV-2 Induces Apoptosis in Cells. Cell. Mol. Immunol. 2020, 17, 881–883. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Simbaña-Rivera, K.; Gomez-Barreno, L.; Rubio-Neira, M.; Guaman, L.P.; Kyriakidis, N.; Muslin, C.; Gomez-Jaramillo, A.M.; Barba, C.; Cevallos, D.; et al. Clinical, Molecular and Epidemiological Characterization of the SARS-CoV2 Virus and the Coronavirus Disease 2019 (COVID-19): A Comprehensive Literature Review. Diagn. Microbiol. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Costa de Oliveira, S. The Impact of Angiotensin-Converting Enzyme 2 (ACE2) Expression Levels in Patients with Comorbidities on COVID-19 Severity: A Comprehensive Review. Microorganisms 2021, 9, 1692. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of Angiotensin-Converting Enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Li, Z. Angiotensin-Converting Enzyme 2 (ACE2): SARS-CoV-2 Receptor and RAS Modulator. Acta Pharm. Sin. B 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Seidu, A.-A.; Hagan, J.E.; Ameyaw, E.K.; Ahinkorah, B.O.; Schack, T. The Role of Testing in the Fight against COVID-19: Current Happenings in Africa and the Way Forward. Int. J. Infect. Dis. 2020, 98, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, O.; Martiny, D.; Rochas, O.; van Belkum, A.; Kozlakidis, Z. Considerations for Diagnostic COVID-19 Tests. Nat. Rev. Microbiol. 2020, 19, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, N.; Jeremiah, S.S.; Ryo, A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020, 323, 2249–2251. [Google Scholar] [CrossRef]
- Nalla, A.K.; Casto, A.M.; Huang, M.-L.W.; Perchetti, G.A.; Sampoleo, R.; Shrestha, L.; Wei, Y.; Zhu, H.; Jerome, K.R.; Greninger, A.L. Comparative Performance of SARS-CoV-2 Detection Assays Using Seven Different Primer-Probe Sets and One Assay Kit. J. Clin. Microbiol. 2020, 58, e00557-20. [Google Scholar] [CrossRef] [Green Version]
- Xiang, F.; Wang, X.; He, X.; Peng, Z.; Yang, B.; Zhang, J.; Zhou, Q.; Ye, H.; Ma, Y.; Li, H.; et al. Antibody Detection and Dynamic Characteristics in Patients with COVID-19. Clin. Infect. Dis. 2020, 71, 1930–1934. [Google Scholar] [CrossRef]
- CDC Using Antibody Tests for COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests.html (accessed on 6 April 2021).
- FDA Antibody (Serology) Testing for COVID-19: Information for Patients and Consumers. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/antibody-serology-testing-covid-19-information-patients-and-consumers (accessed on 6 April 2021).
- 1drop, 1drop 1copy™ COVID-19 QPCR Multi Kit. Available online: https://www.fda.gov/media/137935/download (accessed on 20 August 2020).
- TRUPCR, TRUPCR® SARS-CoV-2 KIT. Available online: https://www.fda.gov/media/139296/download (accessed on 23 August 2020).
- 3D Med ANDiS SARS-CoV-2 and Influenza A/BRT-QPCR Detection Kit. Available online: http://www.3dmedcare.com/UploadImage/covid/04%20DA%20for%20ANDiS%20SARS-CoV-2%20and%20Influenza%20A&B%20RT-qPCR%20Detection%20Kit.pdf (accessed on 24 August 2020).
- Abbott. ABBOTT REALTIME SARS-COV-2 ASSAY. Available online: https://www.molecular.abbott/int/en/products/infectious-disease/RealTime-SARS-CoV-2-Assay#order (accessed on 23 August 2020).
- CareStart™ COVID-19 Antigen. Available online: https://carestartantigen.com (accessed on 1 April 2021).
- Acupath Laboratories Acupath COVID-19 RT-PCR Assay EUA Summary. Available online: https://www.fda.gov/media/139672/download (accessed on 24 August 2020).
- ABL ULTRAGENE COMBO2SCREEN SARS-COV-2 ASSAY. Available online: https://www.ablsa.com/laboratory-applications/ultragene-combo2screen (accessed on 24 August 2020).
- Altona Diagnostics RealStar®SARS-CoV-2 RT-PCR Kit 1.0. Available online: https://altona-diagnostics.com/files/public/Content%20Homepage/-%2002%20RealStar/MAN%20-%20CE%20-%20EN/RealStar%20SARS-CoV-2%20RT-PCR%20Kit%201.0_WEB_CE_EN-S03.pdf (accessed on 24 August 2020).
- Altru Diagnostics Thermo Fisher TaqMan 2019-NCoV Assay Kit v1 (Singleplex). Available online:https://www.fda.gov/media/137546/download (accessed on 23 August 2020).
- Anatolia geneworks Bosphore Novel Coronavirus (2019-NCoV) Detection Kit. Available online:http://www.anatoliageneworks.com/en/kitler.asp?id=360&baslik=Bosphore%20Novel%20Coronavirus%20(2019-nCoV)%20Detection%20Kit&bas=Bosphore%20Novel%20Coronavirus%20(2019-nCoV)%20Detection%20Kit (accessed on 24 August 2020).
- Applied BioCode BioCode® SARS-CoV-2 Assay. Available online: https://www.fda.gov/media/139049/download (accessed on 24 August 2020).
- LineaCOVID-19 LineaTM COVID-19Assay Kit. Available online: https://www.fda.gov/media/138059/download (accessed on 24 August 2020).
- Aspirus Laboratory ASPIRUS SARS-COV-2 RRT-PCR ASSAY. Available online: https://www.fda.gov/media/138526/download (accessed on 24 August 2020).
- Assurance Scientific Laboratories ASSURANCE SARS-COV-2 PANEL. Available online: https://www.fda.gov/media/138154/download (accessed on 24 August 2020).
- Accelarate Technologies A*STAR FORTITUDE KIT 2.0COVID-19 Real-Time RT-PCR Test. Available online: https://www.biovendor.com/file/13373/AW00032-02%20IFU%20ASTAR%20(MiRXES)Fortitude%202.0Watermarked.pdf (accessed on 23 August 2020).
- Avera Institute for Human Genetics AVERA INSTITUTE for HUMAN GENETICS SARS-CoV-2 RT-PCR. Available online: https://www.fda.gov/media/138332/download (accessed on 24 August 2020).
- Avellino Avellino SARS-CoV-2/COVID-19 Test. Available online: https://avellinocoronatest.com/product/ (accessed on 24 August 2020).
- Bag Diagnostics with Secured Results against COVID-19. Available online: https://www.bag-diagnostics.com/files/downloads/Produkt_Flyer/ViroQ_SARS-CoV-2_Broschuere.pdf (accessed on 23 August 2020).
- ThermoFisher Scientific Applied Biosystems™ TaqPath™ COVID-19 CE-IVD RT-PCR Kit. Available online: https://aslm.org/wp-content/uploads/2020/05/ThermoFisher_TaqPathCOVID19_CE-IVD_KIT.pdf (accessed on 24 August 2020).
- Roche Cobas® 8800 System. Available online: https://diagnostics.roche.com/gb/en/products/instruments/cobas-8800.html (accessed on 8 April 2021).
- Eurofins GSD NovaPrime® SARS-CoV-2 (COVID-19) RT-PCR (96 Reactions). Available online: https://www.eurofins-technologies.com/gsd-novaprimer-sars-cov-2-covid-19-real-time-pcr.html (accessed on 24 August 2020).
- QIAGEN QIAstat-Dx® Respiratory SARS-CoV2 Panel Instructions ForUse (Handbook). Available online: https://www.fda.gov/media/136571/download (accessed on 24 August 2020).
- R-Biopharm RIDA® GENE SARS-CoV-2. Available online: https://clinical.r-biopharm.com/products/ridagene-sars-cov-2 (accessed on 24 August 2020).
- SD Biosensor STANDARD M NCoV Real-Time Detection Kit. Available online: http://sdbiosensor.com/xe/product/7653 (accessed on 24 August 2020).
- TBG Biotechnology Corp ExProbe SARS-CoV-2 Testing Kit. Available online: https://www.fda.gov/media/138819/download (accessed on 24 August 2020).
- Zymo Research Quick SARs-Cov-2 RRT-PCR Kit. Available online: https://www.fda.gov/media/137780/download (accessed on 23 August 2020).
- Fulgent Therapeutics COVID-19 by RT-PCR TEST. Available online: https://www.fda.gov/media/138150/download (accessed on 24 August 2020).
- CENTOGENE SARS-CoV-2 Detection Based on E and RdRp Genes. Available online: https://www.fda.gov/media/139725/download (accessed on 24 August 2020).
- GeneXpert Xpert® Xpress SARS-CoV-2. Available online: https://www.fda.gov/media/136314/download (accessed on 24 August 2020).
- ChromaCode HDPCRTM SARS-CoV-2 Assay. Available online: https://www.fda.gov/media/138786/download (accessed on 24 August 2020).
- SpectronRx HymonTM SARS-CoV-2 Test KitInstructionsforUse (Handbook). Available online: https://www.fda.gov/media/138345/download (accessed on 23 August 2020).
- Diagnostic Solutions COVID-19 | SARS-CoV-2. Available online: https://www.fda.gov/media/139516/download (accessed on 23 August 2020).
- DiaSorin Molecular Enabling Swift Action Against TheCOVID-19 Pandemic. Available online: https://molecular.diasorin.com/international/wp-content/uploads/2020/03/OUSC19BR0720-C19-Direct-Brochure-A4-APPROVED-OUS-Only.pdf (accessed on 24 August 2020).
- Eli Lilly Clinical Diagnostics Laboratory LILLY SARS-CoV-2 ASSAY. Available online: https://www.fda.gov/media/140543/download (accessed on 24 August 2020).
- Enzo Life Sciences AMPIPROBE® SARS-CoV-2 Test System. Available online: https://www.fda.gov/media/139828/download (accessed on 24 August 2020).
- EuroRealTime EURORealTime SARS-CoV-2. Available online: https://www.fda.gov/media/138761/download (accessed on 24 August 2020).
- Fosun Pharma Fosun COVID-19 RT-PCR Detection Kit. Available online: https://www.fda.gov/media/137120/download (accessed on 24 August 2020).
- Genematrix NeoPlex COVID-19 Detection Kit (PID: 3218179). Available online: https://www.buykorea.org/bk/byr/product/GOODS_DETAIL-3218179.do (accessed on 24 August 2020).
- GB GB SARS-CoV-2 Real-Time RT-PCR. Available online: https://www.gbc.com.tw/products/real-time-pcr/gb-sars-cov-2-real-time-rt-pcr (accessed on 24 August 2020).
- Genetron Genetron SARS-CoV-2 RNA Test. Available online: https://www.fda.gov/media/138685/download (accessed on 24 August 2020).
- Helix Laboratory Helix COVID-19 Test. Available online: https://www.fda.gov/media/140420/download (accessed on 24 August 2020).
- Hologic SARS-CoV-2 Assay (Panther Fusion™ System). Available online: https://www.hologic.com/sites/default/files/2020-05/AW-21388-001_002_01.pdf (accessed on 24 August 2020).
- InBios Smart Detect™ SARS-CoV-2 RRT-PCR Kit. Available online: https://inbios.com/smart-detecttm-sars-cov-2-rrt-pcr-kit/ (accessed on 23 August 2020).
- IDT SARS-CoV-2 Probes and Other COVID-19 Research Reagents. Available online: https://www.idtdna.com/pages/landing/coronavirus-research-reagents#media (accessed on 24 August 2020).
- Integrity Laboratories SARS-CoV-2 RT-PCR Assay. Available online: https://www.fda.gov/media/136942/download#:~:text=The%20Integrity%20Laboratories%20SARS%2DCoV,detect%20cases%20of%20COVID%2D19. (accessed on 24 August 2020).
- JN Medsys ProTect™ COVID-19 RT-QPCR Kit. Available online: https://jnmedsys.com/covid19 (accessed on 24 August 2020).
- BioPerfectus technologies COVID-19 Coronavirus Real Time PCR Kit. Available online: https://www.fda.gov/media/139279/download (accessed on 24 August 2020).
- KorvaLabs Inc. Clinical Laboratory CURATIVE SARS-COV-2 ASSAY. Available online: https://www.fda.gov/media/137089/download (accessed on 24 August 2020).
- IDYLLA IDYLLA™ SARS-COV-2 TEST. 2020. Available online: https://www.biocartis.com/en-US/meet-idylla/idylla-infectious-disease-assays/idylla-sars-cov-2-test-eua-pending (accessed on 2 September 2020).
- BioFire BioFire® Respiratory Panel 2.1-EZ (RP2.1-EZ). Available online: https://www.fda.gov/media/142696/download (accessed on 21 December 2020).
- Medicon VitaPCRTM Platform. Available online: https://www.mediconire.com/vitapcrtm-platform (accessed on 21 December 2020).
- RCA Laboratory Services GENETWORx Covid-19 Nasal Swab Test. Available online: https://www.fda.gov/media/144553/download (accessed on 21 December 2020).
- Euroimmun EURORealTime SARS-CoV-2/InfluenzaA/B. Available online: https://www.coronavirus-diagnostics.com/documents/Indications/Infections/Coronavirus/MP_2606_D_UK_B.pdf (accessed on 21 December 2020).
- Agena Bioscience Multiplex RT-PCR/MALDI-TOF Test Intended for the Qualitativedetection of Nucleic Acid from SARS-CoV-2. Available online: https://agenabio.com/wp-content/uploads/2020/07/SC2_Panel_IFU-CUS-001_R03.pdf (accessed on 24 August 2020).
- Bio-Rad Qualitative Assay for Use on the QX200TM AndQXDx™ Droplet Digital™ PCR Systems. Available online: https://www.fda.gov/media/137579/download (accessed on 24 August 2020).
- Atila BioSystems IAMP® COVID-19 Detection Kit. Available online: https://www.fda.gov/media/136870/download (accessed on 24 August 2020).
- ID NOW ID NOW COVID-19. Available online: https://www.fda.gov/media/136525/download (accessed on 24 August 2020).
- Color SARS-CoV-2 LAMP Diagnostic Assay. Available online: https://www.color.com/wp-content/uploads/2020/05/LAMP-Diagnostic-Assay.pdf (accessed on 24 August 2020).
- BioSpace Rendu Gets NMPA’s Emergency Approval for Coronavirus Nucleic Acid Detection System. Available online: https://www.biospace.com/article/releases/rendu-gets-nmpa-s-emergency-approval-for-coronavirus-nucleic-acid-detection-system (accessed on 24 August 2020).
- Seasun Biomaterials AQ-TOP™ COVID-19 Rapid Detection Kit. Available online: https://www.fda.gov/media/138307/download (accessed on 24 August 2020).
- Lucira Lucira™ COVID-19All-In-One Test Kit. Available online: https://www.fda.gov/media/143808/download (accessed on 21 December 2020).
- Poplar Healthcare SARS-COV-2 TMA POOLING ASSAY. Available online: https://www.fda.gov/media/140792/download (accessed on 24 August 2020).
- Illumina Illumina COVIDSeq Test. Available online: https://www.illumina.com/products/by-type/ivd-products/covidseq.html (accessed on 24 August 2020).
- Fulgent COVID-19 Testing Solutions. Available online: https://www.fulgentgenetics.com/covid19/molecular (accessed on 23 August 2020).
- Sherlock Biosciences Sherlock Biosciences Receives FDA Emergency Use Authorization for CRISPR SARS-CoV-2 Rapid Diagnostic. Available online: https://sherlock.bio/sherlock-biosciences-receives-fda-emergency-use-authorization-for-crispr-sars-cov-2-rapid-diagnostic/ (accessed on 20 August 2020).
- UCSF SARS-COV-2 RNA DETECTR ASSAY. Available online: https://www.fda.gov/media/139937/download (accessed on 24 August 2020).
- Tahamtan, A.; Ardebili, A. Real-Time RT-PCR in COVID-19 Detection: Issues Affecting the Results. Expert Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef] [Green Version]
- Brennan-Krohn, T. Making Sense of Respiratory Viral Panel Results. Available online: https://asm.org/Articles/2020/March/Making-Sense-of-Respiratory-Viral-Panel-Results (accessed on 6 July 2020).
- Lin, C.-Y.; Hwang, D.; Chiu, N.-C.; Weng, L.-C.; Liu, H.-F.; Mu, J.-J.; Liu, C.-P.; Chi, H. Increased Detection of Viruses in Children with Respiratory Tract Infection Using PCR. Int. J. Environ. Res. Public Health 2020, 17, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahony, J.B. Detection of Respiratory Viruses by Molecular Methods. Clin. Microbiol. Rev. 2008, 21, 716–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- C D C Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html#specimen (accessed on 22 December 2020).
- Azghandi, M.; Kerachian, M.A. Detection of Novel Coronavirus (SARS-CoV-2) RNA in Peripheral Blood Specimens. J. Transl. Med. 2020, 18, 412. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Kim, H.M.; Lee, E.J.; Jo, H.J.; Yoon, Y.; Lee, N.-J.; Son, J.; Lee, Y.-J.; Kim, M.S.; Lee, Y.-P.; et al. Detection and Isolation of SARS-CoV-2 in Serum, Urine, and Stool Specimens of COVID-19 Patients from the Republic of Korea. Osong Public Health Res. Perspect. 2020, 11, 112–117. [Google Scholar] [CrossRef]
- Wozniak, A.; Cerda, A.; Ibarra-Henríquez, C.; Sebastian, V.; Armijo, G.; Lamig, L.; Miranda, C.; Lagos, M.; Solari, S.; Guzmán, A.M.; et al. A Simple RNA Preparation Method for SARS-CoV-2 Detection by RT-QPCR. Sci. Rep. 2020, 10, 16608. [Google Scholar] [CrossRef]
- Mo, Y.; Wan, R.; Zhang, Q. Application of Reverse Transcription-PCR and Real-Time PCR in Nanotoxicity Research. Methods Mol. Biol. 2012, 926, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Barreto, H.G.; de Pádua Milagres, F.A.; de Araújo, G.C.; Daúde, M.M.; Benedito, V.A. Diagnosing the Novel SARS-CoV-2 by Quantitative RT-PCR: Variations and Opportunities. J. Mol. Med. 2020, 98, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Joon, D.; Nimesh, M.; Saluja, D. Loop-Mediated Isothermal Amplification as Alternative to PCR for the Diagnosis of Extra-Pulmonary Tuberculosis. Int. J. Tuberc. Lung Dis. 2015, 19, 986–991. [Google Scholar] [CrossRef]
- Salamin, O.; Kuuranne, T.; Saugy, M.; Leuenberger, N. Loop-Mediated Isothermal Amplification (LAMP) as an Alternative to PCR: A Rapid on-Site Detection of Gene Doping. Drug Test. Anal. 2017, 9, 1731–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Chen, F.; Li, Q.; Wang, L.; Fan, C. Isothermal Amplification of Nucleic Acids. Chem. Rev. 2015, 115, 12491–12545. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.M.; Spoto, G. Isothermal Amplification Methods for the Detection of Nucleic Acids in Microfluidic Devices. Biosensors 2013, 3, 18–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deiman, B.; van Aarle, P.; Sillekens, P. Characteristics and Applications of Nucleic Acid Sequence-Based Amplification (NASBA). Mol. Biotechnol. 2002, 20, 163–179. [Google Scholar] [CrossRef]
- Fakruddin, M. Nucleic acid sequence-based amplification (NASBA)-prospects and applications. Int. J. Life Sci. Pharma Res. 2020, 2, 106–121. [Google Scholar]
- Selvarajah, D.; Naing, C.; Htet, N.H.; Mak, J.W. Loop-Mediated Isothermal Amplification (LAMP) Test for Diagnosis of Uncomplicated Malaria in Endemic Areas: A Meta-Analysis of Diagnostic Test Accuracy. Malar. J. 2020, 19, 211. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.R.; Sethy, K.; Mohapatra, S.; Panda, D. Loop Mediated Isothermal Amplification: An Innovative Gene Amplification Technique for Animal Diseases. Vet. World 2016, 9, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakruddin, M.; Mannan, K.S.B.; Chowdhury, A.; Mazumdar, R.M.; Hossain, M.N.; Islam, S.; Chowdhury, M.A. Nucleic Acid Amplification: Alternative Methods of Polymerase Chain Reaction. J. Pharm. Bioallied Sci. 2013, 5, 245–252. [Google Scholar] [CrossRef]
- Dixit, K.K.; Verma, S.; Singh, O.P.; Singh, D.; Singh, A.P.; Gupta, R.; Negi, N.S.; Das, P.; Sundar, S.; Singh, R.; et al. Validation of SYBR Green I Based Closed Tube Loop Mediated Isothermal Amplification (LAMP) Assay and Simplified Direct-Blood-Lysis (DBL)-LAMP Assay for Diagnosis of Visceral Leishmaniasis (VL). PLoS Negl. Trop. Dis. 2018, 12, e0006922. [Google Scholar] [CrossRef] [Green Version]
- Toonkomdang, S.; Phinyo, P.; Phetsuksiri, B.; Patumanond, J.; Rudeeaneksin, J.; Klayut, W. Pragmatic Accuracy of an In-House Loop-Mediated Isothermal Amplification (LAMP) for Diagnosis of Pulmonary Tuberculosis in a Thai Community Hospital. PLoS ONE 2020, 15, e0236496. [Google Scholar] [CrossRef]
- Thompson, D.; Lei, Y. Mini Review: Recent Progress in RT-LAMP Enabled COVID-19 Detection. Sensors and Actuators Reports 2020, 2, 100017. [Google Scholar] [CrossRef]
- Kashir, J.; Yaqinuddin, A. Loop Mediated Isothermal Amplification (LAMP) Assays as a Rapid Diagnostic for COVID-19. Med. Hypotheses 2020, 141, 109786. [Google Scholar] [CrossRef]
- Joung, J.; Ladha, A.; Saito, M.; Segel, M.; Bruneau, R.; Huang, M.-L.W.; Kim, N.-G.; Yu, X.; Li, J.; Walker, B.D.; et al. Point-of-Care Testing for COVID-19 Using SHERLOCK Diagnostics. medRxiv 2020, 1–21. [Google Scholar] [CrossRef]
- Hao, M.; Qiao, J.; Qi, H. Current and Emerging Methods for the Synthesis of Single-Stranded DNA. Genes 2020, 11, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goo, N.-I.; Kim, D.-E. Rolling Circle Amplification as Isothermal Gene Amplification in Molecular Diagnostics. BioChip J. 2016, 10, 262–271. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, J.; Ye, F.; Feng, T.; Lee, I.; Yin, B. Amplification of Circularizable Probes for the Detection of Target Nucleic Acids and Proteins. Clin. Chim. Acta 2006, 363, 61–70. [Google Scholar] [CrossRef]
- Pokhrel, P.; Hu, C.; Mao, H. Detecting the Coronavirus (COVID-19). ACS Sens. 2020, 5, 2283–2296. [Google Scholar] [CrossRef]
- Qi, X.; Bakht, S.; Devos, K.M.; Gale, M.D.; Osbourn, A. L-RCA (Ligation-Rolling Circle Amplification): A General Method for Genotyping of Single Nucleotide Polymorphisms (SNPs). Nucleic Acids Res. 2001, 29, E116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashkin, K.N.; Strizhkov, B.N.; Gryadunov, D.A.; Surzhikov, S.A.; Grechishnikova, I.V.; Kreindlin, E.Y.; Chupeeva, V.V.; Evseev, K.B.; Turygin, A.Y.; Mirzabekov, A.D. Detection of Single-Nucleotide Polymorphisms in the P53 Gene by LDR/RCA in Hydrogel Microarrays. Mol. Biol. 2005, 39, 26–34. [Google Scholar] [CrossRef]
- Chen, X.; Wang, B.; Yang, W.; Kong, F.; Li, C.; Sun, Z.; Jelfs, P.; Gilbert, G.L. Rolling Circle Amplification for Direct Detection of RpoB Gene Mutations in Mycobacterium Tuberculosis Isolates from Clinical Specimens. J. Clin. Microbiol. 2014, 52, 1540–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Potter, S.J.; Lin, Y.; Cunningham, A.L.; Dwyer, D.E.; Su, Y.; Ma, X.; Hou, Y.; Saksena, N.K. Rapid and Sensitive Detection of Severe Acute Respiratory Syndrome Coronavirus by Rolling Circle Amplification. J. Clin. Microbiol. 2005, 43, 2339–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciftci, S.; Neumann, F.; Abdurahman, S.; Appelberg, K.S.; Mirazimi, A.; Nilsson, M.; Madaboosi, N. Digital Rolling Circle Amplification-Based Detection of Ebola and Other Tropical Viruses. J. Mol. Diagn. 2020, 22, 272–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Hsu, H.; Su, J.; Clapper, J.; Hsu, J. Room Temperature Isothermal Colorimetric Padlock Probe Rolling Circle Amplification for Viral RNA Detection. BioRxiv 2020. [Google Scholar] [CrossRef]
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.S. Molecular Diagnostic Testing for Infectious Diseases Using TMA Technology. Expert Rev. Mol. Diagn. 2001, 1, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-H. Amplification of nucleic acids. In Diagnostic Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 215–247. ISBN 9780128028230. [Google Scholar]
- Gorzalski, A.J.; Tian, H.; Laverdure, C.; Morzunov, S.; Verma, S.C.; VanHooser, S.; Pandori, M.W. High-Throughput Transcription-Mediated Amplification on the Hologic Panther Is a Highly Sensitive Method of Detection for SARS-CoV-2. J. Clin. Virol. 2020, 129, 104501. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, E.; Eissa, S.; Ismail, A.; Zourob, M. Diagnostic Techniques for COVID-19 and New Developments. Talanta 2020, 220, 121392. [Google Scholar] [CrossRef] [PubMed]
- Hologic Aptima® SARS-CoV-2 Assay (Panther® System). Available online: https://www.fda.gov/media/138096/download (accessed on 1 September 2020).
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA Detection Using Recombination Proteins. PLoS Biol. 2006, 4, e204. [Google Scholar] [CrossRef] [PubMed]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase Polymerase Amplification: Basics, Applications and Recent Advances. Trends Analyt. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Heo, S.; Bang, D. Applying a Linear Amplification Strategy to Recombinase Polymerase Amplification for Uniform DNA Library Amplification. ACS Omega 2019, 4, 19953–19958. [Google Scholar] [CrossRef] [Green Version]
- Mayboroda, O.; Gonzalez Benito, A.; Sabaté del Rio, J.; Svobodova, M.; Julich, S.; Tomaso, H.; O’Sullivan, C.K.; Katakis, I. Isothermal Solid-Phase Amplification System for Detection of Yersinia Pestis. Anal. Bioanal. Chem. 2016, 408, 671–676. [Google Scholar] [CrossRef]
- Wu, L.; Ye, L.; Wang, Z.; Cui, Y.; Wang, J. Utilization of Recombinase Polymerase Amplification Combined with a Lateral Flow Strip for Detection of Perkinsus Beihaiensis in the Oyster Crassostrea Hongkongensis. Parasites Vectors 2019, 12, 360. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ma, B.; Fang, J.; Zhi, A.; Chen, E.; Xu, Y.; Yu, X.; Sun, C.; Zhang, M. Recombinase Polymerase Amplification (RPA) Combined with Lateral Flow Immunoassay for Rapid Detection of Salmonella in Food. Foods 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.T.; Nguyen, U.D.; Le, T.T.; Bui, H.T.; Nguyen, A.N.T.; Thi Nguyen, A.N.; Trieu, N.T.; Trieu, L.P.; Bui, S.T.; Nguyen, C.; et al. Establishment of Recombinase Polymerase Amplification Assay for Rapid and Sensitive Detection of Orientia Tsutsugamushi in Southeast Asia. Acta Trop. 2020, 210, 105541. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, F.; Wang, L.; Qian, W.; Qian, C.; Wu, J.; Ying, Y. Instant, Visual, and Instrument-Free Method for On-Site Screening of GTS 40-3-2 Soybean Based on Body-Heat Triggered Recombinase Polymerase Amplification. Anal. Chem. 2017, 89, 4413–4418. [Google Scholar] [CrossRef] [PubMed]
- Rostron, P.; Pennance, T.; Bakar, F.; Rollinson, D.; Knopp, S.; Allan, F.; Kabole, F.; Ali, S.M.; Ame, S.M.; Webster, B.L. Development of a Recombinase Polymerase Amplification (RPA) Fluorescence Assay for the Detection of Schistosoma Haematobium. Parasites Vectors 2019, 12, 514. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, X.; Wang, G.; Zhang, Y.; Shang, Y.; Zhang, Z. Development of a Fluorescent Probe-Based Recombinase Polymerase Amplification Assay for Rapid Detection of Orf Virus. Virol. J. 2015, 12, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, A.S.; Todd, S.; Pollak, N.M.; Marsh, G.A.; Macdonald, J. Ebolavirus Diagnosis Made Simple, Comparable and Faster than Molecular Detection Methods: Preparing for the Future. Virol. J. 2018, 15, 75. [Google Scholar] [CrossRef]
- Zhang, F.; Abudayyeh, O.; Goothenberg, J. A Protocol for Detection of COVID-19 Using CRISPR Diagnostics. Available online: https://www.broadinstitute.org/files/publications/special/COVID-19%20detection%20(updated).pdf (accessed on 10 September 2020).
- Xia, S.; Chen, X. Single-Copy Sensitive, Field-Deployable, and Simultaneous Dual-Gene Detection of SARS-CoV-2 RNA via Modified RT-RPA. Cell Discov. 2020, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Barba, M.; Czosnek, H.; Hadidi, A. Historical Perspective, Development and Applications of next-Generation Sequencing in Plant Virology. Viruses 2014, 6, 106–136. [Google Scholar] [CrossRef] [PubMed]
- First NGS-Based COVID-19 Diagnostic. Nat. Biotechnol. 2020, 38, 777. [CrossRef] [PubMed]
- Canadian Agency for Drugs and Technologies in Health. CADTH Summary of findings—Cost-effectiveness of next generation sequencing. In CADTH Rapid Response Service; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2014. [Google Scholar]
- Illumina Illumina COVIDSeq Test Instructions for Use. Available online: https://www.fda.gov/media/138776/download (accessed on 22 December 2020).
- Deckert, A.; Bärnighausen, T.; Kyei, N.N. Simulation of Pooled-Sample Analysis Strategies for COVID-19 Mass Testing. Bull. World Health Organ. 2020, 98, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shang, X.; Huang, X. Next-Generation Pathogen Diagnosis with CRISPR/Cas-Based Detection Methods. Emerg. Microbes Infect. 2020, 9, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Furmanski, L.; Kellar, J.; Mathewson, S.; Verma, M. Crispr Catalyzes Point-of-Care Testing; BCG: Boston, MA, USA, 2020. [Google Scholar]
- Mendoza, B.J.; Trinh, C.T. In Silico Processing of the Complete CRISPR-Cas Spacer Space for Identification of PAM Sequences. BioRxiv 2018. [Google Scholar] [CrossRef]
- Mojica, F.J.; Juez, G.; Rodríguez-Valera, F. Transcription at Different Salinities of Haloferax Mediterranei Sequences Adjacent to Partially Modified PstI Sites. Mol. Microbiol. 1993, 9, 613–621. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a Target Binding Unleashes Indiscriminate Single-Stranded DNase Activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef] [Green Version]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic Acid Detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandsma, E.; Verhagen, H.J.; van de Laar, T.J.W.; Claas, E.C.J.; Cornelissen, M.; van den Akker, E. Rapid, Sensitive and Specific SARS Coronavirus-2 Detection: A Multi-Center Comparison between Standard QRT-PCR and CRISPR Based DETECTR. J. Infect. Dis. 2020, 223, 206–213. [Google Scholar] [CrossRef]
- Kumar, P.; Malik, Y.S.; Ganesh, B.; Rahangdale, S.; Saurabh, S.; Natesan, S.; Srivastava, A.; Sharun, K.; Yatoo, M.I.; Tiwari, R.; et al. CRISPR-Cas System: An Approach With Potentials for COVID-19 Diagnosis and Therapeutics. Front. Cell. Infect. Microbiol. 2020, 10, 576875. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A.; et al. CRISPR-Cas12-Based Detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.; Smith, B.M.; Jain, P.K. Enhancement of Trans-Cleavage Activity of Cas12a with Engineered CrRNA Enables Amplified Nucleic Acid Detection. Nat. Commun. 2020, 11, 4906. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Bhattacharjee, R.; Baharfar, M.; Liu, G. Current Methods for Diagnosis of Human Coronaviruses: Pros and Cons. Anal. Bioanal. Chem. 2020, 413, 2311–2330. [Google Scholar] [CrossRef]
- Arnaout, R.; Lee, R.A.; Lee, G.R.; Callahan, C.; Yen, C.F.; Smith, K.P.; Arora, R.; Kirby, J.E. SARS-CoV2 Testing: The Limit of Detection Matters. BioRxiv 2020. [Google Scholar] [CrossRef]
- Ding, X.; Yin, K.; Li, Z.; Lalla, R.V.; Ballesteros, E.; Sfeir, M.M.; Liu, C. All-in-One Dual CRISPR-Cas12a (AIOD-CRISPR) Assay: A Case for Rapid, Ultrasensitive and Visual Detection of Novel Coronavirus SARS-CoV-2 and HIV Virus at the Point of Care. BioRxiv 2020. [Google Scholar] [CrossRef]
- Javalkote, V.S.; Kancharla, N.; Bhadra, B.; Shukla, M.; Soni, B.; Goodin, M.; Bandyopadhyay, A.; Dasgupta, S. CRISPR-Based Assays for Rapid Detection of SARS-CoV-2. Methods 2020. [Google Scholar] [CrossRef]
- Ding, X.; Yin, K.; Li, Z.; Lalla, R.V.; Ballesteros, E.; Sfeir, M.M.; Liu, C. Ultrasensitive and Visual Detection of SARS-CoV-2 Using All-in-One Dual CRISPR-Cas12a Assay. Nat. Commun. 2020, 11, 4711. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.J.; Koob, J.G.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F. Author Correction: SHERLOCK: Nucleic Acid Detection with CRISPR Nucleases. Nat. Protoc. 2020, 15, 1311. [Google Scholar] [CrossRef] [Green Version]
- Ackerman, C.M.; Myhrvold, C.; Thakku, S.G.; Freije, C.A.; Metsky, H.C.; Yang, D.K.; Ye, S.H.; Boehm, C.K.; Kosoko-Thoroddsen, T.-S.F.; Kehe, J.; et al. Massively Multiplexed Nucleic Acid Detection with Cas13. Nature 2020, 582, 277–282. [Google Scholar] [CrossRef]
- Metsky, H.C.; Freije, C.A.; Kosoko-Thoroddsen, T.-S.F.; Sabeti, P.C.; Myhrvold, C. CRISPR-Based COVID-19 Surveillance Using a Genomically-Comprehensive Machine Learning Approach. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- MIT News CRISPR-Based Diagnostic Chips Perform Thousands of Tests Simultaneously to Detect Viruses. Available online: https://news.mit.edu/2020/crispr-diagnostic-chips-test-viruses-0429 (accessed on 4 September 2021).
- Rauch, J.N.; Valois, E.; Solley, S.C.; Braig, F.; Lach, R.S.; Baxter, N.J.; Kosik, K.S.; Arias, C.; Acosta-Alvear, D.; Wilson, M.Z. A Scalable, Easy-to-Deploy, Protocol for Cas13-Based Detection of SARS-CoV-2 Genetic Material. J. Clin. Microbiol. 2020, 59, 2–20. [Google Scholar] [CrossRef]
- Fernández-Barat, L.; López-Aladid, R.; Torres, A. The Value of Serology Testing to Manage SARS-CoV-2 Infections. Eur. Respir. J. 2020, 56, 2002411. [Google Scholar] [CrossRef]
- Bao, L.; Deng, W.; Gao, H.; Xiao, C.; Liu, J.; Xue, J.; Lv, Q.; Liu, J.; Yu, P.; Xu, Y.; et al. Reinfection Could Not Occur in SARS-CoV-2 Infected Rhesus Macaques. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, T.; Nissen, K.; Krambrich, J.; Rönnberg, B.; Akaberi, D.; Esmaeilzadeh, M.; Salaneck, E.; Lindahl, J.; Lundkvist, Å. Evaluation of a COVID-19 IgM and IgG Rapid Test; an Efficient Tool for Assessment of Past Exposure to SARS-CoV-2. Infect. Ecol. Epidemiol. 2020, 10, 1754538. [Google Scholar] [CrossRef] [Green Version]
- Taipale, J.; Romer, P.; Linnarsson, S. Population-Scale Testing Can Suppress the Spread of COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, J.; Bao, L.; Shi, Y. Convalescent Plasma as a Potential Therapy for COVID-19. Lancet Infect. Dis. 2020, 20, 398–400. [Google Scholar] [CrossRef]
- Imai, K.; Tabata, S.; Ikeda, M.; Noguchi, S.; Kitagawa, Y.; Matuoka, M.; Miyoshi, K.; Tarumoto, N.; Sakai, J.; Ito, T.; et al. Clinical Evaluation of an Immunochromatographic IgM/IgG Antibody Assay and Chest Computed Tomography for the Diagnosis of COVID-19. J. Clin. Virol. 2020, 128, 104393. [Google Scholar] [CrossRef] [PubMed]
- Beretta, A.; Cranage, M.; Zipeto, D. Is Cross-Reactive Immunity Triggering COVID-19 Immunopathogenesis? Front. Immunol. 2020, 11, 567710. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.; Lindsley, A.; Courter, J.; Assa’ad, A. Clinical Testing for COVID-19. J. Allergy Clin. Immunol. 2020, 146, 23–34. [Google Scholar] [CrossRef] [PubMed]
- FDA Independent Evaluations of COVID-19 Serological Tests. Available online: https://open.fda.gov/apis/device/covid19serology (accessed on 6 April 2021).
- Babson Diagnostics BABSON DIAGNOSTICS AC19G1. Available online: https://www.fda.gov/media/139446/download (accessed on 8 September 2020).
- Beckman Coulter SARS-CoV-2 IgG. Available online: https://www.beckmancoulter.com/products/immunoassay/access-sars-cov-2-igg-antibody-test#/documentos (accessed on 8 September 2020).
- Diazyme DIAZYME DZ-LITE SARS-CoV-2 IgG CLIA KIT. Available online: https://www.fda.gov/media/139865/download (accessed on 8 September 2020).
- InBios SCoV-2 DetectTM IgM ELISA. Available online: https://www.fda.gov/media/139730/download (accessed on 8 September 2020).
- Emory Medical Laboratories SARS-COV-2 RBD IGG FOR ANTIBODY DETECTION. Available online: https://www.fda.gov/media/139053/download (accessed on 8 September 2020).
- Luminex XMAP® SARS-CoV-2 Multi-AntigenIgG Assay Package Insert. Available online: https://www.fda.gov/media/140256/download (accessed on 8 September 2020).
- Siemens Healthineers SARS-CoV-2 IgG (COV2G). Available online: https://www.fda.gov/media/140699/download (accessed on 8 September 2020).
- Siemens SARS-CoV-2 IgG (COV2G). Available online: https://www.fda.gov/media/140704/download (accessed on 8 September 2020).
- EUROIMMUN Anti-SARS-CoV-2 ELISA (IgG). Available online: https://www.fda.gov/media/137609/download (accessed on 8 September 2020).
- Abbott Laboratories SARS-CoV-2 IgG. Available online: https://www.fda.gov/media/137383/download (accessed on 8 September 2020).
- Dia-Sorin LIAISON® SARS-CoV-2 S1/S2 IgG. Available online: https://www.fda.gov/media/137359/download (accessed on 8 September 2020).
- Ortho-Clinical Diagnostics VITROS Immunodiagnostic Products Anti-SARS-CoV-2 IgGReagent Pack. Available online: https://www.fda.gov/media/137363/download (accessed on 8 September 2020).
- Mount Sinai Laboratory COVID-19 ELISA IGG ANTIBODY TEST. Available online: https://www.fda.gov/media/137029/download (accessed on 7 September 2020).
- BIOMÉRIEUX VIDAS® SARS-COV-2 IgG. Available online: https://www.fda.gov/media/140937/download (accessed on 8 September 2020).
- Healgen COVID-19 IgG/IgM Rapid Test Cassette. Available online: https://www.fda.gov/media/138438/download (accessed on 8 September 2020).
- Megna Health RAPID COVID-19 IgM/IgG COMBO TEST KIT. Available online: https://www.fda.gov/media/140297/download (accessed on 9 September 2020).
- Assure Assure COVID-19 IgG/IgM Rapid Test Device. Available online: https://www.fda.gov/media/139792/download (accessed on 8 September 2020).
- Sienna SiennaTM-Clarity COVIBLOCK™. Available online: https://www.fda.gov/media/140082/download (accessed on 8 September 2020).
- AccessBio COVID-19 IgM/IgG COVID-19 IgM/IgG. Available online: https://www.apacor.com/wp-content/uploads/2020/05/IFU-RCIM71-E_Rev.A_20200417.pdf (accessed on 8 September 2020).
- Xiamen Biotime Biotechnology Co., Ltd. BIOTIME SARS-CoV-2 IgG/IgM Rapid Qualitative Test. Available online: https://www.fda.gov/media/140443/download (accessed on 8 September 2020).
- Ab Clinical Labs Vibrant COVID-19 Ab Assay. Available online: https://www.vibrant-america.com/wp-content/uploads/2020/04/VA-COV-001-CovidAb-Analytical-and-Clinical-Studies-Report-FDA-Rev-2.pdf (accessed on 8 September 2020).
- Autobio Diagnostics Anti-SARS-CoV-2 Rapid Test. Available online: https://www.fda.gov/media/137367/download (accessed on 8 September 2020).
- ChemBio Serology Test Evaluation Report for “DPP COVID-19IgM/IgG System. Available online: https://www.accessdata.fda.gov/cdrh_docs/presentations/maf/maf3265-a001.pdf (accessed on 8 September 2020).
- Celex Cellex QSARS-CoV-2 IgG/IgM Rapid Test. Available online: https://www.fda.gov/media/136625/download (accessed on 8 September 2020).
- SIEMENS SARSCoV2 Total Antibody Assay (CV2T). Available online: https://www.fda.gov/media/138757/download (accessed on 8 September 2020).
- Beijing Wantai Biological Pharmacy Enterprise Co., Ltd. WANTAI SARS-CoV-2 Ab ELISA. Available online: https://www.fda.gov/media/140929/download (accessed on 7 September 2020).
- WANTAI Wantai SARS-CoV-2 Diagnostics. Available online: https://www.fda.gov/media/140030/download (accessed on 8 September 2020).
- Wadsworth Center NEW YORK SARS-COV MICROSPHERE IMMUNOASSAY. Available online: https://www.fda.gov/media/137541/download (accessed on 8 September 2020).
- Bio-Rad Platelia SARS-CoV-2 Total Ab. Available online: https://www.fda.gov/media/137493/download (accessed on 8 September 2020).
- Roche Diagnosis Elecsys Anti-SARS-CoV-2. Available online: https://www.fda.gov/media/137605/download (accessed on 8 September 2020).
- Peeling, R.W.; Olliaro, P.L.; Boeras, D.I.; Fongwen, N. Scaling up COVID-19 Rapid Antigen Tests: Promises and Challenges. Lancet Infect. Dis. 2021, 21, E290–E295. [Google Scholar] [CrossRef]
- Chau, C.H.; Strope, J.D.; Figg, W.D. COVID-19 Clinical Diagnostics and Testing Technology. Pharmacotherapy 2020, 40, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Chaimayo, C.; Kaewnaphan, B.; Tanlieng, N.; Athipanyasilp, N.; Sirijatuphat, R.; Chayakulkeeree, M.; Angkasekwinai, N.; Sutthent, R.; Puangpunngam, N.; Tharmviboonsri, T.; et al. Rapid SARS-CoV-2 Antigen Detection Assay in Comparison with Real-Time RT-PCR Assay for Laboratory Diagnosis of COVID-19 in Thailand. Virol. J. 2020, 17, 177. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, Y.; Maejima, M.; Shibusawa, M.; Nagakubo, Y.; Hosaka, K.; Amemiya, K.; Sueki, H.; Hayakawa, M.; Mochizuki, H.; Tsutsui, T.; et al. Comparison of Automated SARS-CoV-2 Antigen Test for COVID-19 Infection with Quantitative RT-PCR Using 313 Nasopharyngeal Swabs, Including from Seven Serially Followed Patients. Int. J. Infect. Dis. 2020, 99, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Porte, L.; Legarraga, P.; Vollrath, V.; Aguilera, X.; Munita, J.M.; Araos, R.; Pizarro, G.; Vial, P.; Dittrich, S.; Weitzel, T. Evaluation of Novel Antigen-Based Rapid Detection Test for the Diagnosis of SARS-CoV-2 in Respiratory Samples. SSRN J. 2020. [Google Scholar] [CrossRef]
- FDA Emergency Use Authorizations for Medical Devices. Available online: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations-medical-devices#covid19ivd (accessed on 13 March 2021).
- Ortho Clinical Diagnostics Introducing Ortho’s VITROS® SARS-CoV-2 Antigen Test. Available online: https://www.orthoclinicaldiagnostics.com/global/covid19 (accessed on 31 March 2021).
- LumiraDx LumiraDx SARS-CoV-2 Ag Test. Available online: https://www.lumiradx.com/uk-en/what-we-do/diagnostics/test-technology/antigen-test (accessed on 6 April 2021).
- BD Veritor™ System for Rapid Detection of SARS-CoV-2. Available online: https://www.fda.gov/media/139755/download (accessed on 8 September 2020).
- Luminostics. Available online: https://luminostics.com (accessed on 1 April 2021).
- Ellume COVID-19 Home Test. Available online: https://www.ellumehealth.com/products/consumer-products/covid-home-test (accessed on 31 March 2021).
- Quidel Sofia SARS Antigen FIA. Available online: https://www.fda.gov/media/137885/download (accessed on 8 September 2020).
- Quidel QuickVue SARS Antigen Test. Available online: https://www.quidel.com/immunoassays/quickvue-sars-antigen-test (accessed on 31 March 2021).
- Abbott BinaxNOW™ COVID-19 Ag Card Will Help You Feel More Confident about Your COVID-19 Status. Available online: https://www.abbott.com/BinaxNOW-Test-NAVICA-App.html (accessed on 30 March 2021).
- Abbott Diagnostics Scarborough, Inc. BinaxNOWTM COVID-19 Ag CARD. Available online: https://www.fda.gov/media/141570/download (accessed on 8 September 2020).
- Quidel Sofia 2 Flu + SARS Antigen Fluorescent Immunoassay (FIA). Available online: https://www.quidel.com/immunoassays/sofia-2-flu-sars-antigen-fia (accessed on 6 April 2021).
- SAMPINUTE. Available online: https://www.celltrion.com/en-us/kit/sampinute (accessed on 1 April 2021).
- Quanterix SARS-CoV-2 N Protein Antigen. Available online: https://www.quanterix.com/simoa-assay-kits/sars-cov-2-n-protein-antigen (accessed on 31 March 2021).
- Nikaeen, G.; Abbaszadeh, S.; Yousefinejad, S. Application of Nanomaterials in Treatment, Anti-Infection and Detection of Coronaviruses. Nanomedicine 2020, 15, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Rothman, R.E. PCR-Based Diagnostics for Infectious Diseases: Uses, Limitations, and Future Applications in Acute-Care Settings. Lancet Infect. Dis. 2004, 4, 337–348. [Google Scholar] [CrossRef]
- Yadavalli, T.; Shukla, D. Role of Metal and Metal Oxide Nanoparticles as Diagnostic and Therapeutic Tools for Highly Prevalent Viral Infections. Nanomedicine 2017, 13, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef] [Green Version]
- Roh, C.; Jo, S.K. Quantitative and Sensitive Detection of SARS Coronavirus Nucleocapsid Protein Using Quantum Dots-Conjugated RNA Aptamer on Chip. J. Chem. Technol. Biotechnol. 2011, 86, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Tallury, P.; Malhotra, A.; Byrne, L.M.; Santra, S. Nanobioimaging and Sensing of Infectious Diseases. Adv. Drug Deliv. Rev. 2010, 62, 424–437. [Google Scholar] [CrossRef]
- Di Gianvincenzo, P.; Marradi, M.; Martínez-Avila, O.M.; Bedoya, L.M.; Alcamí, J.; Penadés, S. Gold Nanoparticles Capped with Sulfate-Ended Ligands as Anti-HIV Agents. Bioorg. Med. Chem. Lett. 2010, 20, 2718–2721. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, G.-L.; Ling, F.; Wang, G.-X. Carbon Nanotube-Based Nanocarrier Loaded with Ribavirin against Grass Carp Reovirus. Antiviral Res. 2015, 118, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sekimukai, H.; Iwata-Yoshikawa, N.; Fukushi, S.; Tani, H.; Kataoka, M.; Suzuki, T.; Hasegawa, H.; Niikura, K.; Arai, K.; Nagata, N. Gold Nanoparticle-Adjuvanted S Protein Induces a Strong Antigen-Specific IgG Response against Severe Acute Respiratory Syndrome-Related Coronavirus Infection, but Fails to Induce Protective Antibodies and Limit Eosinophilic Infiltration in Lungs. Microbiol. Immunol. 2020, 64, 33–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Zhu, J.; Dong, H.; Pei, Z.; Zhou, T.; Hu, G. Rapid Detection of Variant and Classical Porcine Epidemic Diarrhea Virus by Nano-Nest PCR. Pak. Vet. J. 2017, 37, 225–229. [Google Scholar]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Vilaivan, T.; Chailapakul, O.; Henry, C.S. Multiplex Paper-Based Colorimetric DNA Sensor Using Pyrrolidinyl Peptide Nucleic Acid-Induced AgNPs Aggregation for Detecting MERS-CoV, MTB, and HPV Oligonucleotides. Anal. Chem. 2017, 89, 5428–5435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layqah, L.A.; Eissa, S. An Electrochemical Immunosensor for the Corona Virus Associated with the Middle East Respiratory Syndrome Using an Array of Gold Nanoparticle-Modified Carbon Electrodes. Mikrochim. Acta 2019, 186, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, I.-L.; Lin, Y.-C.; Lin, Y.-C.; Jian, C.-Z.; Cheng, I.-C.; Chen, H.-W. A Novel Immunochromatographic Strip for Antigen Detection of Avian Infectious Bronchitis Virus. Int. J. Mol. Sci. 2019, 20, 2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, J.; Li, Y.; Wei, C.; Yang, H.; Yu, J.; Wei, H. Rapid Detection of Viral Antibodies Based on Multifunctional Staphylococcus Aureus Nanobioprobes. Enzyme Microb. Technol. 2016, 95, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Neethirajan, S. Immunosensor Based on Antibody-Functionalized MoS2 for Rapid Detection of Avian Coronavirus on Cotton Thread. IEEE Sens. J. 2018, 18, 4358–4363. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Kang, S.W.; Oh, S.; Lee, J.; Neethirajan, S. Chiral Zirconium Quantum Dots: A New Class of Nanocrystals for Optical Detection of Coronavirus. Heliyon 2018, 4, e00766. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.R.; Nagy, É.; Neethirajan, S. Self-Assembled Star-Shaped Chiroplasmonic Gold Nanoparticles for an Ultrasensitive Chiro-Immunosensor for Viruses. RSC Adv. 2017, 7, 40849–40857. [Google Scholar] [CrossRef] [Green Version]
- Pham, J.; Meyer, S.; Nguyen, C.; Williams, A.; Hunsicker, M.; McHardy, I.; Gendlina, I.; Goldstein, D.Y.; Fox, A.S.; Hudson, A.; et al. Performance Characteristics of a High-Throughput Automated Transcription-Mediated Amplification Test for SARS-CoV-2 Detection. J. Clin. Microbiol. 2020, 58, 10. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.J.; Ratzinger, F.; Schmidt, R.L.J.; Greiner, G.; Landt, O.; Am Ende, A.; Corman, V.M.; Perkmann-Nagele, N.; Watkins-Riedel, T.; Petermann, D.; et al. Development of a Fully Automated High Throughput PCR for the Detection of SARS-CoV-2: The Need for Speed. Virulence 2020, 11, 964–967. [Google Scholar] [CrossRef]
- LGC Biosearch Technologies. Biosearch Tech Fully Automated PCR System for COVID-19. Available online: https://www.biosearchtech.com/covid-19/fully-automated-pcr-system-for-covid-19-detection (accessed on 11 March 2021).
- Pan, M.; Mensah, F.; Kirk, D. 21st Century Technology Offers Hope in Covid-19 Pandemic. Available online: https://www.computeraidedbiology.com/cab-companies-on-covid19 (accessed on 13 June 2020).
- Zhang, Y.; Odiwuor, N.; Xiong, J.; Sun, L.; Nyaruaba, R.O.; Wei, H.; Tanner, N.A. Rapid Molecular Detection of SARS-CoV-2 (COVID-19) Virus RNA Using Colorimetric LAMP. medRxiv 2020. [Google Scholar] [CrossRef]
- Arumugam, A.; Wong, S.S. The Potential Use of Unprocessed Sample for RT-QPCR Detection of COVID-19 without an RNA Extraction Step. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Wang, J.; You, J.; Zhu, S.; Zhou, R.; Tian, Z.; Wu, H.; Lin, Y.; Chen, W.; Xiao, L.; et al. Effect of Large-Scale Testing Platform in Prevention and Control of the COVID-19 Pandemic: An Empirical Study with a Novel Numerical Model. medRxiv 2020. [Google Scholar] [CrossRef]
- Liu, C.; Huang, B.; Zhou, R.; Hao, W.; Qiao, M.; Chen, C.; Zhang, W.; Dong, J.; Zhu, S.; Huang, J.; et al. The Application of a Testing Platform in High-Throughput Nucleic Acid Detection of SARS-CoV-2. OSF Prepr. 2020. [Google Scholar] [CrossRef]
- Hamilton Automated Liquid Handling Equipment: Four Platforms, Unlimited Solutions. Available online: https://www.hamiltoncompany.com/automated-liquid-handling (accessed on 21 August 2020).
- TECAN Lab Automation Solutions for Coronavirus Testing and Research. Available online: https://www.tecan.com/covid19 (accessed on 19 August 2020).
- PR-Newswire. Zymo Research Announces Partnership with Tecan for COVID-19 Viral RNA Extraction, CISION. 2020. Available online: https://www.prnewswire.com/news-releases/zymo-research-announces-partnership-with-tecan-for-covid-19-viral-rna-extraction-301033021.html (accessed on 1 September 2020).
- SynbioBeta Opentrons Partners with Zymo Research To Offer an Affordable, Automated COVID-19 Testing Platform. Available online: https://synbiobeta.com/opentrons-partners-with-zymo-research-to-offer-an-affordable-automated-covid-19-testing-platform (accessed on 21 August 2020).
- Zymo Research Announces Partnership with Tecan for COVID-19 Viral RNA. Available online: https://www.zymoresearch.com/blogs/press-releases/zymo-research-announces-partnership-with-tecan-for-covid-19-viral-rna-extraction (accessed on 8 April 2021).
- Zymo Research Opentrons Partners with Zymo Research to Offer an Affordable Automate. Available online: https://www.zymoresearch.com/blogs/press-releases/opentrons-partners-with-zymo-research-to-offer-an-affordable-automated-covid-19-testing-platform (accessed on 7 April 2021).
- Crone, M.A.; Priestman, M.; Ciechonska, M.; Jensen, K.; Sharp, D.J.; Randell, P.; Storch, M.; Freemont, P. A New Role for Biofoundries in Rapid Prototyping, Development, and Validation of Automated Clinical Diagnostic Tests for SARS-CoV-2. medRxiv 2020. [Google Scholar] [CrossRef]
- London Bio Foundry Covid-19 Resources. Available online: https://www.londonbiofoundry.org/covid19 (accessed on 3 September 2020).
- Hologic Panther Fusion Assays. Available online: https://www.hologic.com/hologic-products/diagnostic-solutions/panther-fusionr-assays (accessed on 8 April 2021).
- Maia Chagas, A.; Molloy, J.C.; Prieto-Godino, L.L.; Baden, T. Leveraging Open Hardware to Alleviate the Burden of COVID-19 on Global Health Systems. PLoS Biol. 2020, 18, e3000730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villanueva-Cañas, J.L.; Gonzalez-Roca, E.; Gastaminza Unanue, A.; Titos, E.; Martínez Yoldi, M.J.; Vergara Gómez, A.; Puig Butillé, J.A. ROBOCOV: An Affordable Open-Source Robotic Platform for COVID-19 Testing by RT-QPCR. BioRxiv 2020. [Google Scholar] [CrossRef]
- Opentrons Hospital Clinic of Barcelona Uses Opentrons To Scale COVID-19 Testing. Available online: https://blog.opentrons.com/barcelona-hospital-clinic-uses-opentrons-robot-for-scalable-covid-19-testing (accessed on 21 August 2020).
- Crawford, E.D.; Acosta, I.; Ahyong, V.; Anderson, E.C.; Arevalo, S.; Asarnow, D.; Axelrod, S.; Ayscue, P.; Azimi, C.S.; Azumaya, C.M.; et al. Rapid Deployment of SARS-CoV-2 Testing: The CLIAHUB. PLoS Pathog. 2020, 16, e1008966. [Google Scholar] [CrossRef] [PubMed]


| Test Name | Manufacture Country | Technology | LoD | Sample | Approval | Ref |
|---|---|---|---|---|---|---|
| 1copy COVID-19 qPCR Multi Kit | Korea 1 drop | RT-qPCR | 0.2 copies/µL | Nasopharyngeal and nasal swab and wash | EUA 1/5/2020 | [40] |
| TRUPCR SARS-CoV-2 Kit | India 3B Blackbio Biotech India Kilpest India subsidiary | RT-qPCR | 10 copies/µL | Nasopharyngeal and oropharyngeal swabs, anterior nasal swab, and mid-turbinate nasal swabs, nasopharyngeal aspirates/washes or nasal aspirates, and bronchoalveolar lavage | EUA 18/6/2020 | [41] |
| SARS-CoV-2 and Influenza A and B RT-qPCR Detection Kit | China 3D Medicines | RT-qPCR | 5 copies/reaction | - | CE mark 3/2020 | [42] |
| Abbott RealTime SARS-CoV-2 EUA test | USA Abbott | RT-qPCR | 0.1 copies/µL | Nasopharyngeal swabs, Oropharyngeal swabs | EUA 18/3/2020 | [43] |
| CareStart COVID-19 MDx RT-PCR | USA Access Bio | RT-qPCR | 5.4 NDU/µL | Nasopharyngeal, oropharyngeal and nasal swabs, and nasopharyngeal wash/aspirate or nasal aspirate and bronchoalveolar lavage | EUA 7/7/2020 | [44] |
| Acupath COVID-19 Real-Time (RT-PCR) Assay | USA Acupath Laboratories | RT-qPCR | 25 copies/µL | Nasopharyngeal swab, nasopharyngeal aspirate, and bronchoalveolar lavage | EUA 29/6/2020 | [45] |
| UltraGene Combo2Screen SARS-CoV-2 assay | Luxemburg Advanced Biological Laboratories | RT-qPCR | 10E-6 TCID50/mL | Nasopharyngeal swab | EUA submission pending, CE mark 5/2020 | [46] |
| RealStar SARS-CoV-2 RT-PCR Kits (1.0 and U.S versions) | Germany Altona Diagnostics | RT-qPCR | 1.0E-01 PFU/mL | Human respiratory swabs | EUA 22/4/2020, CE mark 4/2020 | [47] |
| Altru Dx SARS-CoV-2 RT-PCR assay | USA Altru Diagnostics | RT-qPCR | 0.625 copies/µL | Nasal, midturbinate, nasopharyngeal, and oropharyngeal swab | EUA 30/4/2020 | [48] |
| Bosphore Novel Coronavirus (2019-nCoV) Detection Kit | Turkey Anatolia Geneworks | RT-qPCR | - | Human respiratory sample | CE mark 2/2020 | [49] |
| BioCode SARS-CoV-2 Assay | USA Applied BioCode | RT-qPCR | 1.7E-2 TCID50/mL | Nasopharyngeal swabs (NPS), oropharyngeal swabs (OPS), nasal swabs or bronchoalveolar lavage | EUA 6/15/2020 | [50] |
| Linea COVID-19 RT-PCR test | USA Applied DNA Sciences | RT-qPCR | 1.25 copies/µL | Nasopharyngeal and oropharyngeal swabs, mid-turbinate nasal swabs, nasopharyngeal washes/aspirates or nasal aspirates, and bronchoalveolar lavage | EUA 5/13/2020 | [51] |
| Aspirus SARS-CoV rRT-PCR Assay | USA Aspirus Reference Laboratory | RT-qPCR | 0.5 copies/µL | Nasal, mid-turbinate, nasopharyngeal, oropharyngeal swab specimens and bronchoalveolar lavage specimens | EUA 6/1/2020 | [52] |
| Assurance SARS-CoV-2 Panel | USA Assurance Scientific Laboratories | RT-qPCR | 9 copies/µL | Nasal, nasopharyngeal, or oropharyngeal swabs | EUA 5/15/2020 | [53] |
| A*STAR Fortitude 2.0 | Singapore A*STAR, Tan Tock Seng Hospital of Singapore | RT-qPCR | 25 copies/reaction | Nasal pharyngeal swab | Singapore Health Sciences Authority provisional authorization | [54] |
| Avera Institute for Human Genetics SARS-CoV-2 Assay | USA Avera Institute for Human Genetics | RT-qPCR | 1.6 copies/µL | Nasopharyngeal, nasal, and oropharyngeal swab specimens | EUA 5/22/2020 | [55] |
| Avellino SARS-CoV-2/COVID-19 (AvellinoCoV2) | USA Avellino Lab | RT-qPCR | 18 NDU/µL | Nasopharyngeal swab and oropharyngeal swab | EUA 3/25/2020 | [56] |
| Viro-Q SARS-CoV-2 kit | Germany BAG Diagnostics | RT-qPCR | - | - | CE mark 4/2020 | [57] |
| ThermoFisher—Applied Biosystems TaqPath COVID-19 Combo Kit | USA Rutgers University Clinical Genomics Laboratory | RT-qPCR | 0.250 GCE/µL | Nasopharyngeal swabs, nasopharyngeal aspirate (nasal aspirate), and nasopharyngeal aspirate (nasal aspirate), and bronchoalveolar lavage (BAL) | EUA 4/10/2020 | [58] |
| Cobas SARS-CoV-2 Test | USA Roche | RT-qPCR | 0.003 TCID50/mL | Nasopharyngeal and oropharyngeal swab samples | EUA 3/12/2020, CE mark 2020 | [59] |
| GSD NovaPrime SARS-CoV-2 (COVID-19) Real-Time PCR test | Hungary Gold Standard Diagnostics/Eurofins Technologies | RT-qPCR | 3.75 copies/reaction | Nasal wash/swab, nasopharyngeal wash/swab, oropharyngeal swab and bronchoalveolar lavage | CE mark 5/2020 | [60] |
| QiaStat-Dx Respiratory SARS-CoV-2 Panel | USA Qiagen | RT-qPCR | 180 NDU/µL | Nasopharyngeal swab | under CDC’s EUA | [61] |
| Rida Gene SARS-CoV-2 | Germany R-Biopharma | RT-qPCR | 50 copies/reaction | Human throat and nasopharyngeal swabs | CE mark 5/2020 | [62] |
| Standard M nCoV Real-Time Detection Kit | Korea SD Biosensor | RT-qPCR | 0.25 copies/μL | Nasoropharyngeal, nasal, and mid-turbinate nasal swab, and sputum | EUA 4/23/2020 | [63] |
| ExProbe SARS-CoV-2 Testing Kit | Taiwan TBG Biotechnology | RT-qPCR | - | Nasopharyngeal and oropharyngeal swabs, anterior nasal and mid-turbinate nasal swabs | EUA 6/10/2020 | [64] |
| Quick SARS-CoV-2rRT-PCR Kit | USA Zymo Research | RT-qPCR | 2.5 E3 GEC/μL | Nasal, nasopharyngeal, mid-turbinate or oropharyngeal swabs), and lower respiratory specimens (such as sputum, tracheal aspirates, and bronchoalveolar lavage) | EUA 5/7/2020 | [65] |
| Fulgent COVID-19 by RT-PCR Test | USA Fulgent Genetics/Fulgent Therapeutics | RT-qPCR | 20 copies/µL | Nasal, nasopharyngeal, and oropharyngeal swabs | EUA 5/15/2020 | [66] |
| SARS-CoV-2 RT-PCR test | Germany Centogene | RT-qPCR | 5 copies/µl | Dry oropharyngeal swabs | EUA 7/1/2020 | [67] |
| Xpert Xpress SARS-CoV-2 test | USA Cepheid | RT-qPCR | 0.0001 PFU/µL | Nasopharyngeal, oropharyngeal, nasal, or mid-turbinate swab and/or nasal wash/aspirate | EUA 3/20/2020 | [68] |
| HDPCR SARS-CoV-2 real-time PCR assay | USA ChromaCode | RT-qPCR | 0.250 copies/µL | Nasopharyngeal swabs oropharyngeal swabs, anterior nasal swabs, midturbinate nasal swabs, nasal aspirate, nasal wash, and bronchoalveolar lavage (BAL) specimens | EUA 6/9/2020 | [69] |
| Hymon SARS-CoV-2 Test Kit | China Dba SpectronRx | RT-qPCR | 1.2 copies/µl | Nasal, mid-turbinate, nasopharyngeal, and oropharyngeal swab specimens) | EUA 5/22/2020 | [70] |
| DSL COVID-19 Assay | USA Diagnostic Solutions Laboratory | RT-qPCR | 18 NDU/µL | Nasopharyngeal swab | EUA 6/25/2020 | [71] |
| Simplexa COVID-19 Direct | USA DiaSorin Molecular | RT-qPCR | 6 NDU/µL | Nasal swab, nasopharyngeal swab, nasal wash/aspirate, and BAL | EUA 3/19/2020, CE mark 4/2020 | [72] |
| Lilly SARS-CoV-2 Assay | USA Eli Lilly | RT-qPCR | 1 copy/µL | Nasopharyngeal swabs, oropharyngeal (throat) swabs, anterior nasal swabs, mid-turbinate nasal swabs, nasal aspirates, nasal washes and bronchoalveolar lavage | EUA 7/27/2020 | [73] |
| Ampiprobe SARS-CoV-2 Test System | USA Enzo Biochem/Enzo Life Sciences | RT-qPCR | 0.280 copies/µL | Nasopharyngeal swabs | EUA 7/7/2020 | [74] |
| Euroimmun/PerkinElmer | USA EuroRealTime SARS-CoV-2 | RT-qPCR | 0.150 copies/µL | Nasal, mid-turbinate, nasopharyngeal, oropharyngeal swabs and bronchioalveolar lavage | EUA 6/8/2020, CE mark 3/2020 | [75] |
| Fosun COVID-19 RT-PCR Detection Kit | China Fosun Pharma USA | RT-qPCR | 0.3 copies/µL | Anterior nasal swabs, mid-turbinate nasal swabs, nasopharyngeal swabs, oropharyngeal swabs, sputum, lower respiratory tract aspirates, bronchoalveolar lavage, and nasopharyngeal wash/aspirate or nasal aspirate | EUA 4/17/2020 | [76] |
| NeoPlex COVID-19 Detection Kit | Korea GeneMatrix | RT-qPCR | 5.4 NDU/µL | Nasopharyngeal swabs | EUA 5/14/2020 | [77] |
| GB SARS-CoV-2 Real-Time RT-PCR | Taiwan General Biologicals | RT-qPCR | 0.1 copies/µL | Nasopharyngeal swabs | CE mark 4/2020 | [78] |
| Genetron SARS-CoV-2 RNA Test | China Genetron | RT-qPCR | 10 copies/µL | Oropharyngeal, nasopharyngeal, anterior nasal and mid-turbinate nasal swab | EUA 6/5/2020 | [79] |
| Helix COVID-19 test | USA Helix | RT-qPCR | 1 GCE/µL | Nasopharyngeal and oropharyngeal swabs | EUA 7/23/2020 | [80] |
| Panther Fusion SARS-CoV-2 assay | USA Hologic | RT-qPCR | 1 × 10−5 TCID50/µL | Nasopharyngeal (NP), nasal, oropharyngeal (OP) swab specimens and lower respiratory tract (LRT) specimens | EUA 3/16/2020 | [81] |
| Smart Detect SARS-CoV-2 rRT-PCR Kit | USA InBios International | RT-qPCR | 12 GCE/reaction | Nasopharyngeal swab, anterior nasal swab and mid-turbinate nasal swab | EUA 4/7/2020 | [82] |
| IDT 2019-novel coronavirus kit | USA Integrated DNA Technologies/Danaher | RT-qPCR | - | Oropharyngeal, nasopharyngeal, anterior nasal and mid-turbinate nasal swab | under CDC’s EUA | [83] |
| SARS-CoV-2 Assay | USA Integrity Laboratories | RT-qPCR | 2.5 copies/µL | Nasal, nasopharyngeal and oropharyngeal swab | EUA 4/13/2020 | [84] |
| ProTect Covid-19 kit | Singapour JN Medsys | RT-qPCR | <2% | Upper respiratory nasopharyngeal swabs | Under CDC’s EUA, CE mark 4/2020, Singapore HAS provisional authorization; Philippines FDA | [85] |
| COVID-19 Coronavirus Real Time PCR Kit | China Jiangsu Bioperfectus Technologies | RT-qPCR | 0.350 copies/µL | Nasopharyngeal swabs, oropharyngeal (throat) swabs, anterior nasal swabs, mid-turbinate nasal swabs, nasal aspirates, nasal washes, bronchoalveolar lavage (BAL) fluid and sputum | EUA 18/6/2020 | [86] |
| Curative-Korva SARS-Cov-2 Assay | USA KorvaLabs | RT-qPCR | 0.200 copies/µL | Oropharyngeal (throat) swab, nasopharyngeal swab, nasal swab, and oral fluid specimens | EUA 16/4/2020 | [87] |
| Idylla SARS-CoV-2 Test | Belgium Biocartis | RT-qPCR | 0.5 copies/µL | Nasopharyngeal swab | CE mark 11/2020 | [88] |
| BioFire Respiratory Panel 2.1-EZ (RP2.1-EZ) | USA BioMérieux/BioFire Diagnostics | RT-qPCR | 6 NDU/µL | Nasopharyngeal swab | EUA 2/10/2020 | [89] |
| VitaPCR Influenza A and B/SARS-CoV-2 assay | Singapore Credo Diagnostics Biomedical | RT-qPCR | 2.73 copies/μl | Nasopharyngeal swab | CE mark 10/2020 | [90] |
| Genetworx Covid-19 Nasal Swab Test | USA Genetworx | RT-qPCR | 0.274 copies/µL | Nasal swab | EUA 15/12/2020 | [91] |
| EuroRealTime SARS-CoV-2/Influenza A/B | Germany Euroimmun/PerkinElmer | RT-qPCR | 1.8 NDU/µL | Throat swab | CE mark 12/2020 | [92] |
| MassArray SARS-CoV-2 Panel | USA Agena Bioscience | RT-PCR/MALDI-TOF | 0.3 copies/µL | Nasopharyngeal swab, oropharyngeal swab, and BAL | EUA 10/26/2020 CE mark 9/2020 | [93] |
| Bio-Rad SARS-CoV-2 ddPCR Test | USA Bio-Rad Laboratories | ddPCR | 0.625 copies/µL | Nasopharyngeal, anterior nasal and mid-turbinate swab specimens as well as nasopharyngeal wash/aspirate and nasal aspirate specimens | EUA 1/5/2020 | [94] |
| iAMP COVID-19 Detection Kit | USA Atila BioSystems | Isothermal amplification (OMEGA amplification) | 10 copies/µL | Nasal, nasopharyngeal (NP), and oropharyngeal (OP) swabs | EUA 10/4/2020 | [95] |
| ID Now COVID-19 | USA Abbott | Isothermal amplification (proprietary enzymes) | 0.125 GCE/µL | Direct nasal, nasopharyngeal or throat swabs | EUA 27/3/2020 | [96] |
| Color SARS-CoV-2 LAMP Diagnostic Assay | USA Color | RT-LAMP | 0.75 copies/µL | Nasopharyngeal (NP) swabs, oropharyngeal (OP) swabs, anterior nares (AN) swabs, mid-turbinate nasal (MTN) swabs, NP wash/aspirate or nasal aspirates, and bronchoalveolar lavage specimens | EUA 5/18/2020, amended 24/7/2020 | [97] |
| 2019-nCoV detection kit | China Rendu Biotechnology | RT-LAMP | - | Nasal, nasopharyngeal, and oropharyngeal swab | China NMPA 3/2020 | [98] |
| AQ-TOP COVID-19 Rapid Detection Kit | Korea Seasun Biomaterials | RT-LAMP | 7 copies/µL | Oropharyngeal and nasopharyngeal swab specimens, anterior nasal and mid-turbinate nasal swabs, nasopharyngeal wash/aspirate or nasal aspirate specimens, bronchoalveolar lavage (BAL) and sputum from individuals | EUA 21/5/2020 | [99] |
| Lucira Health | USA Lucira COVID-19 All-In-One Test Kit | RT-LAMP | 0.9 copies/µL | Nasal swab | EUA 17/11/2020 | [100] |
| Poplar SARS-CoV-2 TMA Pooling assay | USA Poplar Healthcare | TMA | - | Nasal, nasopharyngeal, and oropharyngeal swab | EUA 3/8/2020 | [101] |
| COVIDSeq Test | USA Illumina | Next generation gene sequencing | 5.4 NDU/µL | Nasopharyngeal (NP), oropharyngeal (OP), and mid-turbinate (MT) nasal swabs | EUA 9/6/2020 | [102] |
| Fulgent COVID-19 by NGS | USA Fulgent Genetics/MedScan Laboratory | Next generation gene sequencing | 3.6 NDU/µL | Nasal, nasopharyngeal, and oropharyngeal swabs | EUA submission pending | [103] |
| Sherlock CRISPR SARS-CoV-2 kit | USA Sherlock Biosciences | RT-LAMP and CRISPR-Cas 13 | 6.75 copies/µL | Nasopharyngeal and oropharyngeal swab samples | EUA 6/5/2020 | [104] |
| SARS-CoV-2 RNA DETECTR Assay | USA UCSF Clinical Labs at China Basin | CRISPR-Cas12 | 20 copies/µL | Nasopharyngeal swabs, oropharyngeal (throat) swabs, mid-turbinate nasal swabs, anterior nasal swabs, nasopharyngeal wash/aspirate or nasal aspirate | EUA 9/7/2020 | [105] |
| Diagnostic | Country Manufacturer | Tech | Specificity | Sensitivity | Cross Reactivity | Interferences | Date EUA Issued | Ref |
|---|---|---|---|---|---|---|---|---|
| Babson Diagnostics aC19G1 | USA Babson Diagnostics, Inc. | IgG CLIA | 100% | 100% | Anti-HIV 1 + 2, Anti-HCV, CMV IgG, Anti-HBs, Anti-HAV EIA | N/A | 23/6/2020 | [196] |
| Access SARS-CoV-2 IgG | USA Beckman Coulter, Inc. | IgG CLIA | 99.6% | 96.8% | Human chorionic gonadotropin (hCG), HIV antibody, Influenza antibody, Influenza A antibody, Influenza B antibody, Measles antibody, Mycoplasma pneumoniae IgG, Parvovirus B19 antibody, Respiratory pathogen antibodies, Respiratory syncytial virus (RSV) antibody | Hemoglobin, Bilirubin (conjugated), Bilirubin (unconjugated), Triglycerides (Intralipid) | 26/6/2020 | [197] |
| Diazyme DZ-Lite SARS-CoV-2 IgG CLIA Kit | USA, Diazyme Laboratories, Inc. | IgG CLIA | 97.4% | 100% | Influenza A H1N1 IgM/IgG, Influenza A H7N9 IgM/IgG, Rhinovirus Type A IgM/IgG, Rotavirus IgM/IgG, Human coronavirus HKU1 IgM/IgG, Human coronavirus NL63 IgM/IgG, ANA | Triglycerides, Hemoglobin, Rheumatoid Factor, Anti-Mitochondrial, HAMA, Total IgG, Total IgM, Interferon α, Ribavirin | 8/7/2020 | [198] |
| SCoV-2 Detect IgG ELISA | USA, InBios International, Inc. | IgG ELISA | 98.9% | 97.8% | VIH 1 y 2, hepatitis C and B | Hemoglobin Bilirubin Triglycerides Cholesterol | 10/6/2020 | [199] |
| SARS-CoV-2 RBD IgG test | USA, Emory Medical Laboratories | IgG ELISA | 96.4% | 100% | Anti-Influenza B, Anti-HCV, Anti-HBV, Anti-Haemophilus, Anti-Rhinovirus influenzae, ANA, Anti-HIV | N/A | 15/6/2020 | [200] |
| xMAP SARS-CoV-2 Multi-Antigen IgG Assay | USA, Luminex Corporation | IgG FMIA | 99.3% | 96.3% | N/A | (dipotassium EDTA) | 16/7/2020 | [201] |
| ADVIA Centaur SARS-CoV-2 IgG (COV2G) | Germany, Siemens Healthcare Diagnostics Inc. | IgG Semi-quantitative | 99.9% | 100% | Chlamydia trachomatis IgM, Cytomegalovirus (CMV) IgM, Epstein Barr virus (EBV) IgG, Epstein Barr virus (EBV) IgM, Hepatitis A virus (HAV) IgM, Hepatitis B core (Anti-HBc) IgM, Hepatitis B core (Anti-HBc) total antibody, Hepatitis C virus (HCV) antibody, Herpes simplex virus (HSV) IgM | Hemoglobin, Bilirubin (conjugated), Bilirubin (unconjugated), Triglycerides (Intralipid), Biotin | 31/7/2020 | [202] |
| Atellica IM SARS-CoV-2 IgG (COV2G) | Germany, Siemens Healthcare Diagnostics Inc. | IgG Semi-quantitative | 99.9% | 100% | Human chorionic gonadotropin (hCG), Human immunodeficiency virus (HIV) antibody, Influenza antibody, Influenza A antibody, Influenza B antibody, Measles antibody | Hemoglobin, Bilirubin (conjugated), Bilirubin (unconjugated), Triglycerides (Intralipid), Biotin, Cholesterol, Protein, total | 7/31/2020 | [203] |
| Anti-SARS-CoV-2 ELISA (IgG) | Germany, EUROIMMUN US Inc. | Serology IgG | 99.6% | 86.7% | Influenza, Acute EBV infection, Rheumatoid factor, other Human coronavirus | Hemoglobin, triglycerides, and bilirubin | 4/5/2020 | [204] |
| SARS-CoV-2 IgG assay | USA, Abbott Laboratories Inc. | Serology IgG | 99% | 100% | CMV, Hepaitis A,B, RSV, Rubella, Herpes virus | Adenovirus, pregnant female, lupus | 26/4/2020 | [205] |
| LIAISON SARS-CoV-2 S1/S2 IgG | Italy, DiaSorin Inc. | Serology IgG | 99.3% | 97.6% | Anti-Human CoV OC43; Anti-Human CoV HKU1, 4 Anti-Human CoV unknown strain. | Triglycerides, Hemoglobin, Unconjugated bilirubin | 24/4/2020 | [206] |
| VITROS Immunodiagnostic Products Anti-SARS-CoV-2 IgG Reagent Pack | USA, Ortho-Clinical Diagnostics, Inc. | Serology IgG | 100% | 83.3% | Adenovirus Antibody, Influenza A IgG, Influenza A IgM, Influenza B IgG, Influenza B IgM, Coxsackie Virus Antibody, Echovirus Antibody, Polio Virus, Anti-respiratory syncytial virus (RSV), HCV Antibody, Anti Nuclear Antibody | Bilirubin (conjugated), Bilirubin (unconjugated), Biotin, Hemoglobin, Intralipid | 24/4/2020 | [207] |
| COVID-19 ELISA IgG Antibody Test | USA, Mount Sinai Laboratory | Serology IgG | 100% | 92% | Varicella, Infleunza, Hepatitis, HIV, CMV | Ascorbic Acid, Hemoglobin, Bilirubin, Albumin, Triglyceride | 15/4/2020 | [208] |
| SCoV-2 Detect IgM ELISA | USA, InBios International, Inc. | IgM ELISA | 100% | 100% | Anti-Influenza A/B, Anti-Hepatitis B, Anti-Hepatitis C, Anti-Nuclear Antibody, Rheumatoid Factor, Human Anti-Mouse Antibody, Anti-HIV, Anti-Respiratory Syncytial Virus, Normal Human Sera | Hemoglobin, Bilirubin (conjugated and unconjugated), Triglycerides, Buffer (SDB), Cholesterol | 30/6/2020 | [199] |
| VIDAS SARS-CoV-2 IgM | France, bioMérieux SA | IgM ELFA | 99.4% | 100% | SARS-CoV(-1) Infection (2005), SARS-CoV(-1) Infection (2020), HCoV-NL63 Infection, HCoV-229E Infection, HCoV-OC43 Infection HCoV-HKU1 Infection MERS-CoV Infection, Acute EBV infections with heterophile antibodies, ANA | Hemoglobin, Lipids, Albumin, Bilirubin (conjugated), Bilirubin (unconjugated) | 6/8/2020 | [209] |
| RightSign COVID-19 IgG/IgM Rapid Test Cassette | China, Hangzhou Biotest Biotech Co., Ltd. | IgM and IgG Lateral Flow | 100% | 93.3% | Anti-FLU A, Anti-FLU B, Anti-Respiratory Syncytial, Virus Anti-Adenovirus, Anti-HBsAg, Anti-Syphilis, Anti-H. Pylori, Anti-HIV, Anti-HCV, Anti-SARS-COV | Acetaminophen, Caffeine, Albumin, Acetylsalicylic Acid, Gentisic Acid, Ethanol, Ascorbic Acid | 4/6/2020 | [210] |
| LYHER Novel Coronavirus (2019-nCoV) IgM/IgG Antibody Combo Test Kit (Colloidal Gold) | China, Hangzhou Laihe Biotech Co., Ltd. | IgM and IgG Lateral Flow | 98.8% | 96.7% | H1N1-1~H1N1-3, H7N9-1~H7N9-2, ANA-1, Staphyl. -1~Staphyl.-2, EBV-1~EBV-5, RSV-1~RSV-2, Chlamydia-1~Chlamydia-3 | N/A | 19/6/2020 | [211] |
| Assure COVID-19 IgG/IgM Rapid Test Device | China, Assure Tech. (Hangzhou Co., Ltd.) | IgM and IgG Lateral Flow | 100% | 98.8% | Human coronavirus HKU1 IgM/IgG, Human coronavirus NL63 IgM/IgG | Icteric (Bilirubin), Lipemicicines Acetylsalicylic acid Ascorbic acid (Vitamin C), Amoxicillin | 6/7/2020 | [212] |
| Sienna-Clarity COVIBLOCK COVID-19 IgG/IgM Rapid Test Cassette | Finland, Salofa Oy | IgM and IgG Lateral Flow | 99.2% | 94.9% | HCV, HBV, ANA Metapneumovirus | Ascorbic Acid, Hemoglobin, Bilirubin, Albumin, Triglyceride | 13/7/2020 | [213] |
| Rapid COVID-19 IgM/IgG Combo Test Kit | USA, Megna Health, Inc. | IgM and IgG Lateral Flow | 97.5% | 100% | Influenza A Virus, Influenza B Virus, Adenovirus, Rotavirus and Mycoplasma Pneumoniae. | Rheumatoid Factor, Bilirubin, Triglyceride, Hemoglobin | 17/7/2020 | [211] |
| CareStart COVID-19 IgM/IgG | USA, Access Bio, Inc. | IgM and IgG Lateral Flow | 100% | 98.8% | Anti-Influenza A, Anti-HCV, Anti-229E (alpha coronavirus), Anti-OC43 (beta coronavirus), Anti-HKU1 (beta coronavirus), Antinuclear antibodies (ANA), Anti-respiratory syncytial virus, Anti-Rhinovirus | Acetaminophen, HAMA, Acetylsalicylic acid, Hemoglobin, Albendazole, Ibuprofen, Chloroquine diphosphate, Rifampicin | 24/7/2020 | [214] |
| BIOTIME SARS-CoV-2 IgG/IgM Rapid Qualitative Test | China, Xiamen Biotime Biotechnology Co., Ltd. | IgM and IgG Lateral Flow | 98.8% | 100% | Anti-OC43 (beta coronavirus), Anti-HKU1 (beta coronavirus), Antinuclear antibodies (ANA) | N/A | 24/7/2020 | [215] |
| Vibrant COVID-19 Ab Assay | USA, Vibrant America Clinical Labs | IgM and IgG CLIA | 98.3% | 97.1% | Anti-Influenza A, Anti- Influenza B, Anti-HCV, Anti-HBV, ANA, Anti-respiratory syncytial virus, Anti-Haemophilus influenzae | Bilirubin, Triglycerides Hemoglobin Rheumatoid Factor (RF) Cholesterol, HAMA, Ribavirin, Levofloxacin, Azithromycin, Ceftriaxone sodium | 4/6/2020 | [216] |
| Anti-SARS-CoV-2 Rapid Test | China, Autobio Diagnostics Co. Ltd. | Serology IgM and IgG | 97% | 93% | Other human coronavirus, Influenza | HAMA positive sample, Rheumatoid factor, Antinuclear antibody (ANA), Anti-mitochondrial antibody (AMA), Bilirubin | 24/4/2020 | [217] |
| DPP COVID-19 IgM/IgG System | Usa, Chembio Diagnostic System, Inc. | Serology IgM and IgG | 100% | 95% | Human coronavirus, Zika, Influenza A, B, Mononucleosis, Chikungunya, Yellow fever virus, Dengue virus | Biotin Hemoglobin | 14/4/2020 | [218] |
| qSARS-CoV-2 IgG/IgM Rapid Test | USA, Cellex Inc. | Serology IgM and IgG | 96.0% | 93.8% | Human coronavirus, Adenovirus, Influenza A, B, Rhinovirus, Chlamydia pneumoniae, Streptococcus pneumoniae, Mycobacterium tuberculosis, Mycoplasma pneumoniae | Hemoglobin, Bilirubin (conjugated), Triglycerides, Cholesterol | 1/4/2020 | [219] |
| COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) | USA, Healgen Scientific LLC | Serology IgM and IgG | 96.7% | 97% | Influenza A virus IgG, Influenza B virus IgG, Respiratory syncytial virus IgG, Adenovirus IgG, Rhinovirus IgG, Human metapneumovirus IgG, Mycoplasma pneumoniae IgG, Chlamydia pneumoniae IgG, HCV IgG, Haemophilus influenza IgG, HBV core antibody IgG | Ascorbic Acid, Hemoglobin, Bilirubin, Albumin, Triglyceride | 29/5/2020 | [210] |
| Dimension EXL SARS-CoV-2 Total antibody assay (CV2T) | USA, Siemens Healthcare Diagnostics Inc. | Total Antibody CLIA | 99.9% | 100% | Anti-influenza A, Anti-influenza B, AntiHBV Antinuclear, antibody (ANA), Hepatitis B core antigen (anti-HBc) IgM, Hepatitis B surface antigen (HBs Ag), Hepatitis C virus (HCV) antibody, HIV antibody, influenza antibody | Hemoglobin, Bilirubin, conjugated, Bilirubin, Lipemia (Intralipid) | 8/6/2020 | [220] |
| WANTAI SARS-CoV-2 Ab ELISA | China, Beijing Wantai Biological Pharmacy Enterprise Co., Ltd. | Total Antibody ELISA | 100% | 94.5% | HBsAg and antibodies to HIV 1/2, HCV, TP | N/A | 5/8/2020 | [221] |
| WANTAI SARS-CoV-2 Ab Rapid Test | China, Beijing Wantai Biological Pharmacy Enterprise Co., Ltd. | Total Antibody Lateral Flow | 98.8% | 100% | alpha COV 229E, alpha COV NL63, beta COV OC43, beta COV HKU1 Flu A, Flu B, HCV, HBV, ANA | N/A | 10/7/2020 | [222] |
| New York SARS-CoV Microsphere Immunoassay for Antibody Detection | USA, Wadsworth Center, New York State Department of Health | Serology Total Antibody | 99.1% | 99% | HCV, HIV, Measles, Mumps, Rubella, Varicella Zoster virus, WNV, Herpes Simplex virus, ZIKV, Enterovirus, Rheumatoid factor | Biotin Hemoglobin | 30/4/2020 | [223] |
| Platelia SARS-CoV-2 Total Ab assay | USA, Bio-Rad Laboratories, Inc. | Serology Total Antibody | 98% | 99% | Influenza, Mycoplasma pneumoniae, Rheumatoid factor, Other human coronavirus | 29/4/2020 | [224] | |
| Elecsys Anti-SARS-CoV-2 | Germany, Roche Diagnostics | Serology Antibody | 99.8% | 100% | Other human coronavirus, Influenza | Biotin Hemoglobin | 2/5/2020 | [225] |
| Diagnostic | Country, Manufacturer | Technology | Specificity | Sensitivity | Ref |
|---|---|---|---|---|---|
| Lateral Flow, Visual Read | USA, Ortho clinical Diagnostic, Inc. | Chemiluminescence immunoassay | 98.50% | 97.10% | [232] |
| LumiraDx SARS·CoV-2 Ag Test | Spain, LumiraDx UK Ltd. | Chromatographic Digital immunoassay | 96.50% | 98% | [233] |
| BD Veritor System for Rapid Detection | England, Becton Dickinson | Chromatographic immunoassay | 100% | 84% | [234] |
| Clip COVID Rapid Antigen Test | USA, Luminostics, Inc. | Lateral Flow, Immunoluminescent Antigen | 100% | 100% | [235] |
| CareStart COVID-19 Antigen test | USA, Access Bio, Inc. | Lateral Flow, Antigen | 98% | 99% | [44] |
| Ellume COVID-19 Home Test | Australia, Ellume Limited Test | Lateral Flow, Fluorescence | 100% | 100% | [236] |
| Sofia 2 SARS Antigen FIA | Germany, Quidel Corporation | Lateral Flow, Fluorescence Antigen | 100% | 80% | [237] |
| QuickVue SARS Antlgen Test | USA, Quidel Corporatlon | Lateral Flow, Visual Read | 98% | 98% | [238] |
| BinaxNOW COVID-19 Ag Card Home | USA, Abbott Diagnostics | Lateral Flow, Visual Read | 98% | 98% | [239] |
| BinaxNOW COVID-19 Ag card | USA, Abbott Diagnostics Scarborough, Inc. | Lateral Flow, Visual Read | 98.50% | 97.10% | [240] |
| Sofía 2 Flu+ SARS Antlgen FIA | Germany, Quidel Corporatlon | Lateral Flow, Fluorescence, Instrument Read, Multl-Analyte | 100% | 80% | [241] |
| Sampinute COVID-19 Antlgen MIA | USA, Celltrion, Inc. | Magnetic force assisted electrochemical | 100% | 100% | [242] |
| Simoa SARS-CoV-2 N Protein Antigen test | USA, Quanterix | Paramagnetic Microbead-based | 98.50% | 97.10% | [243] |
| Virus | NPs | Readout/Output | LoD | T (min) | Ref |
|---|---|---|---|---|---|
| SARS-CoV | AuNPs | Immunosensing | 10 fmol | 30 | [253] |
| PEDV | AU NPs | Nano nest PCR | 2.21 × 10−7 ngμl−1 | 45 | [254] |
| Mers CoV | AgNPs | Colorimetric | 1.53 nM | 20 | [255] |
| Mers CoV | AgNPs | Electrochemiluminescence | 1.0 pg/mL−1 | 20 | [256] |
| Mers CoV | S.aureus nanobioparticles | Agglutination test | 0.93055556 | 20 | [257] |
| IBV | MoS2 nanosheets | Immunosensing | 4.6 × 102 EID50 per mL | 20 | [258] |
| IBV | Qd-MP NPs and Zr NPs | Photoluminesce | 79.15 EID/50 mL | 20 | [259] |
| IBV | CAu NPs | Chiroimmunosensing | 47.91 EID/50 mL | 20 | [260] |
| IBV | Colloidal Au NPs | ICS | 10 4.4 EID/50 mL | 45 | [257] |
| HCoV | AgNPs | Electrochemiluminescence | 0.4 pgml−1 | 20 | [257,261] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guaman-Bautista, L.P.; Moreta-Urbano, E.; Oña-Arias, C.G.; Torres-Arias, M.; Kyriakidis, N.C.; Malcı, K.; Jonguitud-Borrego, N.; Rios-Solis, L.; Ramos-Martinez, E.; López-Cortés, A.; et al. Tracking SARS-CoV-2: Novel Trends and Diagnostic Strategies. Diagnostics 2021, 11, 1981. https://doi.org/10.3390/diagnostics11111981
Guaman-Bautista LP, Moreta-Urbano E, Oña-Arias CG, Torres-Arias M, Kyriakidis NC, Malcı K, Jonguitud-Borrego N, Rios-Solis L, Ramos-Martinez E, López-Cortés A, et al. Tracking SARS-CoV-2: Novel Trends and Diagnostic Strategies. Diagnostics. 2021; 11(11):1981. https://doi.org/10.3390/diagnostics11111981
Chicago/Turabian StyleGuaman-Bautista, Linda P., Erick Moreta-Urbano, Claudia G. Oña-Arias, Marbel Torres-Arias, Nikolaos C. Kyriakidis, Koray Malcı, Nestor Jonguitud-Borrego, Leonardo Rios-Solis, Espiridion Ramos-Martinez, Andrés López-Cortés, and et al. 2021. "Tracking SARS-CoV-2: Novel Trends and Diagnostic Strategies" Diagnostics 11, no. 11: 1981. https://doi.org/10.3390/diagnostics11111981
APA StyleGuaman-Bautista, L. P., Moreta-Urbano, E., Oña-Arias, C. G., Torres-Arias, M., Kyriakidis, N. C., Malcı, K., Jonguitud-Borrego, N., Rios-Solis, L., Ramos-Martinez, E., López-Cortés, A., & Barba-Ostria, C. (2021). Tracking SARS-CoV-2: Novel Trends and Diagnostic Strategies. Diagnostics, 11(11), 1981. https://doi.org/10.3390/diagnostics11111981








