Klatskin-Mimicking Lesions
Abstract
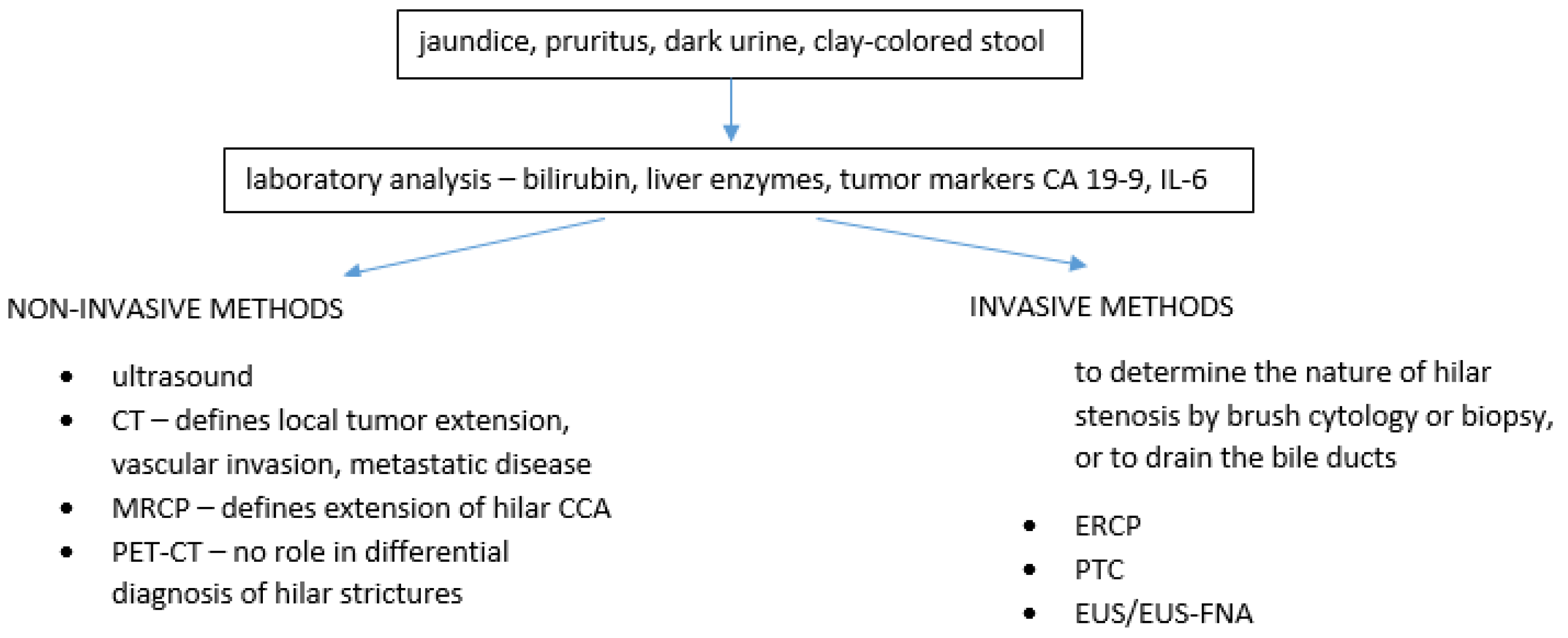
1. Introduction
2. Recurrent Pyogenic Cholangitis
3. Mirizzi Syndrome
4. Stricture in Primary Sclerosing Cholangitis
5. Portal Hypertensive Biliopathy
6. Heterotopic Tissue
7. Ischemic Cholangiopathy
8. Inflammatory-Infiltrative
8.1. Inflammatory Pseudotumor
8.2. IgG4 Sclerosing Cholangitis
8.3. Eosinophilic Cholangiopathy
8.4. Mast Cell Cholangiopathy and Follicular Cholangitis
8.5. Xanthogranulomatous Cholangitis (XGC)
8.6. Sarcoidosis
9. Infective
9.1. Tuberculosis
9.2. AIDS Cholangiopathy
9.3. Other Infective Causes
10. Benign Tumors
10.1. Extrahepatic Biliary Adenomas
10.2. Granular Cell Tumors
10.3. Neurofibroma
10.4. Schwannoma
11. Malignant Tumors
11.1. Intraductal Papillary Neoplasms of the Bile Duct
11.2. Neuroendocrine Tumors
11.3. Lymphoma
11.4. Other Malignancies (Gallbladder Carcinoma, Hepatocellular Carcinoma)
12. Conclusions
Funding
Conflicts of Interest
References
- Gatto, M.; Bragazzi, M.C.; Semeraro, R.; Napoli, C.; Gentile, R.; Torrice, A.; Gaudio, E.; Alvaro, D. Cholangiocarcinoma: Update and future perspectives. Dig. Liver Dis. 2010, 42, 253–260. [Google Scholar] [CrossRef]
- Khan, S.A.; Thomas, H.C.; Davidson, B.R.; Taylor-Robinson, S.D. Cholangiocarcinoma. The Lancet 2005, 366, 1303–1314. [Google Scholar] [CrossRef]
- Cho, M.S.; Kim, S.H.; Park, S.W.; Lim, J.H.; Choi, G.H.; Park, J.S.; Kim, K.S. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J. Gastrointest. Surg. 2012, 16, 1672–1679. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Koea, J.; Holden, A.; Chau, K.; McCall, J. Differential diagnosis of stenosing lesions at the hepatic hilus. World J. Surg. 2004, 28, 466–470. [Google Scholar] [PubMed]
- Paritpokee, N.; Tangkijvanich, P.; Teerasaksilp, S.; Wiwanitkit, V.; Lertmaharit, S.; Tosukhowong, P. Fast liver alkaline phosphatase isoenzyme in diagnosis of malignant biliary obstruction. J. Med Assoc. Thail.= Chotmaihet Thangphaet 1999, 82, 1241–1246. [Google Scholar]
- Heimbach, J.K.; Sanchez, W.; Rosen, C.B.; Gores, G.J. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB 2011, 13, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Szklaruk, J.; Tamm, E.; Charnsangavej, C. Preoperative imaging of biliary tract cancers. Surg. Oncol. Clin. 2002, 11, 865–876. [Google Scholar] [CrossRef]
- Li, J.; Kuehl, H.; Grabellus, F.; Müller, S.P.; Radunz, S.; Antoch, G.; Nadalin, S.; Broelsch, C.E.; Gerken, G.; Paul, A.; et al. Preoperative assessment of hilar cholangiocarcinoma by dual-modality PET/CT. J. Surg. Oncol. 2008, 98, 438–443. [Google Scholar] [CrossRef]
- Lazaridis, K.N.; Gores, G.J. Cholangiocarcinoma. Gastroenterology 2005, 128, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Fritscherravens, A.; Broering, D.C.; Knoefel, W.T.; Rogiers, X.; Swain, P.; Thonke, F.; Bobrowski, C.; Topalidis, T.; Soehendra, N. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am. J. Gastroenterol. 2004, 99, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Are, C.; Gonen, M.; D’Angelica, M.; DeMatteo, R.P.; Fong, Y.; Blumgart, L.H.; Jarnagin, W.R. Differential diagnosis of proximal biliary obstruction. Surgery 2006, 140, 756–763. [Google Scholar] [CrossRef]
- Uhlmann, D.; Wiedmann, M.; Schmidt, F.; Kluge, R.; Tannapfel, A.; Berr, F.; Hauss, J.; Witzigmann, H. Management and outcome in patients with Klatskin-mimicking lesions of the biliary tree. J. Gastrointest. Surg. 2006, 10, 1144–1150. [Google Scholar] [CrossRef]
- Saluja, S.S.; Sharma, R.; Pal, S.; Sahni, P.; Chattopadhyay, T.K. Differentiation between benign and malignant hilar obstructions using laboratory and radiological investigations: A prospective study. HPB 2007, 9, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Kloek, J.J.; van Delden, O.M.; Erdogan, D.; ten Kate, F.J.; Rauws, E.A.; Busch, O.R.; Gouma, D.J.; van Gulik, T.M. Differentiation of malignant and benign proximal bile duct strictures: The diagnostic dilemma. World J. Gastroenterol. 2008, 14, 5032. [Google Scholar] [CrossRef]
- Erdogan, D.E.; Kloek, J.J.; Ten Kate, F.J.W.; Rauws, E.A.J.; Busch, O.R.C.; Gouma, D.J.; van Gulik, T.M. Immunoglobulin G4-related sclerosing cholangitis in patients resected for presumed malignant bile duct strictures. J. Br. Surg. 2008, 95, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Altman, A.; Zangan, S.M. Benign biliary strictures. In Seminars in Interventional Radiology; Thieme Medical Publishers: New York, NY, USA, 2016; Volume 33, pp. 297–306. [Google Scholar]
- Kwan KE, L.; Shelat, V.G.; Tan, C.H. Recurrent pyogenic cholangitis: A review of imaging findings and clinical management. Abdom. Radiol. 2017, 42, 46–56. [Google Scholar] [CrossRef]
- Kaufman, H.S.; Magnuson, T.H.; Lillemoe, K.D.; Frasca, P.; Pitt, H.A. The role of bacteria in gallbladder and common duct stone formation. Ann. Surg. 1989, 209, 584. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kobayashi, A.; Ohto, M.; Tsuchiya, Y.; Saisho, H.; Kimura, K.; Ono, T.; Okuda, K. Bacteriological study of transhepatically aspirated bile. Dig. Dis. Sci. 1985, 29, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.W.; Feintuch, T.A.; Caine, W.P., Jr. Eosinophilic cholangitis, lymphadenopathy, and peripheral eosinophilia: A case report. Am. J. Gastroenterol. (Springer Nat.) 1985, 80, 572–574. [Google Scholar]
- Aljiffry, M.; Renfrew, P.D.; Walsh, M.J.; Laryea, M.; Molinari, M. Analytical review of diagnosis and treatment strategies for dominant bile duct strictures in patients with primary sclerosing cholangitis. HPB 2011, 13, 79–90. [Google Scholar] [CrossRef][Green Version]
- Dhiman, R.K.; Behera, A.; Chawla, Y.K.; Dilawari, J.B.; Suri, S. Portal hypertensive biliopathy. Gut 2007, 56, 1001–1008. [Google Scholar] [CrossRef]
- Dilek, F.H.; Karasu, Ş.; Dilek, O.N. Heterotopic chondroid tissue of the main bile duct mimicking Klatskin tumor: Case report and review of the literature. Clin. J. Gastroenterol. 2019, 12, 205–208. [Google Scholar] [CrossRef]
- Deltenre, P.; Valla, D.C. Ischemic cholangiopathy. Semin. Liver Dis. 2008, 28, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Patnana, M.; Sevrukov, A.B.; Elsayes, K.M.; Viswanathan, C.; Lubner, M.; Menias, C.O. Inflammatory pseudotumor: The great mimicker. Am. J. Roentgenol. 2012, 198, W217–W227. [Google Scholar] [CrossRef] [PubMed]
- Faraj, W.; Ajouz, H.; Mukherji, D.; Kealy, G.; Shamseddine, A.; Khalife, M. Inflammatory pseudo-tumor of the liver: A rare pathological entity. World J. Surg. Oncol. 2011, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Abdalian, R.; Heathcote, E.J. Sclerosing cholangitis: A focus on secondary causes. Hepatology 2006, 44, 1063–1074. [Google Scholar] [CrossRef]
- Amankonah, T.D.; Strom, C.B.; Vierling, J.M.; Petrovic, L.M. Inflammatory pseudotumor of the liver as the first manifestation of Crohn's disease. Am. J. Gastroenterol. 2001, 96, 2520. [Google Scholar] [CrossRef]
- Khosroshahi, A.; Stone, J.H. A clinical overview of IgG4-related systemic disease. Curr. Opin. Rheumatol. 2011, 23, 57–66. [Google Scholar] [CrossRef]
- Ghazale, A.; Chari, S.T.; Zhang, L.; Smyrk, T.C.; Takahashi, N.; Levy, M.J.; Topazian, M.D.; Clain, J.E.; Pearson, R.K.; Petersen, B.T.; et al. Immunoglobulin G4–associated cholangitis: Clinical profile and response to therapy. Gastroenterology 2008, 134, 706–715. [Google Scholar] [CrossRef]
- Leegaard, M. Eosinophilic cholecystitis. Acta Chir. Scand. 1980, 146, 295–296. [Google Scholar] [PubMed]
- Aoki, T.; Kubota, K.; Oka, T.; Hasegawa, K.; Hirai, I.; Makuuchi, M. Follicular cholangitis: Another cause of benign biliary stricture. Hepato-Gastroenterol. 2003, 50, 639–642. [Google Scholar]
- Lee, J.Y.; Lim, J.H.; Lim, H.K. Follicular cholangitis mimicking hilar cholangiocarcinoma. Abdom. Imaging 2005, 30, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Kojima, M.; Gotohda, N.; Takahashi, S.; Nakagohri, T.; Konishi, M.; Ochiai, A.; Kinoshita, T. Incidence, clinical presentation and pathological features of benign sclerosing cholangitis of unknown origin masquerading as biliary carcinoma. J. Hepato-Biliary-Pancreat. Sci. 2010, 17, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Baron, T.H.; Koehler, R.E.; Rodgers, W.H.; Fallon, M.B.; Ferguson, S.M. Mast cell cholangiopathy: Another cause of sclerosing cholangitis. Gastroenterology 1995, 109, 1677–1681. [Google Scholar] [CrossRef]
- Zen, Y.; Ishikawa, A.; Ogiso, S.; Heaton, N.; Portmann, B. Follicular cholangitis and pancreatitis–clinicopathological features and differential diagnosis of an under-recognized entity. Histopathology 2012, 60, 261–269. [Google Scholar] [CrossRef]
- Guzman-Valdivia, G. Xanthogranulomatous cholecystitis: 15 years’ experience. World J. Surg. 2004, 28, 254–257. [Google Scholar] [CrossRef]
- Iannuzzi, M.C.; Rybicki, B.A.; Teirstein, A.S. Sarcoidosis. N. Engl. J. Med. 2007, 357, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Ebert, E.C.; Kierson, M.; Hagspiel, K.D. Gastrointestinal and hepatic manifestations of sarcoidosis. Off. J. Am. Coll. Gastroenterol.|ACG 2008, 103, 3184–3192. [Google Scholar] [CrossRef]
- Petersen, J.M. Klatskin-like biliary sarcoidosis: A cholangioscopic diagnosis. Gastroenterol. Hepatol. 2009, 5, 137–140. [Google Scholar]
- Saluja, S.S.; Ray, S.; Pal, S.; Kukeraja, M.; Srivastava, D.N.; Sahni, P.; Chattopadhyay, T.K. Hepatobiliary and pancreatic tuberculosis: A two decade experience. BMC Surg. 2007, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chong, V.H.; Telisinghe, P.U.; Yapp, S.K.; Jalihal, A. Biliary strictures secondary to tuberculosis and early ampullary carcinoma. Singap. Med. J. 2009, 50, e94–e96. [Google Scholar]
- Margulis, S.J.; Honig, C.L.; Soave, R.; Govoni, A.F.; Mouradian, J.A.; Jacobson, I.M. Biliary tract obstruction in the acquired immunodeficiency syndrome. Ann. Intern. Med. 1986, 105, 207–210. [Google Scholar] [CrossRef]
- Cello, J.P. Acquired immunodeficiency syndrome cholangiopathy: Spectrum of disease. Am. J. Med. 1989, 86, 539–546. [Google Scholar] [CrossRef]
- Urushihara, N.; Ariki, N.; Oyama, T.; Chouda, Y.; Yagi, T.; Inoue, T.; Tomiyama, Y.; Nishiuchi, R.; Oda, M.; Tanaka, N. Secondary sclerosing cholangitis and portal hypertension after O157 enterocolitis: Extremely rare complications of hemolytic uremic syndrome. J. Pediatric Surg. 2001, 36, 1838–1840. [Google Scholar] [CrossRef]
- Bansal, M.; Agarwal, A.; Bariola, R.; Aduli, F.; Govindarajan, R. Biliary actinomycosis mimicking a klatskin tumor. J. Gastrointest. Cancer 2012, 43, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Scheppach, W.; Druge, G.; Wittenberg, G.; Mueller, J.G.; Gassel, A.M.; Gassel, H.J.; Richter, F. Sclerosing cholangitis and liver cirrhosis after extrabiliary infections: Report on three cases. Crit. Care Med. 2001, 29, 438–441. [Google Scholar] [CrossRef]
- Li, K.W.; Wen, T.F.; Li, G.D. Hepatic mucormycosis mimicking hilar cholangiocarcinoma: A case report and literature review. World J. Gastroenterol. 2010, 16, 1039. [Google Scholar] [CrossRef]
- Kim, B.G.; Kang, D.H.; Choi, C.W.; Kim, H.W.; Lee, J.H.; Kim, S.H.; Yeo, H.J.; Lee, S.Y. A case of clonorchiasis with focal intrahepatic duct dilatation mimicking an intrahepatic cholangiocarcinoma. Clin. Endosc. 2011, 44, 55. [Google Scholar] [CrossRef]
- Hai, S.; Yamamoto, S.; Tanaka, H.; Takemura, S.; Ichikawa, T.; Kodai, S.; Shinkawa, H.; Yamamoto, T.; Kubo, S. Adenomyoma of the common hepatic duct mimicking bile duct cancer: Report of a case. Surg. Today 2007, 37, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Linehan, D.C.; Jarnagin, W.R.; Blumgart, L.H. Benign tumors and pseudotumors of the biliary tract. In Surgery of the Liver, Biliary Tract and Pancreas; WB Saunders: Philadelphia, PA, USA, 2007; pp. 751–763. [Google Scholar]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. Tumours of the gallbladder and extrahepatic bile ducts. WHO Classif. Tumours Dig. Syst. 2010, 3, 264–272. [Google Scholar]
- Vasiliadis, K.; Ioannidis, O.; Tsalis, K. Benign Tumours and Pseudotumours Within the Porta Hepatis Masquerading as Perihilar Cholangiocarcinoma. Nezhoubné nádory a pseudotumory v porta hepatis maskující perihilární cholangiokarcinom. Klin. Onkol. 2019, 32, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Loh, A.; Kamar, S.; Dickson, G.H. Solitary benign papilloma (papillary adenoma) of the cystic duct: A rare cause of biliary colic. Br. J. Clin. Pract. 1994, 48, 167–168. [Google Scholar] [PubMed]
- Dowdy, G.S.; Olin, W.G.; Shelton, E.L.; Waldron, G.W. Benign tumors of the extrahepatic bile ducts: Report of three cases and review of the literature. Arch. Surg. 1962, 85, 503–513. [Google Scholar] [CrossRef]
- Munshi, A.G.; Hassan, M.A. Common bile duct adenoma: Case report and brief review of literature. Surg. Laparosc. Endosc. Percutaneous Tech. 2010, 20, e193–e194. [Google Scholar] [CrossRef]
- te Boekhorst, D.S.; Gerhards, M.F.; van Gulik, T.M.; Gouma, D.J. Granular cell tumor at the hepatic duct confluence mimicking Klatskin tumor. Dig. Surg. 2000, 17, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.W.; Goldblum, J.R. Benign tumors of peripheral nerves. In Enzinger and Weiss’s Soft tissue Tumors; Mosby Elsevier: Maryland Heights, MO, USA, 2008; pp. 878–887. [Google Scholar]
- Peyré, C.G.; Wakim, M.; Mateo, R.; Genyk, Y. Unusual cases of jaundice secondary to non-neoplastic bile duct obstruction. Am. Surg. 2004, 70, 620. [Google Scholar]
- Li, F.Y.; Cheng, J.Q.; He, S.; Li, N.; Zhang, M.M.; Zhang, X.L.; Jiang, L.S.; Cheng, N.S.; Xiong, X.Z. Primary neurofibroma of the common bile duct as an unusual cause of obstructive jaundice: A case report. Dig. Dis. Sci. 2005, 50, 1166–1168. [Google Scholar] [CrossRef]
- De Rosa, A.; Gomez, D.; Zaitoun, A.M.; Cameron, I.C. Neurofibroma of the bile duct: A rare cause of obstructive jaundice. Ann. R. Coll. Surg. Engl. 2013, 95, e14–e16. [Google Scholar] [CrossRef] [PubMed]
- Martuza, R.L.; Eldridge, R. Neurofibromatosis 2. New Engl. J. Med. 1988, 318, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Lee, S.S.; Ahn, G.H. Schwannomas of the gastrointestinal tract: Clinicopathological features of 12 cases including a case of esophageal tumor compared with those of gastrointestinal stromal tumors and leiomyomas of the gastrointestinal tract. Pathol. -Res. Pract. 2002, 198, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Joo, K.R.; Chae, M.J.; Jang, J.Y.; Lee, S.G.; Dong, S.H.; Kim, H.J.; Kim, B.H.; Chang, Y.W.; Lee, J.I.; et al. Extrahepatic biliary schwannomas: A case report. J. Korean Med Sci. 2007, 22, 549–552. [Google Scholar] [CrossRef]
- Fonseca, G.M.; Montagnini, A.L.; Rocha, M.S.; Patzina, R.A.; Bernardes MV, A.A.; Cecconello, I.; Jukemura, J. Biliary tract schwannoma: A rare cause of obstructive jaundice in a young patient. World J. Gastroenterol. 2012, 18, 5305. [Google Scholar] [PubMed]
- Fenoglio, L.; Severini, S.; Cena, P.; Migliore, E.; Bracco, C.; Pomero, F.; Panzone, S.; Cavallero, G.B.; Silvestri, A.; Brizio, R.; et al. Common bile duct schwannoma: A case report and review of literature. World J. Gastroenterol. 2007, 13, 1275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ohtsuka, M.; Shimizu, H.; Kato, A.; Yoshitomi, H.; Furukawa, K.; Tsuyuguchi, T.; Sakai, Y.; Yokosuka, O.; Miyazaki, M. Intraductal papillary neoplasms of the bile duct. Int. J. Hepatol. 2014, 2014. [Google Scholar] [CrossRef]
- Kütting, F.; Schmidt, M.; Waldschmidt, D.; Curth, H.; Schramm, C.; Steffen, H.M. Neuroendocrine carcinoma of the gallbladder masquerading as a klatskin tumor in a 74-year-old male. J. Gastrointest. Cancer 2016, 47, 118–122. [Google Scholar] [CrossRef]
- Capella, C.; Solcia, E.; Sobin, L.H.; Arnold, R. Endocrine tumours of the gallbladder and extrahepatic bile ducts. In Pathology & Genetics. Tumours of the Digestive System. World Health Organization Classification of Tumours, 3rd ed.; Hamilton, R., Aaltonen, L.A., Eds.; IARC Press: Lyon, France, 2000; pp. 214–266. [Google Scholar]
- Martignoni, M.E.; Friess, H.; Lübke, D.; Uhl, W.; Maurer, C.; Müller, M.; Richard, H.P.; Reubi, J.C.; Büchler, M.W. Study of a primary gastrinoma in the common hepatic duct–a case report. Digestion 1999, 60, 187–190. [Google Scholar] [CrossRef]
- Tsalis, K.; Vrakas, G.; Geroukis, T.; Cheva, A.; Roidos, G.N.; Lazarides, C. Primary neuroendocrine tumor of the extrahepatic biliary tree mimicking Klatskin tumor. J. Gastrointest. Liver Dis. 2010, 19, 341–342. [Google Scholar]
- Hubert, C.; Sempoux, C.; Berquin, A.; Deprez, P.; Jamar, F.; Gigot, J.F. Bile duct carcinoid tumors: An uncommon disease but with a good prognosis? Hepato-Gastroenterol. 2005, 52, 1042–1047. [Google Scholar]
- Malecki, E.A.; Acosta, R.; Twaddell, W.; Heller, T.; Manning, M.A. Endoscopic diagnosis of a biliary neuroendocrine tumor. Gastrointest. Endosc. (Print) 2009, 70, 1275–1276. [Google Scholar] [CrossRef]
- Chamberlain, R.S.; Blumgart, L.H. Carcinoid tumors of the extrahepatic bile duct: A rare cause of malignant biliary obstruction. Cancer 1999, 86, 1959–1965. [Google Scholar] [CrossRef]
- Maitra, A.; Krueger, J.E.; Tascilar, M.; Offerhaus, G.J.A.; Angeles, A.; Klimstra, D.S.; Hruban, R.H.; Albores, J. Carcinoid tumors of the extrahepatic bile ducts: A study of seven cases. Am. J. Surg. Pathol. 2000, 24, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, A.; Al-Obeidi, S.; Daradkeh, S. Primary non-Hodgkin's lymphoma of the common bile duct: A case report and literature review. Asian J. Surg. 2017, 40, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Mikail, C.; Sefa, T.; Tamer, K.; Anil, S.O.; Gulcin, Y. Primary extrahepatic bile duct lymphoma mimicking Klatskin’s tumor, dramatic response to chemotherapy. Int. J. Surg. Case Rep. 2015, 8, 147–149. [Google Scholar] [CrossRef][Green Version]
- Senthil Kumar, M.P.; Marudanayagam, R. Klatskin-like lesions. HPB Surg. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Uribe, J.; Chai, L.; Xiao, G. Hepatocellular carcinoma invading bile ducts presenting as a klatskin tumor. HPB 2018, 20, S157. [Google Scholar] [CrossRef][Green Version]
- Urasaki, T.; Kodaira, M.; Hibino, M.; Yamagata, S.; Watanabe, Y.; Terazawa, Y.; Sano, M.; Kuriki, K. Poorly Differentiated Gastric Adenocarcinoma Can Mimic Hilar Cholangiocarcinoma. Intern. Med. 2016, 55, 1559–1564. [Google Scholar] [CrossRef][Green Version]
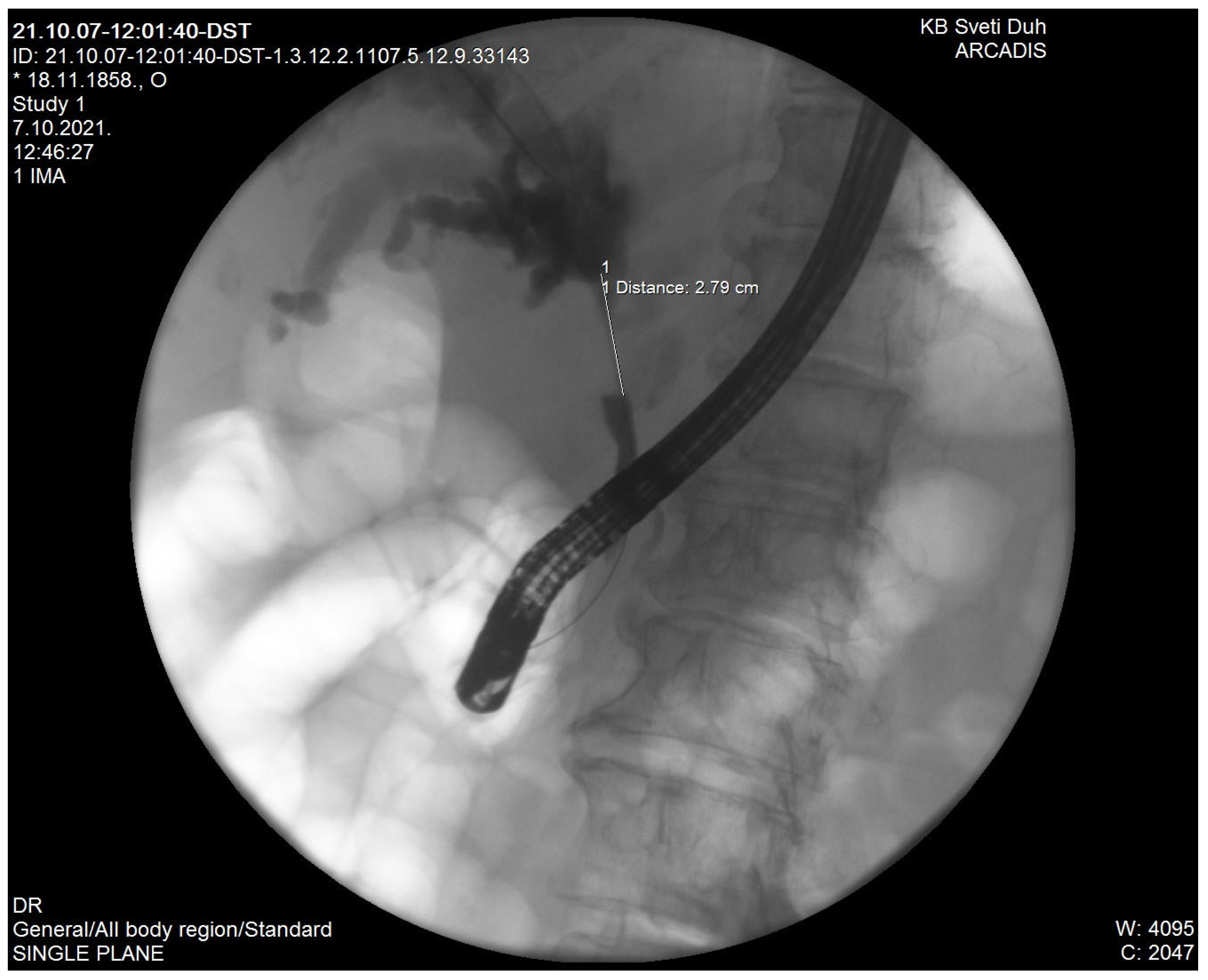
| Recurrent Pyogenic Cholangitis |
| Mirizzi syndrome |
| Stricture in primary sclerosing cholangitis |
| Portal hypertensive biliopathy |
| Heterotopic tissue |
| Ischemic cholangiopathy |
| Inflammatory-infiltrative Inflammatory pseudotumor IgG4 sclerosing cholangitis Eosinophilic cholangiopathy Mast cell cholangiopathy Follicular cholangitis Xanthogranulomatous cholangitis (XGC) Sarcoidosis |
| Infective Tuberculosis AIDS cholangiopathy Other infective causes |
| Benign tumors Extrahepatic biliary adenomas Granular cell tumors Neurofibroma Schwannoma |
| Malignant tumors Intraductal papillary neoplasms of the bile duct Neuroendocrine tumors Lymphoma Other malignancies (gallbladder carcinoma, hepatocellular carcinoma) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marušić, M.; Paić, M.; Knobloch, M.; Vodanović, M. Klatskin-Mimicking Lesions. Diagnostics 2021, 11, 1944. https://doi.org/10.3390/diagnostics11111944
Marušić M, Paić M, Knobloch M, Vodanović M. Klatskin-Mimicking Lesions. Diagnostics. 2021; 11(11):1944. https://doi.org/10.3390/diagnostics11111944
Chicago/Turabian StyleMarušić, Marinko, Matej Paić, Mia Knobloch, and Marko Vodanović. 2021. "Klatskin-Mimicking Lesions" Diagnostics 11, no. 11: 1944. https://doi.org/10.3390/diagnostics11111944
APA StyleMarušić, M., Paić, M., Knobloch, M., & Vodanović, M. (2021). Klatskin-Mimicking Lesions. Diagnostics, 11(11), 1944. https://doi.org/10.3390/diagnostics11111944






