Association of Cervical and Lumbar Paraspinal Muscle Composition Using Texture Analysis of MR-Based Proton Density Fat Fraction Maps
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Magnetic Resonance Imaging
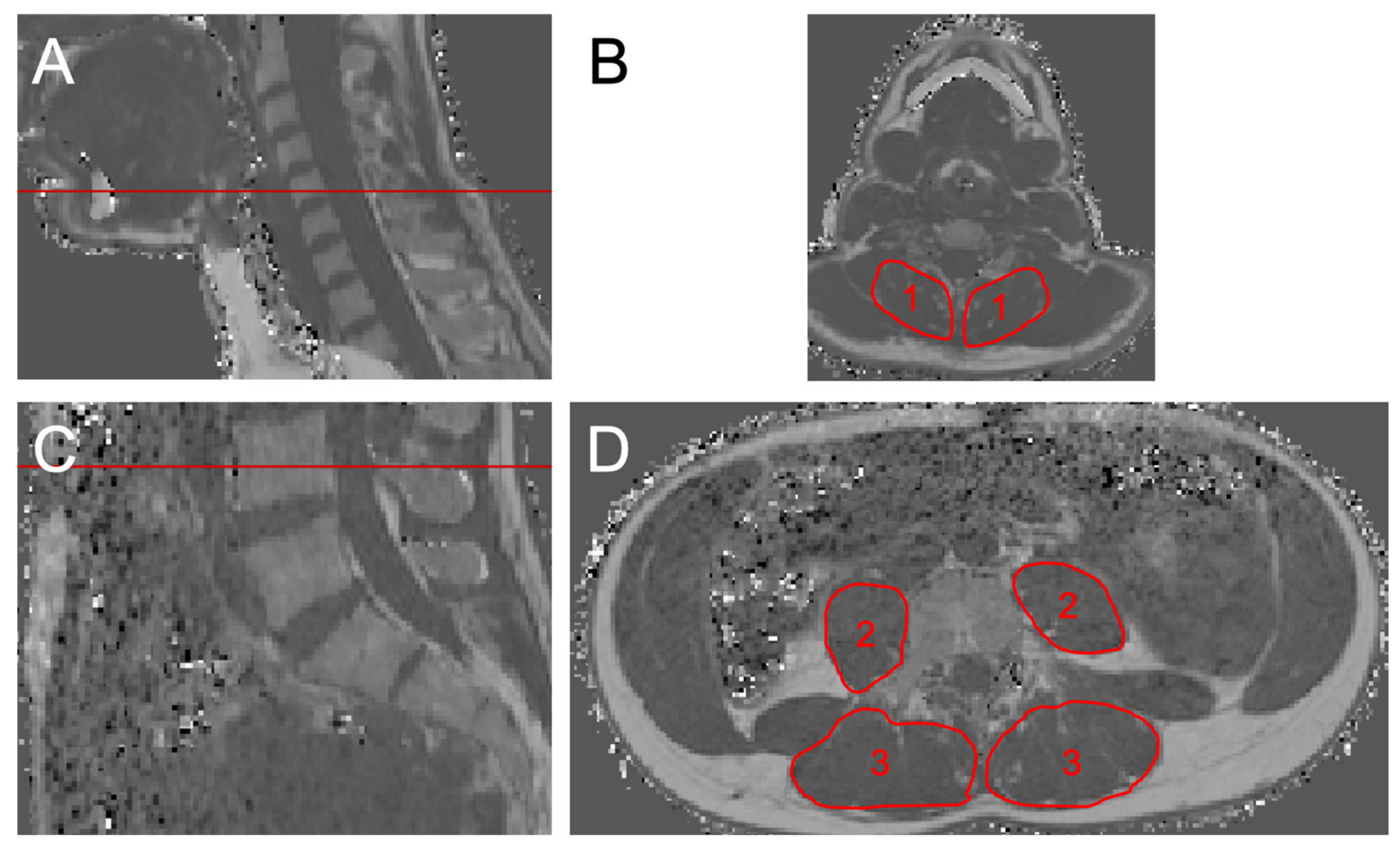
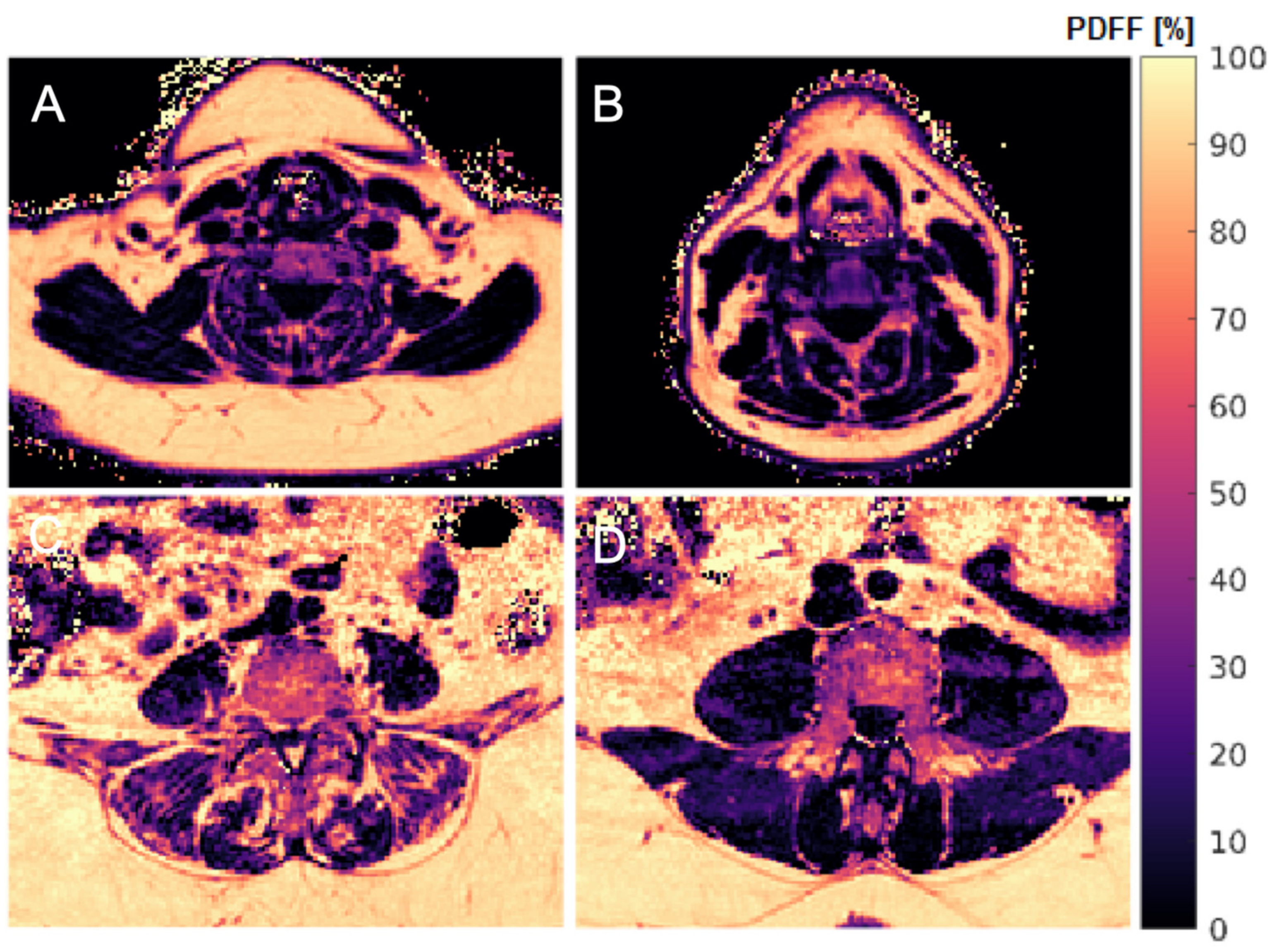
2.3. Muscle Fat Quantification
2.4. Texture Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Gender-Specific Results
3.3. Muscle-Specific Results
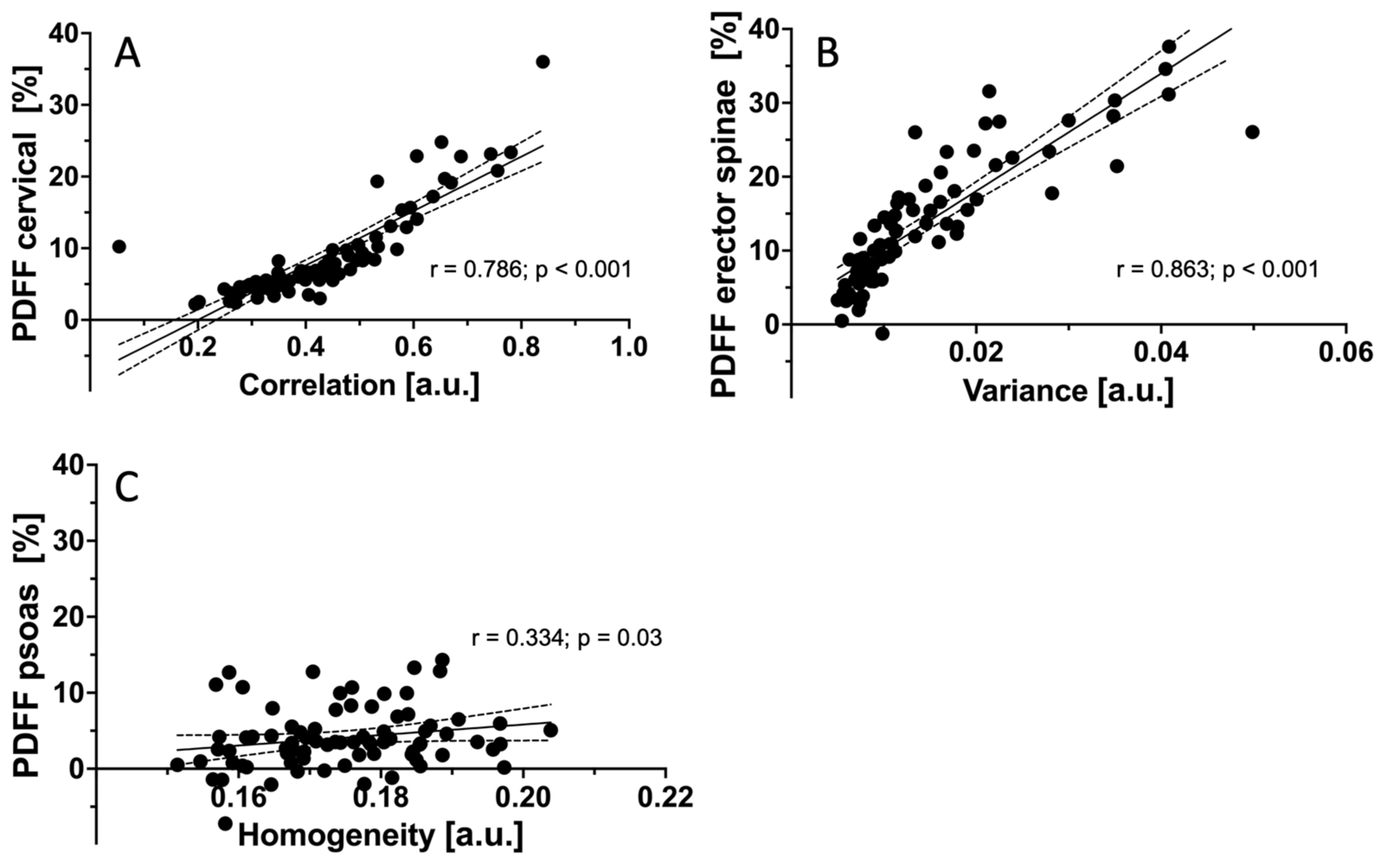
3.4. Correlations of PDFF Measurements and Texture Features between Muscle Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Global Texture Features
Appendix A.2. Second-Order Texture Features
References
- Burian, E.; Syvari, J.; Holzapfel, C.; Drabsch, T.; Kirschke, J.S.; Rummeny, E.J.; Zimmer, C.; Hauner, H.; Karampinos, D.C.; Baum, T.; et al. Gender- and Age-Related Changes in Trunk Muscle Composition Using Chemical Shift Encoding-Based Water(-)Fat MRI. Nutrients 2018, 10, 1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burian, E.; Franz, D.; Greve, T.; Dieckmeyer, M.; Holzapfel, C.; Drabsch, T.; Sollmann, N.; Probst, M.; Kirschke, J.S.; Rummeny, E.J.; et al. Age- and gender-related variations of cervical muscle composition using chemical shift encoding-based water-fat MRI. Eur. J. Radiol. 2020, 125, 108904. [Google Scholar] [CrossRef] [PubMed]
- Le Cara, E.C.; Marcus, R.L.; Dempsey, A.R.; Hoffman, M.D.; Hebert, J.J. Morphology versus function: The relationship between lumbar multifidus intramuscular adipose tissue and muscle function among patients with low back pain. Arch. Phys. Med. Rehabil. 2014, 95, 1846–1852. [Google Scholar] [CrossRef] [Green Version]
- Marcus, R.L.; Addison, O.; Dibble, L.E.; Foreman, K.B.; Morrell, G.; Lastayo, P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J. Aging Res. 2012, 2012, 629637. [Google Scholar] [CrossRef]
- Tuttle, L.J.; Sinacore, D.R.; Mueller, M.J. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J. Aging Res. 2012, 2012, 172957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlaeger, S.; Inhuber, S.; Rohrmeier, A.; Dieckmeyer, M.; Freitag, F.; Klupp, E.; Weidlich, D.; Feuerriegel, G.; Kreuzpointner, F.; Schwirtz, A.; et al. Association of paraspinal muscle water-fat MRI-based measurements with isometric strength measurements. Eur. Radiol. 2019, 29, 599–608. [Google Scholar] [CrossRef]
- Bachettini, N.P.; Bielemann, R.M.; Barbosa-Silva, T.G.; Menezes, A.M.B.; Tomasi, E.; Gonzalez, M.C. Sarcopenia as a mortality predictor in community-dwelling older adults: A comparison of the diagnostic criteria of the European Working Group on Sarcopenia in Older People. Eur. J. Clin. Nutr. 2020, 74, 573–580. [Google Scholar] [CrossRef]
- Peng, Y.C.; Wu, C.H.; Tien, Y.W.; Lu, T.P.; Wang, Y.H.; Chen, B.B. Preoperative sarcopenia is associated with poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy. Eur. Radiol. 2021, 31, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Baum, T.; Cordes, C.; Dieckmeyer, M.; Ruschke, S.; Franz, D.; Hauner, H.; Kirschke, J.S.; Karampinos, D.C. MR-based assessment of body fat distribution and characteristics. Eur. J. Radiol. 2016, 85, 1512–1518. [Google Scholar] [CrossRef]
- Hu, H.H.; Kan, H.E. Quantitative proton MR techniques for measuring fat. NMR Biomed. 2013, 26, 1609–1629. [Google Scholar] [CrossRef] [Green Version]
- Azzabou, N.; Hogrel, J.Y.; Carlier, P.G. NMR based biomarkers to study age-related changes in the human quadriceps. Exp. Gerontol. 2015, 70, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Barnouin, Y.; Butler-Browne, G.; Voit, T.; Reversat, D.; Azzabou, N.; Leroux, G.; Behin, A.; McPhee, J.S.; Carlier, P.G.; Hogrel, J.Y. Manual segmentation of individual muscles of the quadriceps femoris using MRI: A reappraisal. J. Magn. Reson. Imaging 2014, 40, 239–247. [Google Scholar] [CrossRef]
- Fortin, M.; Videman, T.; Gibbons, L.E.; Battie, M.C. Paraspinal muscle morphology and composition: A 15-yr longitudinal magnetic resonance imaging study. Med. Sci. Sports Exerc. 2014, 46, 893–901. [Google Scholar] [CrossRef]
- Dahlqvist, J.R.; Vissing, C.R.; Hedermann, G.; Thomsen, C.; Vissing, J. Fat Replacement of Paraspinal Muscles with Aging in Healthy Adults. Med. Sci. Sports Exerc. 2017, 49, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.J.; Filli, L.; Elliott, J.M.; Nanz, D.; Fischer, M.A.; Marcon, M.; Ulbrich, E.J. Age- and Level-Dependence of Fatty Infiltration in Lumbar Paravertebral Muscles of Healthy Volunteers. AJNR Am. J. Neuroradiol. 2016, 37, 742–748. [Google Scholar] [CrossRef] [Green Version]
- Valentin, S.; Licka, T.; Elliott, J. Age and side-related morphometric MRI evaluation of trunk muscles in people without back pain. Man. Ther. 2015, 20, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Marcon, M.; Ciritsis, B.; Laux, C.; Nanz, D.; Fischer, M.A.; Andreisek, G.; Ulbrich, E.J. Quantitative and qualitative MR-imaging assessment of vastus medialis muscle volume loss in asymptomatic patients after anterior cruciate ligament reconstruction. J. Magn. Reson. Imaging 2015, 42, 515–525. [Google Scholar] [CrossRef]
- Burian, E.; Subburaj, K.; Mookiah, M.R.K.; Rohrmeier, A.; Hedderich, D.M.; Dieckmeyer, M.; Diefenbach, M.N.; Ruschke, S.; Rummeny, E.J.; Zimmer, C.; et al. Texture analysis of vertebral bone marrow using chemical shift encoding-based water-fat MRI: A feasibility study. Osteoporos. Int. 2019, 30, 1265–1274. [Google Scholar] [CrossRef] [Green Version]
- Dieckmeyer, M.; Inhuber, S.; Schlager, S.; Weidlich, D.; Mookiah, M.R.K.; Subburaj, K.; Burian, E.; Sollmann, N.; Kirschke, J.S.; Karampinos, D.C.; et al. Association of Thigh Muscle Strength with Texture Features Based on Proton Density Fat Fraction Maps Derived from Chemical Shift Encoding-Based Water-Fat MRI. Diagnostics 2021, 11, 302. [Google Scholar] [CrossRef]
- Greve, T.; Burian, E.; Zoffl, A.; Feuerriegel, G.; Schlaeger, S.; Dieckmeyer, M.; Sollmann, N.; Klupp, E.; Weidlich, D.; Inhuber, S.; et al. Regional variation of thigh muscle fat infiltration in patients with neuromuscular diseases compared to healthy controls. Qantitative Imaging Med. Surg. 2021, 11, 2610–2621. [Google Scholar] [CrossRef] [PubMed]
- Burian, E.; Syvari, J.; Dieckmeyer, M.; Holzapfel, C.; Drabsch, T.; Sollmann, N.; Kirschke, J.S.; Rummeny, E.J.; Zimmer, C.; Hauner, H.; et al. Age- and BMI-related variations of fat distribution in sacral and lumbar bone marrow and their association with local muscle fat content. Sci. Rep. 2020, 10, 9686. [Google Scholar] [CrossRef]
- Drabsch, T.; Holzapfel, C.; Stecher, L.; Petzold, J.; Skurk, T.; Hauner, H. Associations Between C-Reactive Protein, Insulin Sensitivity, and Resting Metabolic Rate in Adults: A Mediator Analysis. Front. Endocrinol. 2018, 9, 556. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Dimitrov, I.; Sherry, A.D.; Malloy, C.R. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J. Lipid Res. 2008, 49, 2055–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karampinos, D.C.; Yu, H.; Shimakawa, A.; Link, T.M.; Majumdar, S. T(1)-corrected fat quantification using chemical shift-based water/fat separation: Application to skeletal muscle. Magn. Reson. Med. 2011, 66, 1312–1326. [Google Scholar] [CrossRef] [Green Version]
- Castellano, G.; Bonilha, L.; Li, L.M.; Cendes, F. Texture analysis of medical images. Clin. Radiol. 2004, 59, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Lubner, M.G.; Smith, A.D.; Sandrasegaran, K.; Sahani, D.V.; Pickhardt, P.J. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. Radiographics 2017, 37, 1483–1503. [Google Scholar] [CrossRef] [PubMed]
- Varghese, B.A.; Cen, S.Y.; Hwang, D.H.; Duddalwar, V.A. Texture Analysis of Imaging: What Radiologists Need to Know. AJR Am. J. Roentgenol. 2019, 212, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Assefa, D.; Keller, H.; Menard, C.; Laperriere, N.; Ferrari, R.J.; Yeung, I. Robust texture features for response monitoring of glioblastoma multiforme on T1-weighted and T2-FLAIR MR images: A preliminary investigation in terms of identification and segmentation. Med. Phys. 2010, 37, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Thibault, G.; Devic, C.; Fertil, B.; Mari, J.; Sequeira, J. Indices de formes: De la 2D vers la 3D-Application au classement de noyaux de cellules. Journées De L’association Francoph. D’informatique Graph. 2007, 17, 17–24. [Google Scholar]
- Freedman, D. On the histogram as a density estimator: L2 theory. Probab. Theory Relat. Fields 1981, 57, 453–476. [Google Scholar]
- Scott, D.W. On optimal and data-based histograms. Biometrika 1979, 66, 605–610. [Google Scholar] [CrossRef]
- Sturges, H.A. The choice of a class interval. J. Am. Stat. Assoc. 1926, 21, 65–66. [Google Scholar] [CrossRef]
- Vallieres, M.; Freeman, C.R.; Skamene, S.R.; El Naqa, I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys. Med. Biol. 2015, 60, 5471–5496. [Google Scholar] [CrossRef]
- Zhou, H.; Vallieres, M.; Bai, H.X.; Su, C.; Tang, H.; Oldridge, D.; Zhang, Z.; Xiao, B.; Liao, W.; Tao, Y.; et al. MRI features predict survival and molecular markers in diffuse lower-grade gliomas. Neuro-Oncol. 2017, 19, 862–870. [Google Scholar] [CrossRef]
- Vallieres, M.; Kay-Rivest, E.; Perrin, L.J.; Liem, X.; Furstoss, C.; Aerts, H.; Khaouam, N.; Nguyen-Tan, P.F.; Wang, C.S.; Sultanem, K.; et al. Radiomics strategies for risk assessment of tumour failure in head-and-neck cancer. Sci. Rep. 2017, 7, 10117. [Google Scholar] [CrossRef] [Green Version]
- Carlier, P.G.; Azzabou, N.; de Sousa, P.L.; Hicks, A.; Boisserie, J.M.; Amadon, A.; Carlier, R.Y.; Wary, C.; Orlikowski, D.; Laforet, P. Skeletal muscle quantitative nuclear magnetic resonance imaging follow-up of adult Pompe patients. J. Inherit. Metab. Dis. 2015, 38, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Dieckmeyer, M.; Inhuber, S.; Schlaeger, S.; Weidlich, D.; Mookiah, M.R.K.; Subburaj, K.; Burian, E.; Sollmann, N.; Kirschke, J.S.; Karampinos, D.C.; et al. Texture Features of Proton Density Fat Fraction Maps from Chemical Shift Encoding-Based MRI Predict Paraspinal Muscle Strength. Diagnostics 2021, 11, 239. [Google Scholar] [CrossRef]
- Locquet, M.; Beaudart, C.; Hajaoui, M.; Petermans, J.; Reginster, J.Y.; Bruyere, O. Three-Year Adverse Health Consequences of Sarcopenia in Community-Dwelling Older Adults According to 5 Diagnosis Definitions. J. Am. Med. Dir. Assoc. 2019, 20, 43–46.e2. [Google Scholar] [CrossRef]
- Filippin, L.I.; Teixeira, V.N.; da Silva, M.P.; Miraglia, F.; da Silva, F.S. Sarcopenia: A predictor of mortality and the need for early diagnosis and intervention. Aging Clin. Exp. Res. 2015, 27, 249–254. [Google Scholar] [CrossRef]
- Patzelt, L.; Junker, D.; Syvari, J.; Burian, E.; Wu, M.; Prokopchuk, O.; Nitsche, U.; Makowski, M.R.; Braren, R.F.; Herzig, S.; et al. MRI-Determined Psoas Muscle Fat Infiltration Correlates with Severity of Weight Loss during Cancer Cachexia. Cancers 2021, 13, 4433. [Google Scholar] [CrossRef] [PubMed]
| Sex | Mean | SD | p | |
|---|---|---|---|---|
| age | men | 43.7 | 24.6 | n.s. |
| women | 37.1 | 15.0 | ||
| BMI | men | 24.1 | 6.5 | n.s. |
| women | 24.2 | 4.8 | ||
| PDFFcervical | men | 7.9 | 7.2 | n.s. |
| women | 9.5 | 6.1 | ||
| PDFFerector spinae | men | 7.4 | 5.6 | <0.001 |
| women | 16.9 | 9.2 | ||
| PDFFpsoas | men | 3.3 | 4.4 | n.s. |
| women | 4.4 | 3.8 | ||
| Variance (global)cervical | men | 69.2 | 8.7 | <0.001 |
| women | 55.0 | 8.4 | ||
| Variance (global)erector spinae | men | 135.2 | 13.9 | <0.001 |
| women | 117.0 | 13.3 | ||
| Variance (global)psoas | men | 98.6 | 13.6 | <0.001 |
| women | 67.1 | 9.8 | ||
| Skewness (global)cervical | men | −0.65 | 0.9 | n.s. |
| women | −0.29 | 0.8 | ||
| Skewness (global)erector spinae | men | 0.13 | 0.9 | 0.005 |
| women | 0.68 | 0.5 | ||
| Skewness (global)psoas | men | −0.68 | 0.3 | n.s. |
| women | −0.50 | 0.5 | ||
| Kurtosis (global)cervical | men | 3.3 | 1.7 | 0.008 |
| women | 2.3 | 1.4 | ||
| Kurtosis (global)erector spinae | men | 3.2 | 0.8 | <0.001 |
| women | 1.9 | 1.6 | ||
| Kurtosis (global)psoas | men | 1.1 | 0.5 | 0.006 |
| women | 1.6 | 0.7 | ||
| Energycervical | men | 0.018 | 0.001 | 0.011 |
| women | 0.011 | 0.001 | ||
| Energyerector spinae | men | 0.001 | 0.0002 | 0.012 |
| women | 0.0008 | 0.0004 | ||
| Energypsoas | men | 0.0004 | 0.0001 | 0.039 |
| women | 0.0005 | 0.0001 | ||
| Contrastcervical | men | 468.3 | 89.4 | n.s. |
| women | 528.8 | 248.8 | ||
| Contrasterector spinae | men | 336.7 | 50.0 | <0.001 |
| women | 410.0 | 90.4 | ||
| Contrastpsoas | men | 391.9 | 51.8 | n.s. |
| women | 391.2 | 78.2 | ||
| Entropycervical | men | 10.8 | 0.9 | 0.010 |
| women | 11.4 | 0.9 | ||
| Entropyerector spinae | men | 11.3 | 0.5 | <0.001 |
| women | 11.9 | 0.8 | ||
| Entropypsoas | men | 12.1 | 0.2 | n.s. |
| women | 11.9 | 0.3 | ||
| Homogeneitycervical | men | 0.25 | 0.04 | 0.008 |
| women | 0.22 | 0.04 | ||
| Homogeneityerector spinae | men | 0.22 | 0.02 | 0.006 |
| women | 0.20 | 0.02 | ||
| Homogeneitypsoas | men | 0.16 | 0.1 | 0.003 |
| women | 0.17 | 0.1 | ||
| Correlationcervical | men | 0.4 | 0.2 | n.s. |
| women | 0.5 | 0.1 | ||
| Correlationerector spinae | men | 0.5 | 0.1 | <0.001 |
| women | 0.6 | 0.1 | ||
| Correlationpsoas | men | 0.5 | 0.1 | n.s. |
| women | 0.5 | 0.1 | ||
| Variancecervical | men | 0.11 | 0.01 | n.s. |
| women | 0.13 | 0.01 | ||
| Varianceerector spinae | men | 0.01 | 0.001 | <0.001 |
| women | 0.02 | 0.001 | ||
| Variancepsoas | men | 0.01 | 0.001 | n.s. |
| women | 0.01 | 0.001 | ||
| Sum-averagecervical | men | 0.00241 | 0.0003 | n.s. |
| women | 0.00231 | 0.0003 | ||
| Sum-averageerector spinae | men | 0.00208 | 0.0002 | n.s. |
| women | 0.00212 | 0.0002 | ||
| Sum-averagepsoas | men | 0.00256 | 0.0002 | n.s. |
| women | 0.00240 | 0.0002 | ||
| Dissimilaritycervical | men | 11.6 | 1.8 | 0.009 |
| women | 13.4 | 3.7 | ||
| Dissimilarityerector spinae | men | 10.9 | 3.2 | <0.001 |
| women | 12.6 | 2.1 | ||
| Dissimilaritypsoas | men | 13.4 | 0.9 | 0.043 |
| women | 12.9 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burian, E.; Becherucci, E.A.; Junker, D.; Sollmann, N.; Greve, T.; Hauner, H.; Zimmer, C.; Kirschke, J.S.; Karampinos, D.C.; Subburaj, K.; et al. Association of Cervical and Lumbar Paraspinal Muscle Composition Using Texture Analysis of MR-Based Proton Density Fat Fraction Maps. Diagnostics 2021, 11, 1929. https://doi.org/10.3390/diagnostics11101929
Burian E, Becherucci EA, Junker D, Sollmann N, Greve T, Hauner H, Zimmer C, Kirschke JS, Karampinos DC, Subburaj K, et al. Association of Cervical and Lumbar Paraspinal Muscle Composition Using Texture Analysis of MR-Based Proton Density Fat Fraction Maps. Diagnostics. 2021; 11(10):1929. https://doi.org/10.3390/diagnostics11101929
Chicago/Turabian StyleBurian, Egon, Edoardo A. Becherucci, Daniela Junker, Nico Sollmann, Tobias Greve, Hans Hauner, Claus Zimmer, Jan S. Kirschke, Dimitrios C. Karampinos, Karupppasamy Subburaj, and et al. 2021. "Association of Cervical and Lumbar Paraspinal Muscle Composition Using Texture Analysis of MR-Based Proton Density Fat Fraction Maps" Diagnostics 11, no. 10: 1929. https://doi.org/10.3390/diagnostics11101929
APA StyleBurian, E., Becherucci, E. A., Junker, D., Sollmann, N., Greve, T., Hauner, H., Zimmer, C., Kirschke, J. S., Karampinos, D. C., Subburaj, K., Baum, T., & Dieckmeyer, M. (2021). Association of Cervical and Lumbar Paraspinal Muscle Composition Using Texture Analysis of MR-Based Proton Density Fat Fraction Maps. Diagnostics, 11(10), 1929. https://doi.org/10.3390/diagnostics11101929









