Factors Influencing Concordance of PD-L1 Expression between Biopsies and Cytological Specimens in Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Preparation Procedure and PD-L1 Staining
2.3. Quantification of PD-L1 Expression and Estimation of Tumour Cell Proportion
2.4. Statistical Analysis of Data
3. Results
3.1. Characteristics of the Specimens
3.2. Relationship between PD-L1 Expression and Patient and Sample Characteristics
3.3. Concordance of PD-L1 Expression between Paired Histological and Cytological Specimens
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Perez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- US Food and Drug Administration: KEYTRUDA (Pembrolizumab). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125514Orig1s054lbl.pdf (accessed on 5 July 2021).
- Yu, H.; Boyle, T.A.; Zhou, C.; Rimm, D.L.; Hirsch, F.R. PD-L1 Expression in Lung Cancer. J. Thorac. Oncol. 2016, 11, 964–975. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Balar, A.V.; Weber, J.S. PD-1 and PD-L1 antibodies in cancer: Current status and future directions. Cancer Immunol. Immunother. 2017, 66, 551–564. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.; Chirieac, L.R.; D’Amico, T.A.; DeCamp, M.M.; Dilling, T.J.; Dobelbower, M.; et al. Non–Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 504–535. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, L.; Yu, J.; Zhang, Y.; Pang, X.; Ma, C.; Shen, M.; Ruan, S.; Wasan, H.S.; Qiu, S. Clinical efficacy and safety of anti-PD-1/PD-L1 inhibitors for the treatment of advanced or metastatic cancer: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 2083. [Google Scholar] [CrossRef]
- Hasanovic, A.; Rekhtman, N.; Sigel, C.S.; Moreira, A.L. Advances in Fine Needle Aspiration Cytology for the Diagnosis of Pulmonary Carcinoma. Pathol. Res. Int. 2011, 2011, 897292. [Google Scholar] [CrossRef] [PubMed]
- Travis, T.W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.A.; Riely, G.J.; van Schil, P.E.; et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef] [PubMed]
- Skov, B.G.; Høgdall, E.; Clementsen, P.; Krasnik, M.; Larsen, K.R.; Sørensen, J.B.; Skov, T.; Mellemgaard, A. The prevalence of EGFR mutations in non-small cell lung cancer in an unselected Caucasian population. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2015, 123, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Lantuejoul, S.; Tsao, M.; Cooper, W.A.; Girard, N.; Hirsch, F.R.; Roden, A.C.; Lopez-Rios, F.; Jain, D.; Chou, T.-Y.; Motoi, N.; et al. PD-L1 Testing for Lung Cancer in 2019: Perspective From the IASLC Pathology Committee. J. Thorac. Oncol. 2020, 15, 499–519. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, A.; Salatiello, M.; Migliatico, I.; de Luca, C.; Gragnano, G.; Russo, M.; Bellevicine, C.; Malapelle, U.; Troncone, G.; Vigliar, E. PD-L1 and beyond: Immuno-oncology in cytopathology. Cytopathology 2021, 32, 596–603. [Google Scholar] [CrossRef]
- Tejerina, E.; García Tobar, L.; Echeveste, J.I.; de Andrea, C.E.; Vigliar, E.; Lozano, M.D. PD-L1 in Cytological Samples: A Review and a Practical Approach. Front. Med. 2021, 8, 668612. [Google Scholar] [CrossRef] [PubMed]
- Gosney, J.R.; Boothman, A.-; Ratcliffe, M.; Kerr, K.M. Cytology for PD-L1 testing: A systematic review. Lung Cancer 2020, 141, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.S.; Lindquist, K.E.; Seidal, T.; Mager, U.; Mohlin, R.; Tran, L.; Hejny, K.; Holmgren, B.; Violidaki, D.; Dobra, K.; et al. PD-L1 Testing in Cytological Non-Small Cell Lung Cancer Specimens: A Comparison with Biopsies and Review of the Literature. Acta Cytol. 2021, 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Agilent. PD-L1 IHC 28-8 pharmDx Interpretation Manual. Available online: https://www.agilent.com/cs/library/usermanuals/public/29111_pd-l1-ihc-28-8-interpretation-manual.pdf (accessed on 5 July 2021).
- Altman, D.G. Practical Statistics for Medical Research, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 1991. [Google Scholar]
- Vigliar, E.; Malapelle, U.; Troncone, G. Cytopathology meets basic science: Cytopathology Help Desk. Cancer Cytopathol. 2015, 123, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Metovic, J.; Righi, L.; Delsedime, L.; Volante, M.; Papotti, M. Role of Immunocytochemistry in the Cytological Diagnosis of Pulmonary Tumors. Acta Cytol. 2020, 64, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Nehra, M.; Agarwal, D.; Mohan, A. Diagnostic Accuracy of Endobronchial Ultrasound Guided Transbronchial Needle Aspiration in Mediastinal Lymphadenopathy: A Systematic Review and Meta-analysis. Respir. Care 2012, 57, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ying, K.; Shi, L.; Zhang, L.; Zhou, L. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: A meta-analysis. Eur. J. Cancer 2013, 49, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Porcel, J.M. Malignant pleural effusions because of lung cancer. Curr. Opin. Pulm. Med. 2016, 22, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Krogerus, L.; Kholová, I. Cell Block in Cytological Diagnostics: Review of Preparatory Techniques. Acta Cytol. 2018, 62, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Moreira, A.L. The Role of Ancillary Techniques in Pulmonary Cytopathology. Acta Cytol. 2020, 64, 166–174. [Google Scholar] [CrossRef]
- Huang, M.; Wei, S. Overview of Molecular Testing of Cytology Specimens. Acta Cytol. 2020, 64, 136–146. [Google Scholar] [CrossRef]
- Torous, V.F.; Rangachari, D.; Gallant, B.P.; Shea, M.; Costa, D.B.; VanderLaan, P.A. PD-L1 testing using the clone 22C3 pharmDx kit for selection of patients with non–small cell lung cancer to receive immune checkpoint inhibitor therapy: Are cytology cell blocks a viable option? J. Am. Soc. Cytopathol. 2018, 7, 133–141. [Google Scholar] [CrossRef]
- Grosu, H.B.; Arriola, A.; Stewart, J.; Ma, J.; Bassett, R.; Hernandez, M.; Ost, D.; Roy-Chowdhuri, S. PD-L1 detection in histology specimens and matched pleural fluid cell blocks of patients with NSCLC. Respirology 2019, 24, 1198–1203. [Google Scholar] [CrossRef]
- Zou, Y.; Xu, L.; Tang, Q.; You, Q.; Wang, X.; Ding, W.; Zhao, J.; Ren, G. Cytology cell blocks from malignant pleural effusion are good candidates for PD-L1 detection in advanced NSCLC compared with matched histology samples. BMC Cancer 2020, 20, 344. [Google Scholar] [CrossRef]
- Kim, M.; Koh, J.; Kim, S.; Go, H.; Jeon, Y.K.; Chung, D.H. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer 2015, 88, 24–33. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Aubry, M.C.; Moser, J.C.; Harrington, S.M.; Dronca, R.S.; Park, S.S.; Dong, H. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann. Oncol. 2016, 27, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Takamori, S.; Toyokawa, G.; Okamoto, I.; Takada, K.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Akamine, T.; Katsura, M.; Mukae, N.; et al. Discrepancy in Programmed Cell Death-Ligand 1 Between Primary and Metastatic Non-small Cell Lung Cancer. Anticancer. Res. Int. J. Cancer Res. Treat. 2017, 37, 4223–4228. [Google Scholar]
- Kim, H.R.; Cha, Y.J.; Hong, M.H.; Gandhi, M.; Levinson, S.; Jung, I.; Lee, J.G.; Lee, C.Y.; Cho, B.C.; Ha, S.-J.; et al. Concordance of programmed death-ligand 1 expression between primary and metastatic non-small cell lung cancer by immunohistochemistry and RNA in situ hybridization. Oncotarget 2017, 8, 87234–87243. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Agulnik, J.; Kasymjanova, G.; Fiset, P.; Camilleri-Broet, S.; Redpath, M.; Cohen, V.; Small, D.; Pepe, C.; Sakr, L.; et al. The metastatic site does not influence PD-L1 expression in advanced non-small cell lung carcinoma. Lung Cancer 2019, 132, 36–38. [Google Scholar] [CrossRef]
- Hong, L.; Negrao, M.; Dibaj, S.S.; Chen, R.; Reuben, A.; Bohac, J.M.; Liu, X.; Skoulidis, F.; Gay, C.M.; Cascone, T.; et al. Programmed Death-Ligand 1 Heterogeneity and Its Impact on Benefit From Immune Checkpoint Inhibitors in NSCLC. J. Thorac. Oncol. 2020, 15, 1449–1459. [Google Scholar] [CrossRef]
- Evans, M.; O’Sullivan, B.; Hughes, F.; Mullis, T.; Smith, M.; Trim, N.; Taniere, P. The Clinicopathological and Molecular Associations of PD-L1 Expression in Non-small Cell Lung Cancer: Analysis of a Series of 10,005 Cases Tested with the 22C3 Assay. Pathol. Oncol. Res. 2020, 26, 79–89. [Google Scholar] [CrossRef]
- Tsao, M.; Kerr, K.M.; Kockx, M.; Beasley, M.-B.; Borczuk, A.C.; Botling, J.; Bubendorf, L.; Chirieac, L.; Chen, G.; Chou, T.-Y.; et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J. Thorac. Oncol. 2018, 13, 1302–1311. [Google Scholar] [CrossRef]
- Russell-Goldman, E.; Kravets, S.; Dahlberg, S.E.; Sholl, L.M.; Vivero, M. Cytologic-histologic correlation of programmed death-ligand 1 immunohistochemistry in lung carcinomas: PD-L1 Staining in Cytology Specimens. Cancer Cytopathol. 2018, 126, 253–263. [Google Scholar] [CrossRef]
- Gagné, A.; Orain, M.; Ionescu, D.; Tsao, M.; Joubert, D.; Joubert, P. Comprehensive assessment of PD-L1 immunohistochemistry on paired tissue and cytology specimens from non-small cell lung cancer. Lung Cancer 2020, 146, 276–284. [Google Scholar] [CrossRef]
- Sinclair, W.; Kobalka, P.; Ren, R.; Beshai, B.; Limbach, A.A.L.; Wei, L.; Mei, P.; Li, Z. Interobserver agreement in programmed cell death-ligand 1 immunohistochemistry scoring in nonsmall cell lung carcinoma cytologic specimens. Diagn. Cytopathol. 2021, 49, 219–225. [Google Scholar] [CrossRef]
- Rashed, H.E.; Abdelrahman, A.E.; Abdelgawad, M.; Balata, S.; Shabrawy, M.E. Prognostic significance of programmed cell death ligand 1 (pd-l1), cd8 tumor-infiltrating lymphocytes and p53 in non-small cell lung cancer: An immunohistochemical study. Turk. J. Pathol. 2017, 1, 211–222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gandini, S.; Massi, D.; Mandalà, M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol.-Hematol. 2016, 100, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, G.; Wang, Y.; Wang, Y.; Zhao, S.; Haihong, P.; Zhao, H. PD-L1 expression in lung cancer and its correlation with driver mutations: A meta-analysis. Sci. Rep. 2017, 7, 10255. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Han, X.; Liu, C.; Gao, N.; Zhao, J.; Zhang, X.; Jiang, L.; Ren, L.; Li, P.; Wang, N. PD-L1 expression in pleural effusions of pulmonary adenocarcinoma and survival prediction: A controlled study by pleural biopsy. Sci. Rep. 2018, 8, 11206. [Google Scholar] [CrossRef]
- Velcheti, V.; Patwardhan, P.D.; Liu, F.X.; Chen, X.; Cao, X.; Burke, T. Real-world PD-L1 testing and distribution of PD-L1 tumor expression by immunohistochemistry assay type among patients with metastatic non-small cell lung cancer in the United States. PLoS ONE 2018, 13, e0206370. [Google Scholar] [CrossRef]
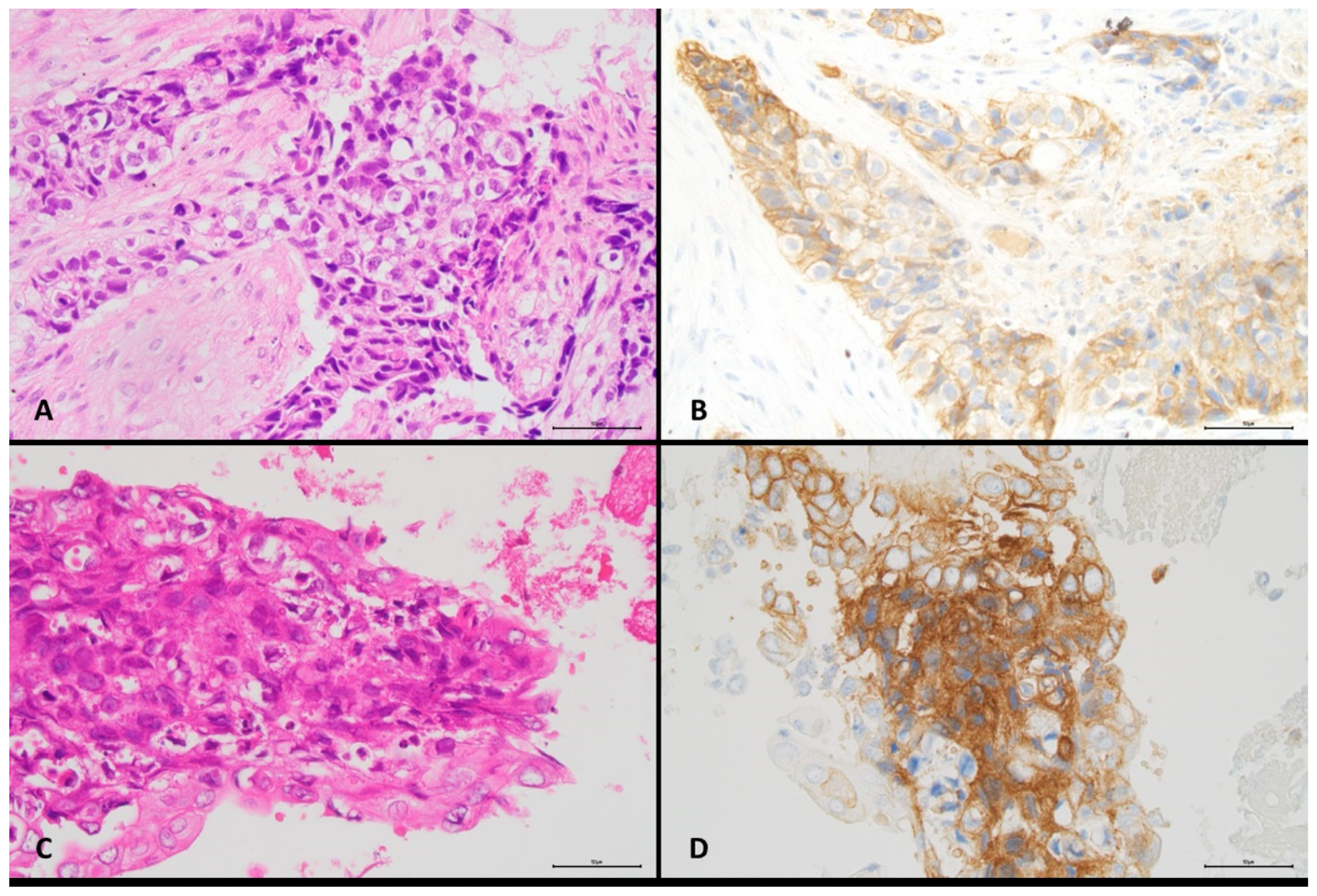
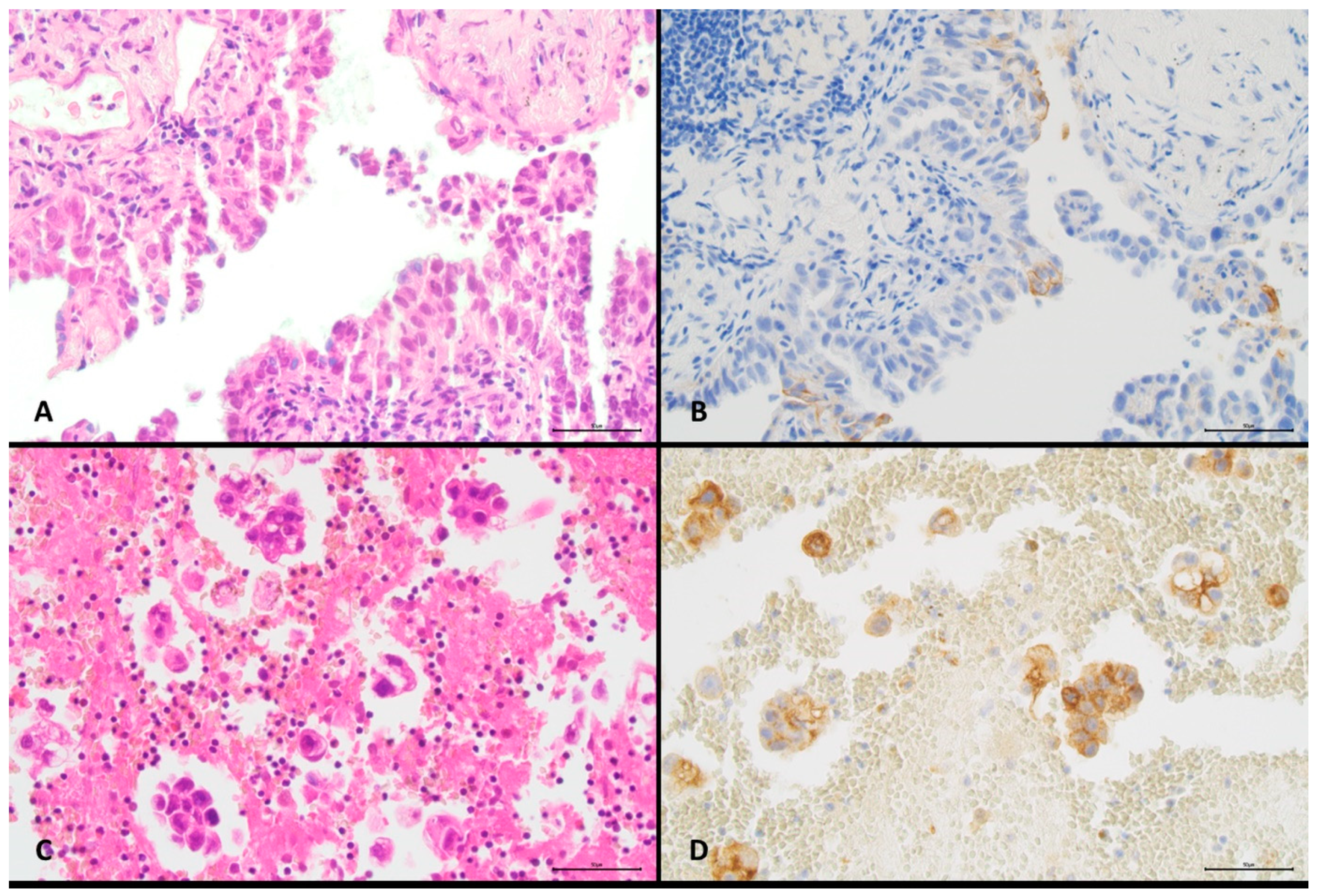
| Characteristics | All Cases | PD-L1 Positive Tumour Cells | Cell Content Number of Tumour Cells | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1% | 1–4% | 5–9% | 10–49% | ≥50% | 100–300 | >300–500 | >500–1000 | >1000 | ||
| Histological subtype, n (%) | ||||||||||
| Adenocarcinoma | 66/100 | |||||||||
| Cytological samples | 25/66 (38) | 7/66 (11) | 5/66 (8) | 8/66 (12) | 21/66 (32) | 34/66 (52) | 12/66 (18) | 5/66 (8) | 15/66 (23) | |
| Biopsy samples | 26/66 (39) | 8/66 (12) | 5/66 (8) | 7/66 (11) | 20/66 (30) | 34/66 (52) | 11/66 (17) | 16/66 (24) | 5/66 (8) | |
| Squamous cell carcinoma | 34/100 | |||||||||
| Cytological samples | 22/34 (65) | 1/34 (3) | 2/34 (6) | 3/34 (9) | 6/34 (18) | 13/34 (38) | 11/34 (32) | 4/34 (12) | 6/34 (18) | |
| Biopsy samples | 18/34 (53) | 4/34 (12) | 0/34 (0) | 7/34 (21) | 5/34 (15) | 10/34 (29) | 7/34 (21) | 7/34 (21) | 10/34 (29) | |
| Type of cytological specimen, n (%) | ||||||||||
| Pleural fluid | 19/100 | 9/19 (47) | 3/19 (16) | 2/19 (11) | 1/19 (5) | 4/19 (21) | 3/19 (16) | 5/19 (26) | 2/19 (11) | 9/19 (47) |
| Bronchial brush | 17/100 | 11/17 (65) | 0/17 (0) | 1/17 (6) | 1/17 (6) | 4/17 (24) | 8/17 (47) | 2/17 (12) | 5/17 (29) | 2/17 (12) |
| Bronchoalveolar lavages | 64/100 | 27/64 (42) | 5/64 (8) | 4/64 (6) | 9/64 (14) | 19/64 (30) | 36/64 (56) | 16/64 (25) | 2/64 (3) | 10/64 (16) |
| Type of biopsy, n (%) | ||||||||||
| Bronchial biopsy | 80/100 | 36/80 (45) | 10/80 (13) | 4/80 (5) | 9/80 (11) | 21/80 (26) | 37/80 (46) | 15/80 (19) | 18/80 (23) | 10/80 (13) |
| Transthoracic core biopsy | 20/100 | 8/20 (40) | 2/20 (10) | 1/20 (5) | 5/20 (25) | 4/20 (20) | 7/20 (35) | 3/20 (15) | 5/20 (25) | 5/20 (25) |
| PD-L1 | All Cases | Pleural Effusions | Bronchial Brushes | Bronchoalveolar Lavages | |
|---|---|---|---|---|---|
| Cutoff ≥1% positive cells (95% CI) | Kappa (κ) | 0.70 (0.56–0.83) | 0.48 (0.08–0.87) | 0.55 (0.21–0.90) | 0.81 (0.66–0.95) |
| OPA | 85 (77–91) | 74 (51–89) | 76 (52–91) | 91 (81–96) | |
| PPA | 89 (77–95) | 70 (39–90) | 100 (56–100) | 92 (78–98) | |
| NPA | 81 (67–90) | 78 (44–95) | 64 (35–85) | 89 (71–97) | |
| McNemar | 0.61 | 1.00 | 0.134 | 0.683 | |
| Cutoff ≥5% positive cells (95% CI) | Kappa (κ) | 0.53 (0.37–0.70) | 0.42 (−0.01–0.84) | 0.65 (0.32–0.99) | 0.53 (0.33–0.74) |
| OPA | 77 (68–84) | 74 (51–89) | 82 (58–95) | 77 (65–85) | |
| PPA | 73 (59–84) | 57 (25–84) | 100 (56–100) | 72 (54–85) | |
| NPA | 80 (67–89) | 83 (54–97) | 73 (43–91) | 81 (64–91) | |
| McNemar | 1.00 | 1.00 | 0.248 | 0.606 | |
| Cutoff ≥10% positive cells (95% CI) | Kappa (κ) | 0.51 (0.34–0.69) | 0.07 (−0.39–0.52) | 0.64 (0.29–0.99) | 0.56 (0.35–0.76) |
| OPA | 77 (68–84) | 68 (46–85) | 82 (58–95) | 78 (66–87) | |
| PPA | 71 (55–83) | 20 (2–64) | 100 (51–100) | 75 (56–88) | |
| NPA | 81 (69–89) | 86 (59–97) | 75 (46–92) | 81 (65–91) | |
| McNemar | 1.00 | 0.683 | 0.248 | 0.789 | |
| Cutoff ≥50% positive cells (95% CI) | Kappa (κ) | 0.53 (0.34–0.72) | −0.16 (−0.33–0.00) | 0.85 (0.57–1.00) | 0.58 (0.36–0.80) |
| OPA | 82 (73–89) | 68 (46–85) | 94 (71–100) | 83 (72–90) | |
| PPA | 63 (44–79) | 00 (00–55) | 100 (45–100) | 68 (46–85) | |
| NPA | 89 (80–95) | 87 (61–98) | 92 (65–100) | 89 (76–96) | |
| McNemar | 0.81 | 0.683 | 1.00 | 1.00 | |
| PD-L1 | All Cases | Pleural Effusions | Bronchial Brushes | Bronchoalveolar Lavages | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concordant | Discordant | p Values | Concordant | Discordant | p Values | Concordant | Discordant | p Values | Concordant | Discordant | p Values | ||
| Cut-off ≥1% positive cells | All cases, n (%) | 85/100 (85) | 15/100 (15) | 14/19 (74) | 5/19 (26) | 13/17 (77) | 4/17 (24) | 58/64 (91) | 6/64 (9) | ||||
| Histological subtype, n (%) | 0.77 | n/a | 0.60 | 0.40 | |||||||||
| Adenocarcinoma | 55/66 (83) | 11/66 (17) | 14/19 (74) | 5/19 (26) | 6/7 (86) | 1/7 (14) | 35/40 (88) | 5/40 (13) | |||||
| Squamous cell carcinoma | 30/34 (88) | 4/34 (12) | 0/0 (00) | 0/0 (00) | 7/10 (70) | 3/10 (30) | 23/24 (96) | 1/24 (4) | |||||
| Type of biopsy, n (%) | 0.17 | 0.63 | 1.00 | 0.51 | |||||||||
| Bronchial biopsy | 70/80 (88) | 10/80 (13) | 8/10 (80) | 2/10 (20) | 10/13 (77) | 3/13 (23) | 52/57 (91) | 5/57 (9) | |||||
| Transthoracic core biopsy | 15/20 (75) | 5/20 (25) | 6/9 (67) | 3/9 (33) | 3/4 (75) | 1/4 (25) | 6/7 (86) | 1/7 (14) | |||||
| Specimen cell amount “Cell blocks”, n (%) | 0.34 | 0.23 | 0.28 | 0.52 | |||||||||
| 100–300 | 37/47 (79) | 10/47 (21) | 1/3 (33) | 2/3 (67) | 5/8 (63) | 3/8 (38) | 31/36 (86) | 5/36 (14) | |||||
| >300–500 | 20/23 (87) | 3/23 (13) | 4/5 (80) | 1/5 (20) | 1/2 (50) | 1/2 (50) | 15/16 (94) | 1/16 (6) | |||||
| >500–1000 | 8/9 (89) | 1/9 (11) | 1/2 (50) | 1/2 (50) | 5/5 (100) | 0/5 (00) | 2/2 (100) | 0/2 (00) | |||||
| >1000 | 20/21 (95) | 1/21 (5) | 8/9 (89) | 1/9 (11) | 2/2 (100) | 0/2 (00) | 10/10 (100) | 0/10 (00) | |||||
| Specimen cell amount “Biopsies”, n (%) | 0.46 | 0.05 | 0.21 | 0.97 | |||||||||
| 100–300 | 37/44 (84) | 7/44 (16) | 9/10 (90) | 1/10 (10) | 3/6 (50) | 3/6 (50) | 25/28 (89) | 3/28 (11) | |||||
| >300–500 | 16/18 (89) | 2/18 (11) | 1/2 (50) | 1/2 (50) | 4/4 (100) | 0/4 (00) | 11/12 (92) | 1/12 (8) | |||||
| >500–1000 | 21/23 (91) | 2/23 (9) | 4/5 (80) | 1/5 (20) | 3/3 (100) | 0/3 (00) | 14/15 (93) | 1/15 (7) | |||||
| >1000 | 11/15 (73) | 4/15 (27) | 0/2 (00) | 2/2 (100) | 3/4 (75) | 1/4 (25) | 8/9 (89) | 1/9 (11) | |||||
| Cut-off ≥5% positive cells | All cases, n (%) | 77/100 (77) | 23/100 (23) | 14/19 (74) | 5/19 (26) | 14/17 (82) | 3/17 (18) | 49/64 (77) | 15/64 (23) | ||||
| Histological subtype, n (%) | 0.21 | n/a | 1.00 | 0.14 | |||||||||
| Adenocarcinoma | 48/66 (73) | 18/66 (27) | 14/19 (74) | 5/15 (26) | 6/7 (86) | 1/7 (14) | 28/40 (70) | 12/40 (30) | |||||
| Squamous cell carcinoma | 29/34 (85) | 5/34 (15) | 0/0 (00) | 0/0 (00) | 8/10 (80) | 2/10 (20) | 21/24 (88) | 3/24 (13) | |||||
| Type of biopsy, n (%) | 0.77 | 0.63 | 1.00 | 1.00 | |||||||||
| Bronchial biopsy | 62/80 (78) | 18/80 (23) | 8/10 (80) | 2/10 (20) | 11/13 (85) | 2/13 (15) | 43/57 (75) | 14/57 (25) | |||||
| Transthoracic core biopsy | 15/20 (75) | 5/20 (25) | 6/9 (67) | 3/9 (33) | 3/4 (75) | 1/4 (25) | 6/7 (86) | 1/7 (14) | |||||
| Specimen cell amount “Cell blocks”, n (%) | 0.35 | 0.48 | 0.36 | 0.43 | |||||||||
| 100–300 | 34/47 (72) | 13/47 (28) | 3/3 (100) | 0/3 (00) | 6/8 (75) | 2/8 (25) | 25/36 (69) | 11/36 (31) | |||||
| >300–500 | 18/23 (78) | 5/23 (22) | 3/5 (60) | 2/5 (40) | 1/2 (50) | 1/2 (50) | 14/16 (88) | 2/16 (13) | |||||
| >500–1000 | 9/9 (100) | 0/9 (00) | 2/2 (100) | 0/2 (00) | 5/5 (100) | 0/5 (00) | 2/2 (100) | 0/2 (00) | |||||
| >1000 | 16/21 (76) | 5/21 (24) | 6/9 (67) | 3/9 (33) | 2/2 (100) | 0/2 (00) | 8/10 (80) | 2/10 (20) | |||||
| Specimen cell amount “Biopsies”, n (%) | 0.32 | 0.69 | 0.45 | 0.045 | |||||||||
| 100–300 | 35/44 (80) | 9/44 (21) | 8/10 (80) | 2/10 (20) | 4/6 (67) | 2/6 (33) | 23/28 (82) | 5/28 (18) | |||||
| >300–500 | 11/18 (61) | 7/18 (39) | 1/2 (50) | 1/2 (50) | 4/4 (100) | 0/4 (00) | 6/12 (50) | 6/12 (50) | |||||
| >500–1000 | 18/23 (78) | 5/23 (22) | 4/5 (80) | 1/5 (20) | 3/3 (100) | 0/3 (00) | 11/15 (73) | 4/15 (27) | |||||
| >1000 | 13/15 (87) | 2/15 (13) | 1/2 (50) | 1/2 (50) | 3/4 (75) | 1/4 (25) | 9/9 (100) | 0/9 (00) | |||||
| Cut-off ≥10% positive cells | All cases, n (%) | 77/100 (77) | 23/100 (23) | 13/19 (68) | 6/19 (32) | 14/17 (82) | 3/17 (18) | 50/64 (78) | 14/64 (22) | ||||
| Histological subtype, n (%) | 0.21 | n/a | 1.00 | 0.22 | |||||||||
| Adenocarcinoma | 48/66 (73) | 18/66 (27) | 13/19 (68) | 6/19 (32) | 6/7 (86) | 1/7 (14) | 29/40 (73) | 11/40 (28) | |||||
| Squamous cell carcinoma | 29/34 (85) | 5/34 (15) | 0/0 (00) | 0/0 (00) | 8/10 (80) | 2/10 (20) | 21/24 (88) | 3/24 (13) | |||||
| Type of biopsy, n (%) | 0.77 | 1.00 | 1.00 | 1.00 | |||||||||
| Bronchial biopsy | 62/80 (78) | 18/80 (23) | 7/10 (70) | 3/10 (30) | 11/13 (85) | 2/13 (15) | 44/57 (77) | 13/57 (23) | |||||
| Transthoracic core biopsy | 15/20 (75) | 5/20 (25) | 6/9 (67) | 3/9 (33) | 3/4 (75) | 1/4 (25) | 6/7 (86) | 1/7 (14) | |||||
| Specimen cell amount “Cell blocks”, n (%) | 0.75 | 0.78 | 0.57 | 0.53 | |||||||||
| 100–300 | 35/47 (75) | 12/47 (26) | 2/3 (67) | 1/3 (33) | 7/8 (88) | 1/8 (13) | 26/36 (72) | 10/36 (28) | |||||
| >300–500 | 17/23 (74) | 6/23 (26) | 3/5 (60) | 2/5 (40) | 1/2 (50) | 1/2 (50) | 13/16 (81) | 3/16 (19) | |||||
| >500–1000 | 8/9 (89) | 1/9 (11) | 2/2 (100) | 0/2 (00) | 4/5 (80) | 1/5 (20) | 2/2 (100) | 0/2 (00) | |||||
| >1000 | 17/21 (81) | 4/21 (19) | 6/9 (67) | 3/9 (33) | 2/2 (100) | 0/2 (00) | 9/10 (90) | 1/10 (10) | |||||
| Specimen cell amount “Biopsies”, n (%) | 0.18 | 0.70 | 0.82 | 0.15 | |||||||||
| 100–300 | 34/44 (77) | 10/44 (23) | 7/10 (70) | 3/10 (30) | 5/6 (83) | 1/6 (17) | 22/28 (79) | 6/28 (21) | |||||
| >300–500 | 11/18 (61) | 7/18 (39) | 1/2 (50) | 1/2 (50) | 3/4 (75) | 1/4 (25) | 7/12 (58) | 5/12 (42) | |||||
| >500–1000 | 18/23 (78) | 5/23 (22) | 3/5 (60) | 2/5 (40) | 3/3 (100) | 0/3 (00) | 12/15 (80) | 3/15 (20) | |||||
| >1000 | 14/15 (93) | 1/15 (7) | 2/2 (100) | 0/2 (00) | 3/4 (75) | 1/4 (25) | 9/9 (100) | 0/9 (00) | |||||
| Cut-off ≥50% positive cells | All cases, n (%) | 82/100 (82) | 18/100 (18) | 13/19 (68) | 6/19 (32) | 16/17 (94) | 1/17 (6) | 53/64 (83) | 11/64 (17) | ||||
| Histological subtype, n (%) | 0.11 | n/a | 0.41 | 0.51 | |||||||||
| Adenocarcinoma | 51/66 (77) | 15/66 (23) | 13/19 (68) | 6/19 (32) | 6/7 (86) | 1/7 (14) | 32/40 (80) | 8/40 (20) | |||||
| Squamous cell carcinoma | 31/34 (91) | 3/34 (9) | 0/0 (00) | 0/0 (00) | 10/10 (100) | 0/10 (00) | 21/24 (88) | 3/24 (13) | |||||
| Type of biopsy, n (%) | 0.75 | 1.00 | 1.00 | 1.00 | |||||||||
| Bronchial biopsy | 66/80 (83) | 14/80 (18) | 7/10 (70) | 3/10 (30) | 12/13 (92) | 1/13 (8) | 47/57 (83) | 10/57 (18) | |||||
| Transthoracic core biopsy | 16/20 (80) | 4/20 (20) | 6/9 (67) | 3/9 (33) | 4/4 (100) | 0/4 (00) | 6/7 (86) | 1/7 (14) | |||||
| Specimen cell amount “Cell blocks”, n (%) | 0.31 | 0.78 | 0.47 | 0.35 | |||||||||
| 100–300 | 41/47 (87) | 6/47 (13) | 2/3 (67) | 1/3 (33) | 8/8 (100) | 0/8 (00) | 31/36 (86) | 5/36 (14) | |||||
| >300–500 | 16/23 (70) | 7/23 (30) | 3/5 (60) | 2/5 (40) | 2/2 (100) | 0/2 (00) | 11/16 (69) | 5/16 (31) | |||||
| >500–1000 | 8/9 (89) | 1/9 (11) | 2/2 (100) | 0/2 (00) | 4/5 (80) | 1/5 (20) | 2/2 (100) | 0/2 (00) | |||||
| >1000 | 17/21 (81) | 4/21 (19) | 6/9 (67) | 3/9 (33) | 2/2 (100) | 0/2 (00) | 9/10 (90) | 1/10 (10) | |||||
| Specimen cell amount “Biopsies”, n (%) | 0.017 | 0.70 | 0.33 | 0.016 | |||||||||
| 100–300 | 35/44 (80) | 9/44 (21) | 7/10 (70) | 3/10 (30) | 6/6 (100) | 0/6 (00) | 22/28 (79) | 6/28 (21) | |||||
| >300–500 | 11/18 (61) | 7/18 (39) | 1/2 (50) | 1/2 (50) | 3/4 (75) | 1/4 (25) | 7/12 (58) | 5/12 (42) | |||||
| >500–1000 | 21/23 (91) | 2/23 (9) | 3/5 (60) | 2/5 (40) | 3/3 (100) | 0/3 (00) | 15/15 (100) | 0/15 (00) | |||||
| >1000 | 15/15 (100) | 0/15 (00) | 2/2 (100) | 0/2 (00) | 4/4 (100) | 0/4 (00) | 9/9 (100) | 0/9 (00) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, M.S.I.; Hejny, K.; Johansson, F.; Mufti, J.; Vidis, A.; Mager, U.; Dejmek, A.; Seidal, T.; Brunnström, H. Factors Influencing Concordance of PD-L1 Expression between Biopsies and Cytological Specimens in Non-Small Cell Lung Cancer. Diagnostics 2021, 11, 1927. https://doi.org/10.3390/diagnostics11101927
Mansour MSI, Hejny K, Johansson F, Mufti J, Vidis A, Mager U, Dejmek A, Seidal T, Brunnström H. Factors Influencing Concordance of PD-L1 Expression between Biopsies and Cytological Specimens in Non-Small Cell Lung Cancer. Diagnostics. 2021; 11(10):1927. https://doi.org/10.3390/diagnostics11101927
Chicago/Turabian StyleMansour, Mohammed S. I., Kim Hejny, Felicia Johansson, Joudy Mufti, Ante Vidis, Ulrich Mager, Annika Dejmek, Tomas Seidal, and Hans Brunnström. 2021. "Factors Influencing Concordance of PD-L1 Expression between Biopsies and Cytological Specimens in Non-Small Cell Lung Cancer" Diagnostics 11, no. 10: 1927. https://doi.org/10.3390/diagnostics11101927
APA StyleMansour, M. S. I., Hejny, K., Johansson, F., Mufti, J., Vidis, A., Mager, U., Dejmek, A., Seidal, T., & Brunnström, H. (2021). Factors Influencing Concordance of PD-L1 Expression between Biopsies and Cytological Specimens in Non-Small Cell Lung Cancer. Diagnostics, 11(10), 1927. https://doi.org/10.3390/diagnostics11101927








