SERPINE2 Overexpression Is Associated with Poor Prognosis of Urothelial Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Survival Analysis of SERPINE2 in UBUC
2.2. Patient Cohort
2.3. TMA Construction
2.4. IHC Method
2.5. IHC Evaluation
2.6. Statistical Analysis
3. Results
3.1. Survival Analysis of SERPINE2 in UBUC Using TCGA Dataset
3.2. Clinicopathological Findings for UTUC
3.3. Clinicopathological Findings for UBUC
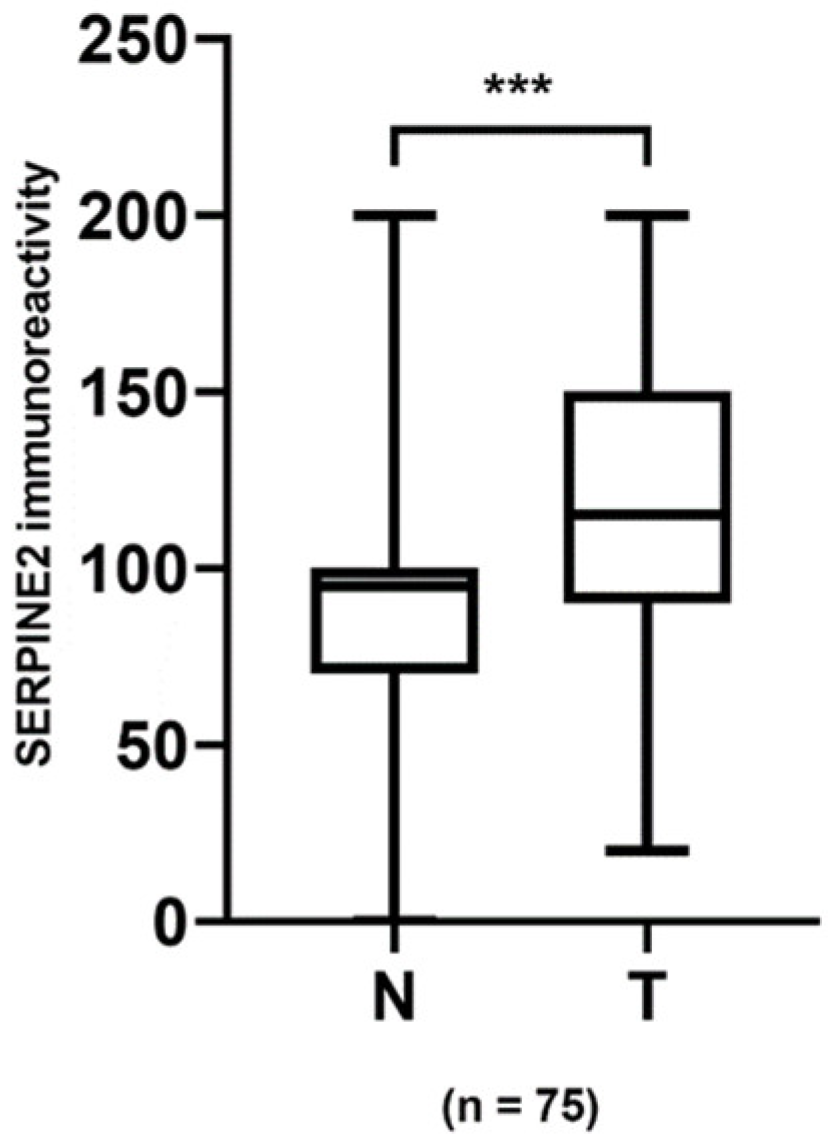
3.4. Association of SERPINE2 Immunoreactivity with UTUC and UBUC
3.5. Survival Analysis for UTUC and UBUC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Roupret, M.; Babjuk, M.; Comperat, E.; Zigeuner, R.; Sylvester, R.; Burger, M.; Cowan, N.; Bohle, A.; Van Rhijn, B.W.; Kaasinen, E.; et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur. Urol. 2013, 63, 1059–1071. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Comperat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Alfred Witjes, J.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Roupret, M.; Babjuk, M.; Comperat, E.; Zigeuner, R.; Sylvester, R.J.; Burger, M.; Cowan, N.C.; Gontero, P.; Van Rhijn, B.W.G.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur. Urol. 2018, 73, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Guenther, J.; Nick, H.; Monard, D. A glia-derived neurite-promoting factor with protease inhibitory activity. EMBO J. 1985, 4, 1963–1966. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, S.; Boulaftali, Y.; Venisse, L.; Arocas, V.; Meilhac, O.; Michel, J.B.; Jandrot-Perrus, M.; Bouton, M.C. Macrophages and platelets are the major source of protease nexin-1 in human atherosclerotic plaque. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1844–1850. [Google Scholar] [CrossRef] [PubMed]
- Fayard, B.; Bianchi, F.; Dey, J.; Moreno, E.; Djaffer, S.; Hynes, N.E.; Monard, D. The serine protease inhibitor protease nexin-1 controls mammary cancer metastasis through LRP-1-mediated MMP-9 expression. Cancer Res. 2009, 69, 5690–5698. [Google Scholar] [CrossRef]
- McKee, C.M.; Ding, Y.; Zhou, J.; Li, C.; Huang, L.; Xin, X.; He, J.; Allen, J.E.; El-Deiry, W.S.; Cao, Y. Protease nexin 1 induces apoptosis of prostate tumor cells through inhibition of X-chromosome-linked inhibitor of apoptosis protein. Oncotarget 2015, 6, 3784–3796. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Conde, J.; Scotece, M.; Abella, V.; Lois, A.; Lopez, V.; Pino, J.; Gomez, R.; Gomez-Reino, J.J.; Gualillo, O. SERPINE2 Inhibits IL-1alpha-Induced MMP-13 Expression in Human Chondrocytes: Involvement of ERK/NF-kappaB/AP-1 Pathways. PLoS ONE 2015, 10, e0135979. [Google Scholar] [CrossRef]
- Gomez, D.; Kessler, K.; Borges, L.F.; Richard, B.; Touat, Z.; Ollivier, V.; Mansilla, S.; Bouton, M.C.; Alkoder, S.; Nataf, P.; et al. Smad2-Dependent Protease Nexin-1 Overexpression Differentiates Chronic Aneurysms from Acute Dissections of Human Ascending Aorta. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2222–2232. [Google Scholar] [CrossRef]
- Boulaftali, Y.; Ho-Tin-Noe, B.; Pena, A.; Loyau, S.; Venisse, L.; François, D.; Richard, B.; Arocas, V.; Collet, J.P.; Jandrot-Perrus, M.; et al. Platelet Protease Nexin-1, a Serpin That Strongly Influences Fibrinolysis and Thrombolysis. Circulation 2011, 123, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.B.; Gronke, R.S. Protease nexins and cellular regulation. Semin. Thromb. Hemost. 1986, 12, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Zhu, Q.; Li, X.; Zhu, G.; Deng, S.; Wang, Y.; Ni, L.; Chen, X.; Zhang, Y.; Xia, T.; et al. Protease Nexin I is a feedback regulator of EGF/PKC/MAPK/EGR1 signaling in breast cancer cells metastasis and stemness. Cell Death Dis. 2019, 10, 649. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, M.; Biebl, A.; Neesse, A.; Wagner, M.; Iwamura, T.; Leder, G.; Adler, G.; Gress, T.M. SERPINE2 (protease nexin I) promotes extracellular matrix production and local invasion of pancreatic tumors in vivo. Cancer Res. 2003, 63, 4945–4951. [Google Scholar]
- Bergeron, S.; Lemieux, E.; Durand, V.; Cagnol, S.; Carrier, J.C.; Lussier, J.G.; Boucher, M.J.; Rivard, N. The serine protease inhibitor serpinE2 is a novel target of ERK signaling. Mol. Cancer 2010, 9, 271. [Google Scholar] [CrossRef]
- Wang, K.; Wang, B.; Xing, A.Y.; Xu, K.S.; Li, G.X.; Yu, Z.H. Prognostic significance of SERPINE2 in gastric cancer and its biological function in SGC7901 cells. J. Cancer Res. Clin. Oncol. 2015, 141, 805–812. [Google Scholar] [CrossRef]
- Nagahara, A.; Nakayama, M.; Oka, D.; Tsuchiya, M.; Kawashima, A.; Mukai, M.; Nakai, Y.; Takayama, H.; Nishimura, K.; Jo, Y.; et al. SERPINE2 is a possible candidate promotor for lymph node metastasis in testicular cancer. Biochem. Biophys. Res. Commun. 2010, 391, 1641–1646. [Google Scholar] [CrossRef]
- Wu, Q.W. Serpine2, a potential novel target for combating melanoma metastasis. Am. J. Transl. Res. 2016, 8, 1985–1997. [Google Scholar]
- Mao, M.; Wang, W. SerrpinE2 promotes multiple cell proliferation and drug resistance in osteosarcoma. Mol. Med. Rep. 2016, 14, 881–887. [Google Scholar] [CrossRef]
- Stępień, T.; Brożyna, M.; Kuzdak, K.; Motylewska, E.; Komorowski, J.; Stępień, H.; Ławnicka, H. Elevated Concentrations of SERPINE2/Protease Nexin-1 and Secretory Leukocyte Protease Inhibitor in the Serum of Patients with Papillary Thyroid Cancer. Dis. Markers 2017, 2017, 4962137. [Google Scholar] [CrossRef]
- Gao, S.; Krogdahl, A.; Sorensen, J.A.; Kousted, T.M.; Dabelsteen, E.; Andreasen, P.A. Overexpression of protease nexin-1 mRNA and protein in oral squamous cell carcinomas. Oral Oncol. 2008, 44, 309–313. [Google Scholar] [CrossRef][Green Version]
- Shen, Y.; Wang, X.; Xu, J.; Lu, L. SerpinE2, a poor biomarker of endometrial cancer, promotes the proliferation and mobility of EC cells. Cancer Biomark. 2017, 19, 271–278. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, A.; Huang, F.; Gong, T.; Liu, Z. SERPINE2 promotes esophageal squamous cell carcinoma metastasis by activating BMP4. Cancer Lett. 2020, 469, 390–398. [Google Scholar] [CrossRef]
- Dokuni, R.; Nagano, T.; Jimbo, N.; Sato, H.; Kiriu, T.; Yasuda, Y.; Yamamoto, M.; Tachihara, M.; Kobayashi, K.; Maniwa, Y.; et al. High expression level of serpin peptidase inhibitor clade E member 2 is associated with poor prognosis in lung adenocarcinoma. Respir. Res. 2020, 21, 331. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, D.; Yang, S.; Sun, Y.; Liu, Y.; Shi, J.; Hu, C.; Pan, J.; Liu, T.; Jin, B.; et al. BAP31 Promotes Tumor Cell Proliferation by Stabilizing SERPINE2 in Hepatocellular Carcinoma. Front. Cell. Dev. Biol. 2020, 8, 607906. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Teng, H.; Liu, M.; Liu, B.; Zhang, D.; Xu, Z.; Wang, Y.; Zhou, H. Prognostic Value of Immune-Related Genes in the Tumor Microenvironment of Bladder Cancer. Front. Oncol. 2020, 10, 1302. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Humphrey, P.; Ulbright, T.; Reuter, V. WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2016; Volume 8, ISBN 978-92-832-2437-2. [Google Scholar]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: Chicago, IL, USA, 2017; ISBN 978-3-319-40617-6. [Google Scholar]
- Huang, M.; Long, Y.; Jin, Y.; Ya, W.; Meng, D.; Qin, T.; Su, L.; Zhou, W.; Wu, J.; Huang, C.; et al. Comprehensive analysis of the lncRNA-miRNA-mRNA regulatory network for bladder cancer. Transl. Androl. Urol. 2021, 10, 1286–1301. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yang, J.; Xu, X.; Wang, Y.; Yu, M.; Zhu, Y. A robust 11-genes prognostic model can predict overall survival in bladder cancer patients based on five cohorts. Cancer Cell Int. 2020, 20, 402. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Oosterlinck, W.; Sylvester, R.; Kaasinen, E.; Böhle, A.; Palou-Redorta, J.; European Association of Urology (EAU). EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur. Urol. 2008, 54, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, P.; Ho-Tin-Noé, B.; Vranckx, R.; Bouton, M.C.; Meilhac, O.; Lijnen, H.R.; Guillin, M.C.; Michel, J.B.; Anglés-Cano, E. Protease nexin-1 inhibits plasminogen activation-induced apoptosis of adherent cells. J. Biol. Chem. 2004, 279, 10346–10356. [Google Scholar] [CrossRef]
- Smirnova, T.; Bonapace, L.; MacDonald, G.; Kondo, S.; Wyckoff, J.; Ebersbach, H.; Fayard, B.; Doelemeyer, A.; Coissieux, M.M.; Heideman, M.R.; et al. Serpin E2 promotes breast cancer metastasis by remodeling the tumor matrix and polarizing tumor associated macrophages. Oncotarget 2016, 7, 82289–82304. [Google Scholar] [CrossRef]
- Vaillant, C.; Valdivieso, P.; Nuciforo, S.; Kool, M.; Schwarzentruber-Schauerte, A.; Méreau, H.; Cabuy, E.; Lobrinus, J.A.; Pfister, S.; Zuniga, A.; et al. Serpine2/PN-1 Is Required for Proliferative Expansion of Pre-Neoplastic Lesions and Malignant Progression to Medulloblastoma. PLoS ONE 2015, 10, e0124870. [Google Scholar] [CrossRef]
- Xu, D.; McKee, C.M.; Cao, Y.; Ding, Y.; Kessler, B.M.; Muschel, R.J. Matrix metalloproteinase-9 regulates tumor cell invasion through cleavage of protease nexin-1. Cancer Res. 2010, 70, 6988–6998. [Google Scholar] [CrossRef]
- Barkan, G.A.; Wojcik, E.M.; Nayar, R.; Savic-Prince, S.; Quek, M.L.; Kurtycz, D.F.; Rosenthal, D.L. The Paris System for Reporting Urinary Cytology: The Quest to Develop a Standardized Terminology. Acta Cytol. 2016, 60, 185–197. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, J.J.; Goldsmith, J.C.; Wilson, J.S.; Bryan, R.T.; Ward, D.G. A Systematic Review of the Diagnostic and Prognostic Value of Urinary Protein Biomarkers in Urothelial Bladder Cancer. Bladder Cancer 2016, 2, 301–317. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Upper Urinary Tract Urothelial Carcinoma | Urinary Bladder Urothelial Carcinoma | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | Mean ± SEM | Median | p | No. | Mean ± SEM | Median | p | |
| Age | ||||||||
| <65 | 53 | 80.36 ± 9.44 | 68 | 0.065 | 22 | 93.86 ± 15.34 | 83 | 0.558 |
| ≥65 | 64 | 58.47 ± 5.76 | 60 | 62 | 78.39 ± 7.58 | 83 | ||
| Gender | ||||||||
| Male | 57 | 55.96 ± 6.40 | 45 | 0.078 | 62 | 84.27 ± 8.14 | 88 | 0.478 |
| Female | 60 | 76.17 ± 7.66 | 73 | 22 | 77.27 ± 13.02 | 65 | ||
| Location | ||||||||
| Pelvis | 73 | 74.32 ± 7.06 | 70 | 0.083 | ||||
| Ureter | 44 | 53.07 ± 6.33 | 53 | |||||
| Invasion | ||||||||
| Absent | 27 | 36.48 ± 8.36 | 15 | 0.001 * | 32 | 52.97 ± 8.87 | 38 | 0.001 * |
| Present | 90 | 75.28 ± 5.81 | 70 | 52 | 100.58 ± 8.83 | 95 | ||
| Histological grade | ||||||||
| Low | 29 | 37.41 ± 7.23 | 25 | 0.001 * | 24 | 58.13 ± 8.158 | 63 | 0.066 |
| High | 88 | 75.85 ± 5.93 | 75 | 60 | 92.17 ± 8.78 | 83 | ||
| UIS | ||||||||
| Absent | 50 | 54.80 ± 7.54 | 48 | 0.033 * | 59 | 69.07 ± 7.64 | 70 | 0.003 * |
| Present | 67 | 74.93 ± 6.72 | 75 | 25 | 114.00 ± 12.55 | 95 | ||
| Primary tumor status | ||||||||
| Ta-1 | 63 | 49.05 ± 5.86 | 35 | <0.001 * | 58 | 68.97 ± 7.69 | 68 | 0.003 * |
| T2-4 | 54 | 86.48 ± 7.83 | 78 | 26 | 112.50 ± 12.42 | 95 | ||
| Nodal metastasis | ||||||||
| Absent | 112 | 64.46 ± 5.07 | 60 | 0.122 | 80 | 80.13 ± 6.92 | 80 | 0.162 |
| Present | 5 | 108.00 ± 32.58 | 95 | 4 | 128.75 ± 38.48 | 105 | ||
| Distant metastasis | ||||||||
| Absent | 96 | 66.04 ± 5.60 | 60 | 0.921 | 68 | 80.22 ± 7.72 | 83 | 0.418 |
| Present | 21 | 67.62 ± 12.39 | 65 | 16 | 91.88 ± 15.33 | 83 | ||
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Variables | No. | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p |
| Age | |||||
| <65 | 53 | Reference | Reference | ||
| ≥65 | 64 | 2.907 (1.529–5.527) | 0.001 * | 3.657 (1.898–7.048) | <0.001 * |
| Gender | |||||
| Male | 57 | Reference | |||
| Female | 60 | 1.224 (0.721–2.076) | 0.454 | ||
| Location | |||||
| Pelvis | 73 | Reference | |||
| Ureter | 44 | 1.002 (0.764–1.313) | 0.991 | ||
| Invasion | |||||
| Absent | 27 | Reference | Reference | ||
| Present | 90 | 2.245 (1.017–4.959) | 0.045 * | 0.181 (0.051–0.638) | 0.008 * |
| Histological grade | |||||
| Low | 29 | Reference | Reference | ||
| High | 88 | 4.866 (1.759–13.459) | 0.002 * | 13.815 (3.092–61.720) | 0.001 * |
| UIS | |||||
| Absent | 50 | Reference | Reference | ||
| Present | 67 | 2.088 (1.181–3.694) | 0.011 * | 1.294 (0.710–2.361) | 0.400 |
| Primary tumor status | |||||
| Ta-1 | 63 | Reference | Reference | ||
| T2-4 | 54 | 2.386 (1.379–4.127) | 0.002* | 1.705 (0.871–3.337) | 0.120 |
| Nodal metastasis | |||||
| Absent | 112 | Reference | Reference | ||
| Present | 5 | 6.441 (2.435–17.040) | <0.001 * | 2.609 (0.739–7.153) | 0.062 |
| Distant metastasis | |||||
| Absent | 96 | Reference | Reference | ||
| Present | 21 | 3.997 (2.248–7.110) | <0.001 * | 3.556 (1.892–6.687) | <0.001 * |
| SERPINE2 expression | |||||
| Low | 58 | Reference | Reference | ||
| High | 59 | 2.243 (1.297–3.879) | 0.004 * | 2.541 (1.405–4.597) | 0.002 * |
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Variables | No. | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p |
| Age | |||||
| <65 | 22 | Reference | Reference | ||
| ≥65 | 62 | 4.442 (1.578–12.501) | 0.005* | 4.844 (1.646–14.254) | 0.004 * |
| Gender | |||||
| Male | 62 | Reference | |||
| Female | 22 | 0.643 (0.296–1.398) | 0.265 | ||
| Invasion | |||||
| Absent | 32 | Reference | |||
| Present | 52 | 1.968 (0.996–3.887) | 0.051 | ||
| Histological grade | |||||
| Low | 24 | Reference | Reference | ||
| High | 60 | 2.317 (1.024–5.245) | 0.044 * | 1.434 (0.578–3.556) | 0.437 |
| UIS | |||||
| Absent | 59 | Reference | |||
| Present | 25 | 1.853 (0.973–3.529) | 0.060 | ||
| Primary tumor status | |||||
| Ta-1 | 58 | Reference | Reference | ||
| T2-4 | 26 | 3.560 (1.885–6.722) | <0.001 * | 1.062 (0.394–2.862) | 0.905 |
| Nodal metastasis | |||||
| Absent | 80 | Reference | Reference | ||
| Present | 4 | 5.130 (1.792–14.686) | 0.002 * | 1.251 (0.386–4.061) | 0.709 |
| Distant metastasis | |||||
| Absent | 68 | Reference | Reference | ||
| Present | 16 | 9.036 (4.356–18.743) | <0.001 * | 8.454 (2.917–24.506) | <0.001 * |
| SERPINE2 expression | |||||
| Low | 42 | Reference | Reference | ||
| High | 42 | 2.203 (1.156–4.198) | 0.016 * | 2.301 (1.117–4.742) | 0.024 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, H.-W.; Hsia, K.-T.; Liao, J.-B.; Yeh, C.-C.; Kuo, W.-T.; Yang, Y.-F. SERPINE2 Overexpression Is Associated with Poor Prognosis of Urothelial Carcinoma. Diagnostics 2021, 11, 1928. https://doi.org/10.3390/diagnostics11101928
Chuang H-W, Hsia K-T, Liao J-B, Yeh C-C, Kuo W-T, Yang Y-F. SERPINE2 Overexpression Is Associated with Poor Prognosis of Urothelial Carcinoma. Diagnostics. 2021; 11(10):1928. https://doi.org/10.3390/diagnostics11101928
Chicago/Turabian StyleChuang, Hao-Wen, Kan-Tai Hsia, Jia-Bin Liao, Chih-Ching Yeh, Wei-Ting Kuo, and Yi-Fang Yang. 2021. "SERPINE2 Overexpression Is Associated with Poor Prognosis of Urothelial Carcinoma" Diagnostics 11, no. 10: 1928. https://doi.org/10.3390/diagnostics11101928
APA StyleChuang, H.-W., Hsia, K.-T., Liao, J.-B., Yeh, C.-C., Kuo, W.-T., & Yang, Y.-F. (2021). SERPINE2 Overexpression Is Associated with Poor Prognosis of Urothelial Carcinoma. Diagnostics, 11(10), 1928. https://doi.org/10.3390/diagnostics11101928






