A Novel Strategy for the Diagnosis of Pulmonary High-Grade Neuroendocrine Tumor
Abstract
1. Introduction
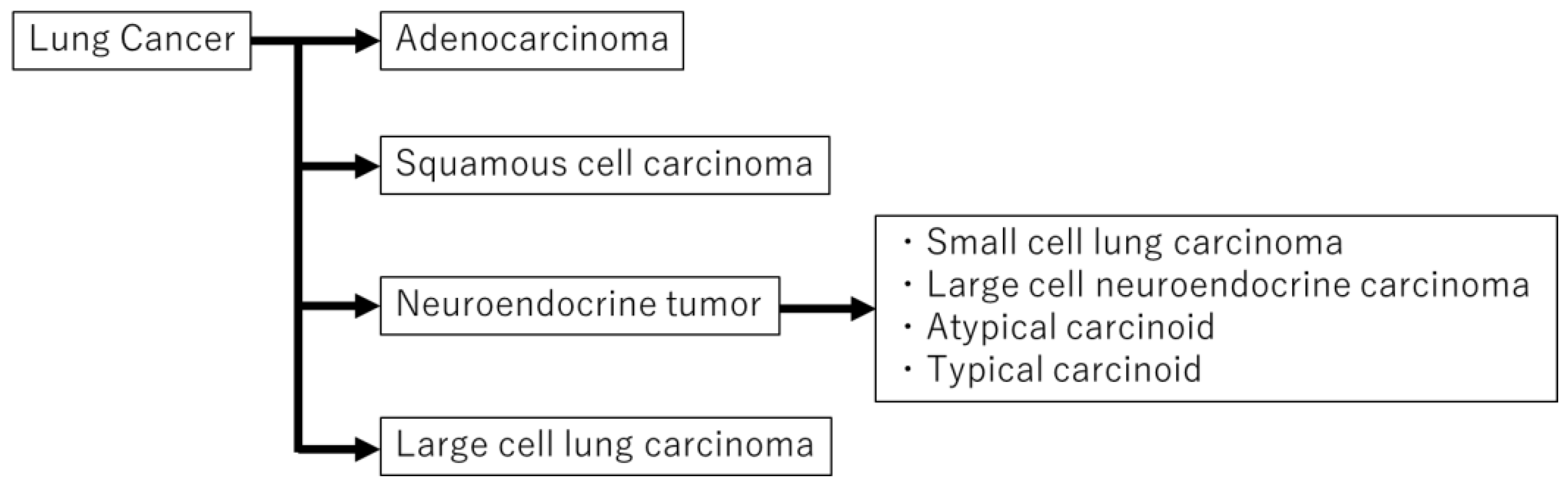
2. Diagnostic Methods of Histologic Type of Lung Cancer
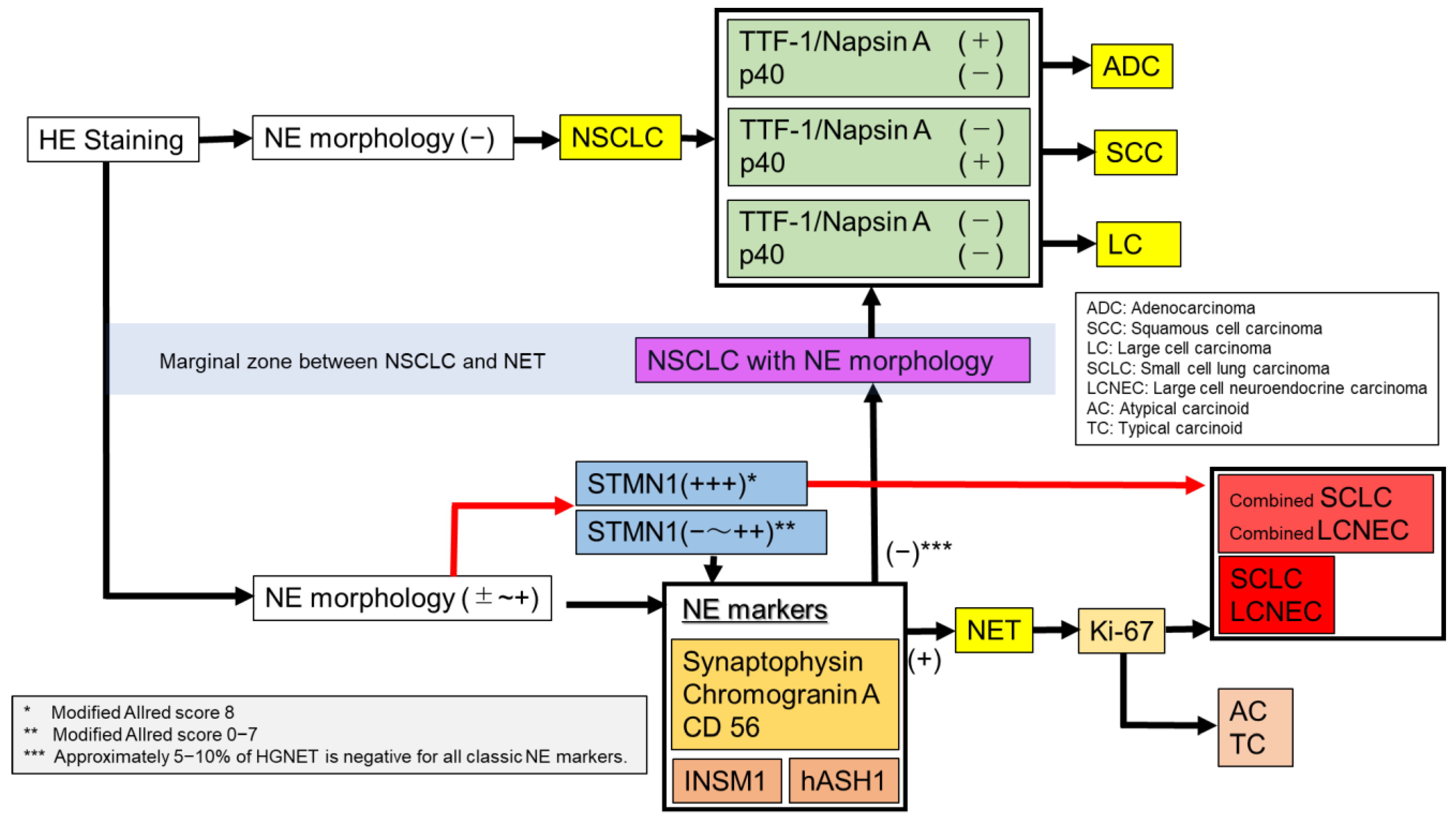
2.1. Hematoxlin and Eosin (HE) Staining (Mitosis, Necrosis, and Neuroendocrine Morphology)
2.2. Classic Neuroendocrine Markers (Synaptophysin, CD56 and Chromogranin A)
2.3. TTF-1 and Napsin A: Markers for Adenocarcinoma
2.4. p40, p63, and CK5/6: Markers for Squamous Cell Carcinoma (SCC)
2.5. Insulinoma-Associated Protein 1 (INSM1)
2.6. Human Achaete-Scute Homolog1 (hASH1)
2.7. Ki-67
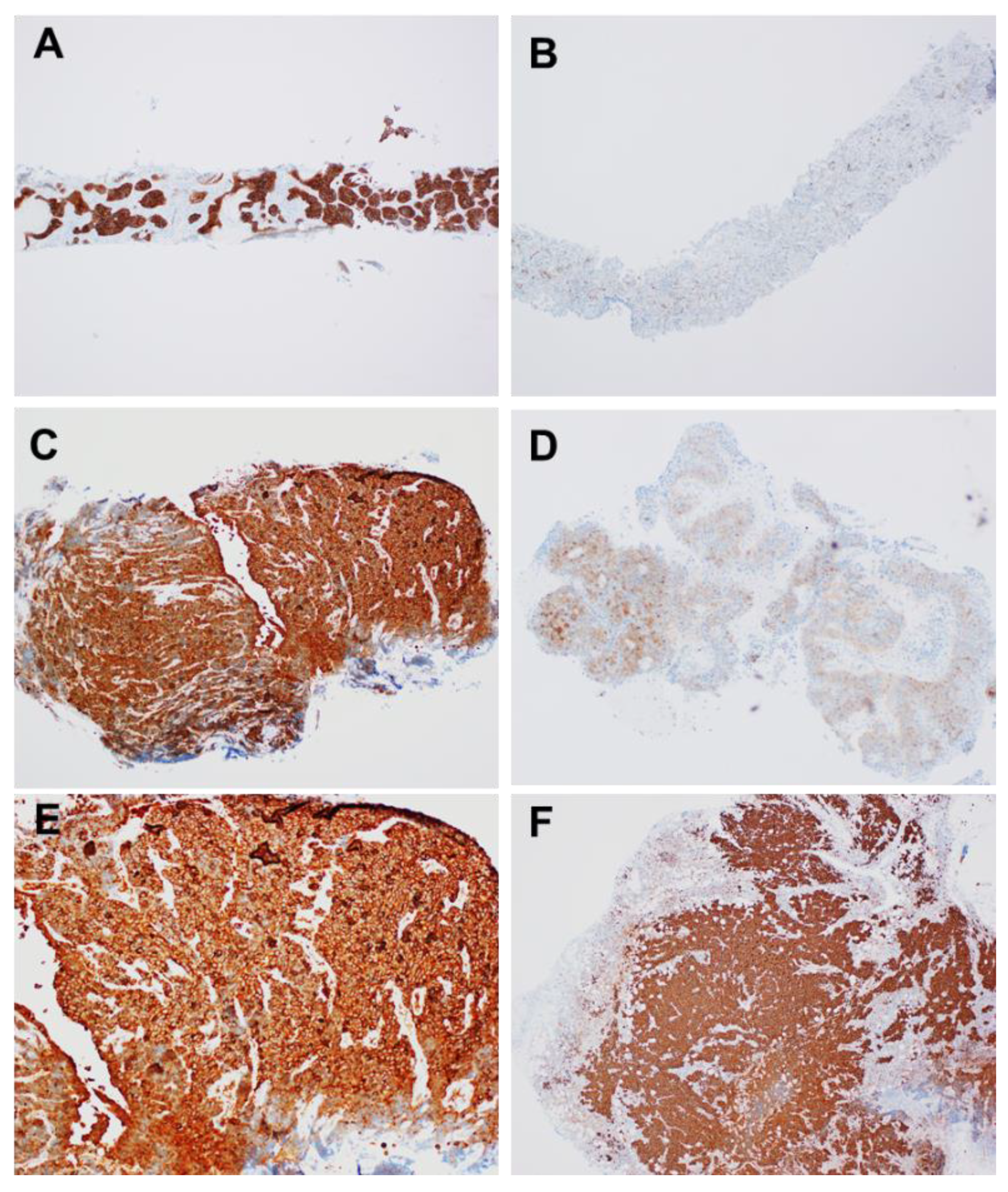
2.8. Stathmin-1 (STMN1)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, K.; Mino-Kenudson, M. Update on large cell neuroendocrine carcinoma. Transl. Lung Cancer Res. 2017, 6, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D. Advances in neuroendocrine lung tumors. Ann. Oncol. 2010, 21, vii65–vii71. [Google Scholar] [CrossRef]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive genomic profiles of small cell lung cancer. Nat. Cell Biol. 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Iyoda, A.; Hiroshima, K.; Baba, M.; Saitoh, Y.; Ohwada, H.; Fujisawa, T. Pulmonary large cell carcinomas with neuroendocrine features are high-grade neuroendocrine tumors. Ann. Thorac. Surg. 2002, 73, 1049–1054. [Google Scholar] [CrossRef]
- Iyoda, A.; Hiroshima, K.; Toyozaki, T.; Haga, Y.; Fujisawa, T.; Ohwada, H. Clinical characterization of pulmonary large cell neuroendocrine carcinoma and large cell carcinoma with neuroendocrine morphology. Cancer 2001, 91, 1992–2000. [Google Scholar] [CrossRef]
- Sequist, L.V.; Waltman, B.A.; Dias-Santagata, D.; Digumarthy, S.; Turke, A.B.; Fidias, P.; Bergethon, K.; Shaw, A.T.; Gettinger, S.; Cosper, A.K.; et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci. Transl. Med. 2011, 3, 75ra26. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D. Pathology and diagnosis of neuroendocrine tumors: Lung neuroendocrine. Thorac. Surg. Clin. 2014, 24, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Virtanen, C.; Honjoh, D.; Miyoshi, T.; Satoh, Y.; Okumura, S.; Nakagawa, K.; Nomura, H.; Ishikawa, Y. Two prognostically significant subtypes of high-grade lung neuroendocrine tumours independent of small-cell and large-cell neuroendocrine carcinomas identified by gene expression profiles. Lancet 2004, 363, 775–781. [Google Scholar] [CrossRef]
- Miyoshi, T.; Umemura, S.; Matsumura, Y.; Mimaki, S.; Tada, S.; Makinoshima, H.; Ishii, G.; Udagawa, H.; Matsumoto, S.; Yoh, K.; et al. Genomic Profiling of Large-Cell Neuroendocrine Carcinoma of the Lung. Clin. Cancer Res. 2016, 23, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Kameya, T.; Matsuno, Y.; Noguchi, M.; Tada, H.; Ishikawa, Y.; Yokose, T.; Jiang, S.-X.; Inoue, T.; Nakagawa, K.; et al. Neuroendocrine Neoplasms of the Lung: A Prognostic Spectrum. J. Clin. Oncol. 2006, 24, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gazdar, A.F.; Bunn, P.A.; Minna, J.D. Small-cell lung cancer: What we know, what we need to know and the path forward. Nat. Rev. Cancer 2017, 17, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csőszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.-H.; et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J. Clin. Oncol. 2020, 38, 2369–2379. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.W.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab, with or without tremelimumab, plus platinum–etoposide versus platinum–etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): Updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 51–65. [Google Scholar] [CrossRef]
- Travis, W.D.; Linnoila, R.I.; Tsokos, M.G.; Hitchcock, C.L.; Cutler, G.B.; Nieman, L.; Chrousos, G.; Pass, H.; Doppman, J. Neuroendocrine Tumors of the Lung With Proposed Criteria for Large-Cell Neuroendocrine Carcinoma. Am. J. Surg. Pathol. 1991, 15, 529–553. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.; Sause, W.T. Large cell neuroendocrine tumors of the lung clinical significance and histopathologic definition. Cancer 1985, 56, 1624–1629. [Google Scholar] [CrossRef]
- Warren, W.H.; E Gould, V.; Faber, L.P.; Kittle, C.F.; A Memoli, V. Neuroendocrine neoplasms of the bronchopulmonary tract. A classification of the spectrum of carcinoid to small cell carcinoma and intervening variants. J. Thorac. Cardiovasc. Surg. 1985, 89, 819–825. [Google Scholar] [CrossRef]
- Takei, H.; Asamura, H.; Maeshima, A.; Suzuki, K.; Kondo, H.; Niki, T.; Yamada, T.; Tsuchiya, R.; Matsuno, Y. Large cell neuroendocrine carcinoma of the lung: A clinicopathologic study of eighty-seven cases. J. Thorac. Cardiovasc. Surg. 2002, 124, 285–292. [Google Scholar] [CrossRef]
- Rossi, A.; Cavazza, A.; Marchioni, A.; Longo, L.; Migaldi, M.; Sartori, G.; Bigiani, N.; Schirosi, L.; Casali, C.; Morandi, U.; et al. Role of Chemotherapy and the Receptor Tyrosine Kinases KIT, PDGFRα, PDGFRβ, and Met in Large-Cell Neuroendocrine Carcinoma of the Lung. J. Clin. Oncol. 2005, 23, 8774–8785. [Google Scholar] [CrossRef]
- Fasano, M.; Della Corte, C.M.; Papaccio, F.; Ciardiello, F.; Morgillo, F. Pulmonary Large-Cell Neuroendocrine Carcinoma: From Epidemiology to Therapy. J. Thorac. Oncol. 2015, 10, 1133–1141. [Google Scholar] [CrossRef]
- Raman, V.; Jawitz, O.; Yang, C.-F.J.; Tong, B.C.; D’Amico, T.A.; Berry, M.F.; Harpole, D.H. Adjuvant Therapy for Patients With Early Large Cell Lung Neuroendocrine Cancer: A National Analysis. Ann. Thorac. Surg. 2019, 108, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Grand, B.; Cazes, A.; Mordant, P.; Foucault, C.; Dujon, A.; Guillevin, E.F.; Barthes, F.L.P.; Riquet, M. High grade neuroendocrine lung tumors: Pathological characteristics, surgical management and prognostic implications. Lung Cancer 2013, 81, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-M.; Ahn, M.-J.; Ahn, J.S.; Um, S.-W.; Kim, H.; Kim, H.K.; Choi, Y.S.; Han, J.; Kim, J.; Kwon, O.J.; et al. Chemotherapy for pulmonary large cell neuroendocrine carcinoma: Similar to that for small cell lung cancer or non-small cell lung cancer? Lung Cancer 2012, 77, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-T.; Li, Y.; Yan, L.-X.; Zhu, Z.-F.; Dong, X.-R.; Chu, Q.; Wu, L.; Zhang, H.-M.; Xu, C.-W.; Lin, G.; et al. Disparity in clinical outcomes between pure and combined pulmonary large-cell neuroendocrine carcinoma: A multi-center retrospective study. Lung Cancer 2020, 139, 118–123. [Google Scholar] [CrossRef]
- Iyoda, A.; Hiroshima, K.; Moriya, Y.; Sekine, Y.; Shibuya, K.; Iizasa, T.; Nakatani, Y.; Fujisawa, T. Prognostic impact of large cell neuroendocrine histology in patients with pathologic stage Ia pulmonary non–small cell carcinoma. J. Thorac. Cardiovasc. Surg. 2006, 132, 312–315. [Google Scholar] [CrossRef]
- Staaf, J.; Tran, L.; Söderlund, L.; Nodin, B.; Jirström, K.; Vidarsdottir, H.; Planck, M.; Mattsson, J.S.M.; Botling, J.; Micke, P.; et al. Diagnostic Value of Insulinoma-Associated Protein 1 (INSM1) and Comparison With Established Neuroendocrine Markers in Pulmonary Cancers. Arch. Pathol. Lab. Med. 2020, 144, 1075–1085. [Google Scholar] [CrossRef]
- Shimizu, K.; Goto, Y.; Kawabata-Iwakawa, R.; Ohtaki, Y.; Nakazawa, S.; Yokobori, T.; Obayashi, K.; Kawatani, N.; Yajima, T.; Kaira, K.; et al. Stathmin-1 Is a Useful Diagnostic Marker for High-Grade Lung Neuroendocrine Tumors. Ann. Thorac. Surg. 2019, 108, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Rooper, L.; Sharma, R.; Li, Q.K.; Illei, P.B.; Westra, W.H. INSM1 Demonstrates Superior Performance to the Individual and Combined Use of Synaptophysin, Chromogranin and CD56 for Diagnosing Neuroendocrine Tumors of the Thoracic Cavity. Am. J. Surg. Pathol. 2017, 41, 1561–1569. [Google Scholar] [CrossRef]
- Kadota, K.; Nitadori, J.-I.; Rekhtman, N.; Jones, D.R.; Adusumilli, P.S.; Travis, W.D. Reevaluation and Reclassification of Resected Lung Carcinomas Originally Diagnosed as Squamous Cell Carcinoma Using Immunohistochemical Analysis. Am. J. Surg. Pathol. 2015, 39, 1170–1180. [Google Scholar] [CrossRef]
- Ionescu, D.N.; Treaba, D.; Gilks, C.B.; Leung, S.; Renouf, D.; Laskin, J.; Wood-Baker, R.; Gown, A.M. Nonsmall Cell Lung Carcinoma With Neuroendocrine Differentiation—An Entity of No Clinical or Prognostic Significance. Am. J. Surg. Pathol. 2007, 31, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Cappel, J.; Findeis-Hosey, J.; McMahon, L.; Yang, Q.; Xiao, G.-Q.; Xu, H.; Li, F. hASH1 is a specific immunohistochemical marker for lung neuroendocrine tumors. Hum. Pathol. 2016, 48, 142–147. [Google Scholar] [CrossRef]
- Yatabe, Y.; Dacic, S.; Borczuk, A.C.; Warth, A.; Russell, P.A.; Lantuejoul, S.; Beasley, M.B.; Thunnissen, E.; Pelosi, G.; Rekhtman, N.; et al. Best Practices Recommendations for Diagnostic Immunohistochemistry in Lung Cancer. J. Thorac. Oncol. 2019, 14, 377–407. [Google Scholar] [CrossRef]
- Sakakibara, R.; Kobayashi, M.; Takahashi, N.; Inamura, K.; Ninomiya, H.; Wakejima, R.; Kitazono, S.; Yanagitani, N.; Horiike, A.; Ichinose, J.; et al. Insulinoma-associated Protein 1 (INSM1) Is a Better Marker for the Diagnosis and Prognosis Estimation of Small Cell Lung Carcinoma Than Neuroendocrine Phenotype Markers Such as Chromogranin A, Synaptophysin, and CD56. Am. J. Surg. Pathol. 2020, 44, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.L.; Dingemans, A.-M.C.; Van Suylen, R.-J.; Bakker, M.A.D.; Damhuis, R.A.M.; Broek, E.C.V.D.; Speel, E.-J.; Thunnissen, E. Is the sum of positive neuroendocrine immunohistochemical stains useful for diagnosis of large cell neuroendocrine carcinoma (LCNEC) on biopsy specimens? Histopathol. 2018, 74, 555–566. [Google Scholar] [CrossRef]
- Folpe, A.L.; Gown, A.M.; Lamps, L.W.; Garcia, R.; Dail, D.H.; Zarbo, R.J.; A Schmidt, R. Thyroid transcription factor-1: Immunohistochemical evaluation in pulmonary neuroendocrine tumors. Mod. Pathol. 1999, 12, 5–8. [Google Scholar]
- Stahlman, M.T.; Gray, M.E.; Whitsett, J.A. Expression of thyroid transcription factor-1(TTF-1) in fetal and neonatal human lung. J. Histochem. Cytochem. 1996, 44, 673–678. [Google Scholar] [CrossRef]
- Brasch, F.; Ochs, M.; Kähne, T.; Guttentag, S.; Schauer-Vukasinovic, V.; Derrick, M.; Johnen, G.; Kapp, N.; Müller, K.-M.; Richter, J.; et al. Involvement of Napsin A in the C- and N-terminal Processing of Surfactant Protein B in Type-II Pneumocytes of the Human Lung. J. Biol. Chem. 2003, 278, 49006–49014. [Google Scholar] [CrossRef]
- Tran, L.; Mattsson, J.S.; Nodin, B.; Jönsson, P.; Planck, M.; Jirström, K.; Botling, J.; Micke, P.; Brunnström, H. Various Antibody Clones of Napsin A, Thyroid Transcription Factor 1, and p40 and Comparisons With Cytokeratin 5 and p63 in Histopathologic Diagnostics of Non–Small Cell Lung Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 648–659. [Google Scholar] [CrossRef]
- Agoff, S.N.; Lamps, L.W.; Philip, A.T.; Amin, M.B.; A Schmidt, R.; True, L.D.; Folpe, A.L. Thyroid Transcription Factor-1 Is Expressed in Extrapulmonary Small Cell Carcinomas but Not in Other Extrapulmonary Neuroendocrine Tumors. Mod. Pathol. 2000, 13, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, O.; Dietel, M. Expression of thyroid transcription factor-1 in pulmonary and extrapulmonary small cell carcinomas and other neuroendocrine carcinomas of various primary sites. Histopathol. 2000, 36, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Schmidt, L.A.; Hatanaka, K.; Thomas, D.; Lagstein, A.; Myers, J.L. Evaluation of napsin A, TTF-1, p63, p40, and CK5/6 immunohistochemical stains in pulmonary neuroendocrine tumors. Am. J. Clin. Pathol. 2014, 142, 320–324. [Google Scholar] [CrossRef]
- La Rosa, S.; Chiaravalli, A.M.; Placidi, C.; Papanikolaou, N.; Cerati, M.; Capella, C. TTF1 expression in normal lung neuroendocrine cells and related tumors: Immunohistochemical study comparing two different monoclonal antibodies. Virchows Archiv 2010, 457, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, N.G. Value of thyroid transcription factor-1 immunostaining in tumor diagnosis: A review and update. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 429–444. [Google Scholar] [CrossRef]
- Smits, A.J.; Vink, A.; Tolenaars, G.; Herder, G.J.; Kummer, J.A. Different Cutoff Values for Thyroid Transcription Factor-1 Antibodies in the Diagnosis of Lung Adenocarcinoma. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 416–421. [Google Scholar] [CrossRef]
- Klebe, S.; Swalling, A.; Jonavicius, L.; Henderson, D.W. An immunohistochemical comparison of two TTF-1 monoclonal antibodies in atypical squamous lesions and sarcomatoid carcinoma of the lung, and pleural malignant mesothelioma*. J. Clin. Pathol. 2016, 69, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.A.; Teruya-Feldstein, J.; Westra, W.H.; Pelosi, G.; Travis, W.D.; Rekhtman, N. p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod. Pathol. 2012, 25, 405–415. [Google Scholar] [CrossRef]
- Pelosi, G.; Rossi, G.; Cavazza, A.; Righi, L.; Maisonneuve, P.; Barbareschi, M.; Graziano, P.; Pastorino, U.; Garassino, M.; de Braud, F.; et al. DeltaNp63 (p40) distribution inside lung cancer: A driver biomarker approach to tumor characterization. Int. J. Surg. Pathol. 2013, 21, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Butnor, K.J.; Burchette, J.L. p40 (DeltaNp63) and keratin 34betaE12 provide greater diagnostic accuracy than p63 in the evaluation of small cell lung carcinoma in small biopsy samples. Hum. Pathol. 2013, 44, 1479–1486. [Google Scholar] [CrossRef]
- Goto, Y.; De Silva, M.; Toscani, A.; Prabhakar, B.; Notkins, A.; Lan, M. A novel human insulinoma-associated cDNA, IA-1, encodes a protein with “zinc-finger” DNA-binding motifs. J. Biol. Chem. 1992, 267, 15252–15257. [Google Scholar] [CrossRef]
- Jia, S.; Wildner, H.; Birchmeier, C. Insm1 controls the differentiation of pulmonary neuroendocrine cells by repressing Hes1. Dev. Biol. 2015, 408, 90–98. [Google Scholar] [CrossRef]
- Fujino, K.; Motooka, Y.; Hassan, W.; Abdalla, M.O.; Sato, Y.; Kudoh, S.; Hasegawa, K.; Niimori-Kita, K.; Kobayashi, H.; Kubota, I.; et al. Insulinoma-Associated Protein 1 Is a Crucial Regulator of Neuroendocrine Differentiation in Lung Cancer. Am. J. Pathol. 2015, 185, 3164–3177. [Google Scholar] [CrossRef]
- Ishii, J.; Sato, H.; Sakaeda, M.; Shishido-Hara, Y.; Hiramatsu, C.; Kamma, H.; Shimoyamada, H.; Fujiwara, M.; Endo, T.; Aoki, I.; et al. POU domain transcription factor BRN2 is crucial for expression of ASCL1, ND1 and neuroendocrine marker molecules and cell growth in small cell lung cancer. Pathol. Int. 2013, 63, 158–168. [Google Scholar] [CrossRef]
- Borromeo, M.D.; Savage, T.K.; Kollipara, R.K.; He, M.; Augustyn, A.; Osborne, J.K.; Girard, L.; Minna, J.D.; Gazdar, A.F.; Cobb, M.; et al. ASCL1 and NEUROD1 Reveal Heterogeneity in Pulmonary Neuroendocrine Tumors and Regulate Distinct Genetic Programs. Cell Rep. 2016, 16, 1259–1272. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Dermawan, J.K.; Lanigan, C.P.; Farver, C.F. Insulinoma-associated protein 1 (INSM1) is a sensitive and highly specific marker of neuroendocrine differentiation in primary lung neoplasms: An immunohistochemical study of 345 cases, including 292 whole-tissue sections. Mod. Pathol. 2019, 32, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ball, D.W.; Azzoli, C.G.; Baylin, S.B.; Chi, D.; Dou, S.; Donis-Keller, H.; Cumaraswamy, A.; Borges, M.; Nelkin, B.D. Identification of a human achaete-scute homolog highly expressed in neuroendocrine tumors. Proc. Natl. Acad. Sci. USA 1993, 90, 5648–5652. [Google Scholar] [CrossRef]
- Duchrow, M.; Schlüter, C.; Key, G.; Kubbutat, M.H.; Wohlenberg, C.; Flad, H.D.; Gerdes, J. Cell proliferation-associated nuclear antigen defined by antibody Ki-67: A new kind of cell cycle-maintaining proteins. Arch. Immunol. Ther. Exp. 1995, 43, 117–121. [Google Scholar]
- Warth, A.; Cortis, J.; Soltermann, A.; Meister, M.; Budczies, J.; Stenzinger, A.; Goeppert, B.; Thomas, M.; Herth, F.; Schirmacher, P.; et al. Tumour cell proliferation (Ki-67) in non-small cell lung cancer: A critical reappraisal of its prognostic role. Br. J. Cancer 2014, 111, 1222–1229. [Google Scholar] [CrossRef]
- Duchrow, M.; Schluter, C.; Wohlenberg, C.; Flad, H.D.; Gerdes, J. Molecular characterization of the gene locus of the human cell proliferation-associated nuclear protein defined by monoclonal antibody Ki-67. Cell Prolif. 1996, 29, 1–12. [Google Scholar] [CrossRef]
- Yerushalmi, R.; Woods, R.; Ravdin, P.M.; Hayes, M.M.; Gelmon, K.A. Ki67 in breast cancer: Prognostic and predictive potential. Lancet Oncol. 2010, 11, 174–183. [Google Scholar] [CrossRef]
- Wei, D.-M.; Chen, W.-J.; Meng, R.-M.; Zhao, N.; Zhang, X.-Y.; Liao, D.-Y.; Chen, G. Augmented expression of Ki-67 is correlated with clinicopathological characteristics and prognosis for lung cancer patients: An up-dated systematic review and meta-analysis with 108 studies and 14,732 patients. Respir. Res. 2018, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.; Brambilla, E.; Busam, K.J.; De Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, F.; Pan, C.; He, Z.; Pan, X.; Zhu, Q.; Wu, W.; Chen, L. Tumor cell proliferation (Ki-67) expression and its prognostic significance in histological subtypes of lung adenocarcinoma. Lung Cancer 2021, 154, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Aslan, D.L.; Gulbahce, H.E.; Pambuccian, S.E.; Manivel, J.C.; Jessurun, J. Ki-67 immunoreactivity in the differential diagnosis of pulmonary neuroendocrine neoplasms in specimens with extensive crush artifact. Am. J. Clin. Pathol. 2005, 123, 874–878. [Google Scholar] [CrossRef]
- Pelosi, G.; Rindi, G.; Travis, W.D.; Papotti, M. Ki-67 Antigen in Lung Neuroendocrine Tumors: Unraveling a Role in Clinical Practice. J. Thorac. Oncol. 2014, 9, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Jiang, S.-X.; Kameya, T.; Asamura, H.; Sato, Y.; Nagai, K.; Okayasu, I. Divergent cyclin B1 expression and Rb/p16/cyclin D1 pathway aberrations among pulmonary neuroendocrine tumors. Mod. Pathol. 2004, 17, 1259–1267. [Google Scholar] [CrossRef][Green Version]
- Martin, B.; Paesmans, M.; Mascaux, C.; Berghmans, T.; Lothaire, P.; Meert, A.-P.; Lafitte, J.-J.; Sculier, J.-P. Ki-67 expression and patients survival in lung cancer: Systematic review of the literature with meta-analysis. Br. J. Cancer 2004, 91, 2018–2025. [Google Scholar] [CrossRef]
- Haga, Y.; Hiroshima, K.; Iyoda, A.; Shibuya, K.; Shimamura, F.; Iizasa, T.; Fujisawa, T.; Ohwada, H. Ki-67 expression and prognosis for smokers with resected stage i Non–Small cell lung cancer. Ann. Thorac. Surg. 2003, 75, 1727–1732. [Google Scholar] [CrossRef]
- Pelosi, G.; Rodriguez, J.; Viale, G.; Rosai, J. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: A major pitfall in the management of lung cancer patients. Am. J. Surg. Pathol. 2005, 29, 179–187. [Google Scholar] [CrossRef]
- Lin, O.; Olgac, S.; Green, I.; Zakowski, M.F.; Klimstra, D.S. Immunohistochemical staining of cytologic smears with MIB-1 helps distinguish low-grade from high-grade neuroendocrine neoplasms. Am. J. Clin. Pathol. 2003, 120, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Belmont, L.D.; Mitchison, T.J. Identification of a Protein That Interacts with Tubulin Dimers and Increases the Catastrophe Rate of Microtubules. Cell 1996, 84, 623–631. [Google Scholar] [CrossRef]
- Curmi, P.A.; Gavet, O.; Charbaut, E.; Ozon, S.; Lachkar-Colmerauer, S.; Manceau, V.; Siavoshian, S.; Maucuer, A.; Sobel, A. Stathmin and its Phosphoprotein Family. General Properties, Biochemical and Functional Interaction with Tubulin. Cell Struct. Funct. 1999, 24, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Cassimeris, L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr. Opin. Cell Biol. 2002, 14, 18–24. [Google Scholar] [CrossRef]
- Curmi, P.A.; Andersen, S.S.L.; Lachkar, S.; Gavet, O.; Karsenti, E.; Knossow, M.; Sobel, A. The Stathmin/Tubulin Interaction in Vitro. J. Biol. Chem. 1997, 272, 25029–25036. [Google Scholar] [CrossRef]
- Obayashi, S.; Horiguchi, J.; Higuchi, T.; Katayama, A.; Handa, T.; Altan, B.; Bai, T.; Bao, P.; Bao, H.; Yokobori, T.; et al. Stathmin1 expression is associated with aggressive phenotypes and cancer stem cell marker expression in breast cancer patients. Int. J. Oncol. 2017, 51, 781–790. [Google Scholar] [CrossRef]
- Biaoxue, R.; Hua, L.; Wenlong, G.; Shuanying, Y. Overexpression of stathmin promotes metastasis and growth of malignant solid tumors: A systemic review and meta-analysis. Oncotarget 2016, 7, 78994–79007. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Ohtaki, Y.; Altan, B.; Yokobori, T.; Nagashima, T.; Arai, M.; Mogi, A.; Kuwano, H. Prognostic impact of stathmin 1 expression in patients with lung adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2017, 154, 1406–1417.e3. [Google Scholar] [CrossRef]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar]
- Uribarri, M.; Hormaeche, I.; Zalacain, R.; Lopez-Vivanco, G.; Martinez, A.; Nagore, D.; Ruiz-Argüello, M.B. A New Biomarker Panel in Bronchoalveolar Lavage for an Improved Lung Cancer Diagnosis. J. Thorac. Oncol. 2014, 9, 1504–1512. [Google Scholar] [CrossRef]
| TC | AC | LCNEC | SCLC | |
|---|---|---|---|---|
| Mitosis | 0–1 | <2 | >10 | >10 |
| Necrosis | − | ± | + | + |
| NE morphology | + | + | + | + |
| Combined NSCLC | − | − | ± | ± |
| Ki-67 LI | ~5% | ~20% | 40–80% | 50–100% |
| TTF-1 | − | − | +(50%) | +(85%) |
| p40 | − | − | − | − |
| Synaptophysin | +(90–100%) | +(90–100%) | +(80–90%) | +(80–90%) |
| Chromogranin A | +(90–100%) | +(90–100%) | +(80–90%) | +(80–90%) |
| CD56 | +(90–100%) | +(90–100%) | +(80–90%) | +(80–90%) |
| INSM1 | +(90–100%) | +(90–100%) | +(70–80%) | +(80–90%) |
| hASH1 | +(60–70%) | +(60–70%) | +(70–80%) | +(70–80%) |
| Stathmin1 (IHC) | Weak | Not evaluated | Strong (100%) | Strong (100%) |
| Stathmin1 (Modified Allred Score) | <2 | Not evaluated | 8 Strong positive | 8 Strong positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, K.; Shimizu, K.; Ide, S.; Mishima, S.; Matsuoka, S.; Takeda, T.; Eguchi, T.; Hamanaka, K.; Uehara, T. A Novel Strategy for the Diagnosis of Pulmonary High-Grade Neuroendocrine Tumor. Diagnostics 2021, 11, 1945. https://doi.org/10.3390/diagnostics11111945
Miura K, Shimizu K, Ide S, Mishima S, Matsuoka S, Takeda T, Eguchi T, Hamanaka K, Uehara T. A Novel Strategy for the Diagnosis of Pulmonary High-Grade Neuroendocrine Tumor. Diagnostics. 2021; 11(11):1945. https://doi.org/10.3390/diagnostics11111945
Chicago/Turabian StyleMiura, Kentaro, Kimihiro Shimizu, Shogo Ide, Shuji Mishima, Shunichiro Matsuoka, Tetsu Takeda, Takashi Eguchi, Kazutoshi Hamanaka, and Takeshi Uehara. 2021. "A Novel Strategy for the Diagnosis of Pulmonary High-Grade Neuroendocrine Tumor" Diagnostics 11, no. 11: 1945. https://doi.org/10.3390/diagnostics11111945
APA StyleMiura, K., Shimizu, K., Ide, S., Mishima, S., Matsuoka, S., Takeda, T., Eguchi, T., Hamanaka, K., & Uehara, T. (2021). A Novel Strategy for the Diagnosis of Pulmonary High-Grade Neuroendocrine Tumor. Diagnostics, 11(11), 1945. https://doi.org/10.3390/diagnostics11111945








