Diagnostic Performance of Dual-Energy Subtraction Radiography for the Detection of Pulmonary Emphysema: An Intra-Individual Comparison
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Data Acquisition
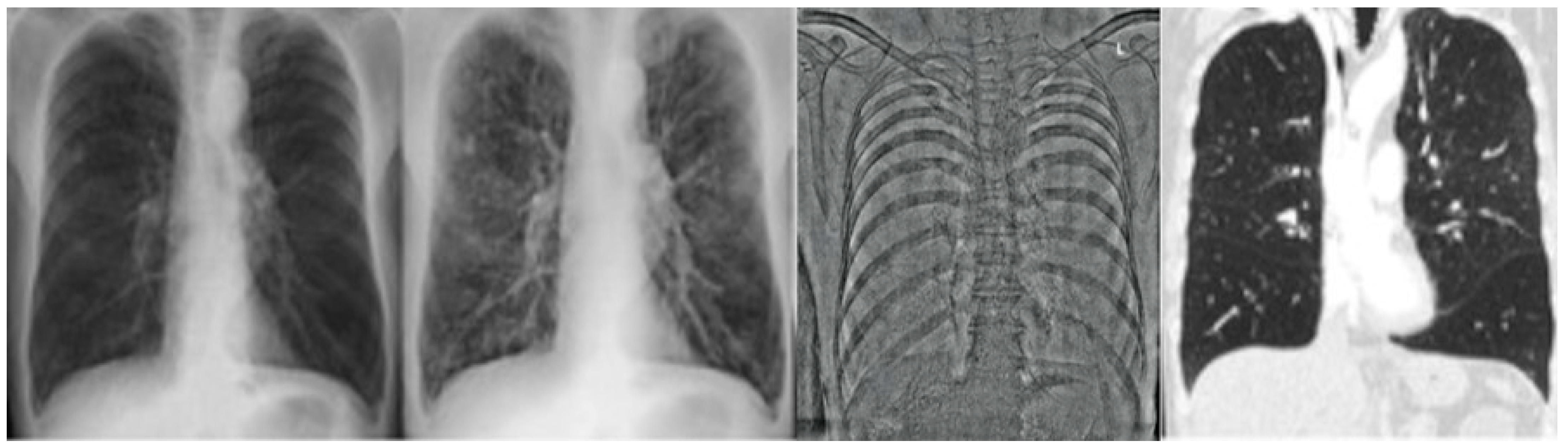
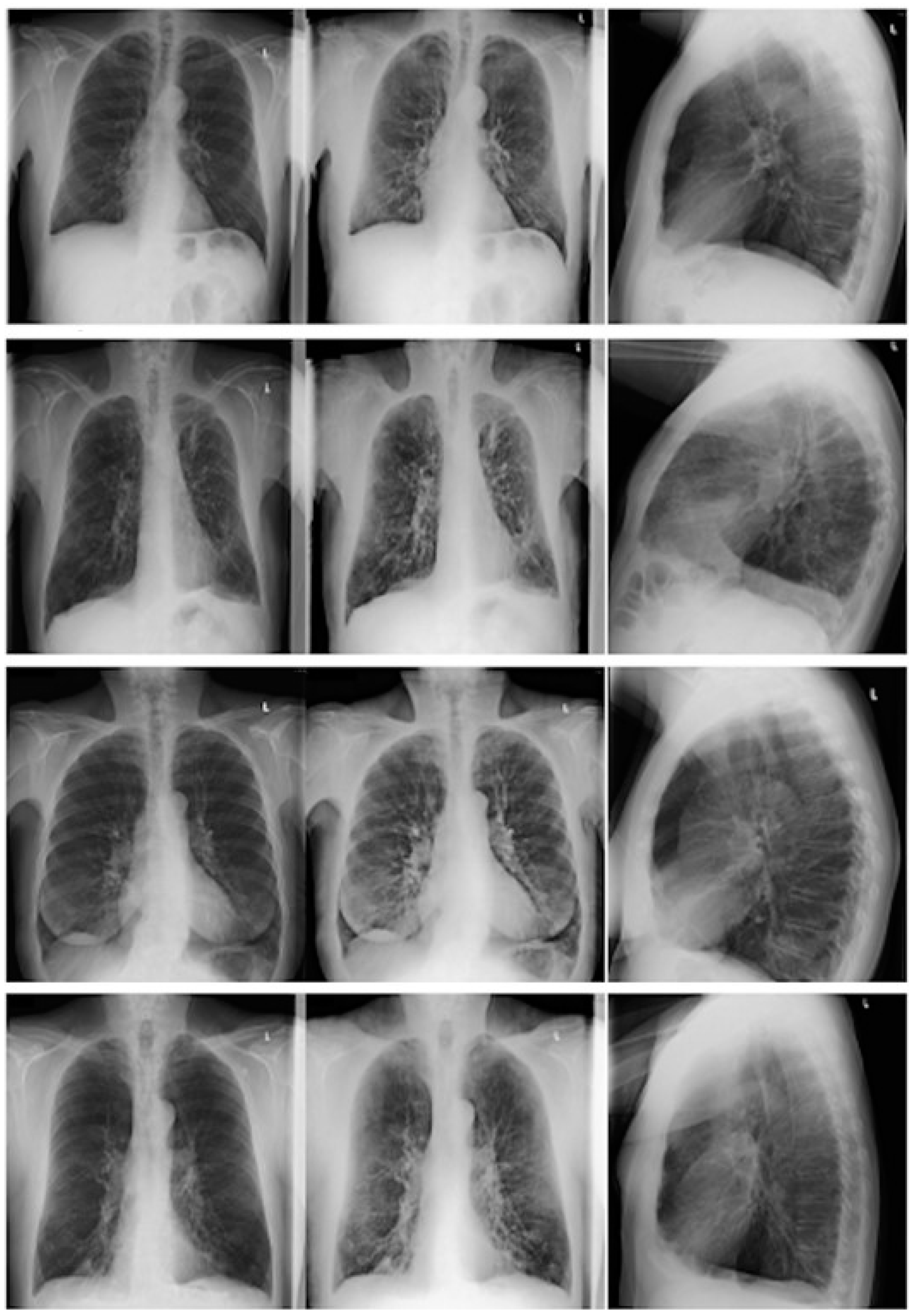
2.2.1. CR and DE Images
2.2.2. CT Images
2.3. Image Analysis
2.3.1. CR/DE Image Analysis
2.3.2. CT Image Analysis
2.3.3. Statistical Analysis
3. Results
3.1. Patient Population
3.2. CT Images: Standard of Reference
3.3. Presence of Emphysema
3.4. Emphysema Grading
3.5. CR and DE Image Analysis
3.5.1. Interreader Agreement
3.5.2. Presence of Emphysema and Location of the Most Affected Lung Quadrant
3.5.3. Severity of Emphysema between CR/DE and CT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Snider, G.L. Nosology for our day: Its application to chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003, 167, 678–683. [Google Scholar] [CrossRef]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2014.
- Sullivan, S.D.; Ramsey, S.D.; Lee, T.A. The economic burden of COPD. Chest 2000, 117, 5S–9S. [Google Scholar] [CrossRef]
- Calverley, P.M.; Walker, P. Chronic obstructive pulmonary disease. Lancet 2003, 362 (Suppl. 2), 1053–1061. [Google Scholar] [CrossRef]
- Plantier, L.; Boczkowski, J.; Crestani, B. Defect of alveolar regeneration in pulmonary emphysema: Role of lung fibroblasts. Int. J. Chronic Obstr. Pulm. Dis. 2007, 2, 463–469. [Google Scholar]
- Gorbunova, V.; Jacobs, S.S.A.M.; Lo, P.; Dirksen, A.; Nielsen, M.; Bab-Hadiashar, A.; de Bruijne, M. Early Detection of Emphysema Progression. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI 2010 13th International Conference, Beijing, China, 20–24 September 2010; Volume 13, pp. 193–200. [Google Scholar]
- Mohsen, L.A.; Gawad, E.A.A.; Ibrahiem, M.A. CT quantification of emphysema: Is semi-quantitative scoring a reliable enough method? Egypt. J. Radiol. Nucl. Med. 2014, 45, 673–678. [Google Scholar] [CrossRef]
- Irion, K.L.; Marchiori, E.; Hochhegger, B.; da Silva Porto, N.; da Silva Moreira, J.; Anselmi, C.E.; Allen Holemans, J.; Irion, P.O. CT Quantification of Emphysema in Young Subjects with No Recognizable Chest Disease. Am. J. Roentgenol. 2008, 192, W90–W96. [Google Scholar] [CrossRef] [PubMed]
- Madani, A.; Keyzer, C.; Gevenois, P.A. Quantitative computed tomography assessment of lung structure and function in pulmonary emphysema. Eur. Respir. J. 2001, 18, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Thurlbeck, W.M.; Müller, N.L. Emphysema: Definition, imaging, and quantification. AJR Am. J. Roentgenol. 1994, 163, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Alkadhi, H.; Marincek, B.; Stolzmann, P. Dual-Energy Bildgebung Nicht nur fürs Handgepäck. Schweiz. Med. Forum. Schlaglichter 2008, 8, 1019–1020. [Google Scholar]
- Welte, T.; Vogelmeier, C.; Papi, A. COPD: Early diagnosis and treatment to slow disease progression. Int. J. Clin. Pract. 2015, 69, 336–349. [Google Scholar] [CrossRef]
- Frauenfelder, T.; Nguyen, T.D.L.; Delaloye, B. CT der Lunge: Von der morphologischen Darstellung zur Quantifizierung. Swiss Med. Forum 2013, 686–688. [Google Scholar] [CrossRef]
- Wells, J.M.; Washko, G.R.; Han, M.K.; Abbas, N.; Nath, H.; Mamary, A.J.; Regan, E.; Bailey, W.C.; Martinez, F.J.; Westfall, E.; et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N. Engl. J. Med. 2012, 367, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Peinado, V.I.; Ramírez, J.; Melgosa, T.; Roca, J.; Rodriguez-Roisin, R.; Barberà, J.A. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur. Respir. J. 2002, 19, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, J.E.; Collins, J.; Brooks, G.N.; Yandow, D.R.; Broderick, L.S. Dual-energy subtraction chest radiography: What to look for beyond calcified nodules. Radiographics 2006, 26, 79–92. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, H.; Armato, S.G. Temporal subtraction chest radiography. Eur. J. Radiol. 2009, 72, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Martini, K.; Baessler, M.; Baumueller, S.; Frauenfelder, T. Diagnostic accuracy and added value of dual-energy subtraction radiography compared to standard conventional radiography using computed tomography as standard of reference. PLoS ONE 2017, 12, e0174285. [Google Scholar] [CrossRef]
- Gilkeson, R.C.; Novak, R.D.; Sachs, P. Digital radiography with dual-energy subtraction: Improved evaluation of cardiac calcification. AJR Am. J. Roentgenol. 2004, 183, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, F.; Freund, T.; Röttgen, R.; Engert, U.; Felix, R.; Ricke, J. Dual-energy chest radiography with a flat-panel digital detector: Revealing calcified chest abnormalities. AJR Am. J. Roentgenol. 2003, 181, 1519–1524. [Google Scholar] [CrossRef]
- Kelcz, F.; Zink, F.E.; Peppler, W.W.; Kruger, D.G.; Ergun, D.L.; Mistretta, C.A. Conventional chest radiography vs dual-energy computed radiography in the detection and characterization of pulmonary nodules. AJR Am. J. Roentgenol. 1994, 162, 271–278. [Google Scholar] [CrossRef]
- Kimura, T.; Kawakami, T.; Kikuchi, A.; Ooev, R.; Akiyama, M.; Horikoshi, H. A Study on Diagnostic Assist Systems of Chronic Obstructive Pulmonary Disease from Medical Images by Deep Learning. J. Comput. Commun. 2018, 6, 21–31. [Google Scholar] [CrossRef][Green Version]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice, 3rd ed.; Pearson/Prentice Hall: Washington, DC, USA, 2009. [Google Scholar]
- Makita, H.; Nasuhara, Y.; Nagai, K.; Ito, Y.; Hasegawa, M.; Betsuyaku, T.; Onodera, Y.; Hizawa, N.; Nishimura, M.; Group, H.C.C.S. Characterisation of phenotypes based on severity of emphysema in chronic obstructive pulmonary disease. Thorax 2007, 62, 932–937. [Google Scholar] [CrossRef]
- Benoit, T.M.; Straub, G.; von Garnier, C.; Franzen, D. Schweres Lungenemphysem: Noch lange kein Endbahnhof! Swiss Med. Forum 2020, 20, 7–11. [Google Scholar] [CrossRef]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; Montes de Oca, M.; Mendez, R.A.; Pinto Plata, V.; Cabral, H.J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef]
- Mair, G.; Miller, J.J.; McAllister, D.; Maclay, J.; Connell, M.; Murchison, J.T.; MacNee, W. Computed tomographic emphysema distribution: Relationship to clinical features in a cohort of smokers. Eur. Respir. J. 2009, 33, 536–542. [Google Scholar] [CrossRef]
- de Torres, J.P.; Bastarrika, G.; Zagaceta, J.; Sáiz-Mendiguren, R.; Alcaide, A.B.; Seijo, L.M.; Montes, U.; Campo, A.; Zulueta, J.J. Emphysema presence, severity, and distribution has little impact on the clinical presentation of a cohort of patients with mild to moderate COPD. Chest 2011, 139, 36–42. [Google Scholar] [CrossRef]
- Gurney, J.W.; Jones, K.K.; Robbins, R.A.; Gossman, G.L.; Nelson, K.J.; Daughton, D.; Spurzem, J.R.; Rennard, S.I. Regional distribution of emphysema: Correlation of high-resolution CT with pulmonary function tests in unselected smokers. Radiology 1992, 183, 457–463. [Google Scholar] [CrossRef]
- Šileikienė, V.; Urbonas, M.; Matačiūnas, M.; Norkūnienė, J. Relationships between pulmonary function test parameters and quantitative computed tomography measurements of emphysema in subjects with chronic obstructive pulmonary disease. Acta Med. Litu. 2017, 24, 209–218. [Google Scholar] [CrossRef][Green Version]
- Thurlbeck, W.M.; Simon, G. Radiographic appearance of the chest in emphysema. AJR Am. J. Roentgenol. 1978, 130, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Linan, D.; Jun, L.; Wushuai, J.; Lu, Z.; Mingschu, W.; Hongli, S.; Shugian, L. Emphysema early diagnosis using X-reay diffraction enhanced imaging at synchrotron light source. BioMed. Eng. OnLine 2014, 13, 82. [Google Scholar]
- Gezer, M.C.; Algin, O.; Durmaz, A.; Arslan, H. Efficiency and reporting confidence analysis of sequential dual-energy subtraction for thoracic X-ray examinations. Qatar Med. J. 2019, 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Huda, W.; Abrahams, R.B. Radiographic techniques, contrast, and noise in X-ray imaging. AJR Am. J. Roentgenol. 2015, 204, W126–W131. [Google Scholar] [CrossRef]
- Del Ciello, A.; Franchi, P.; Contegiacomo, A.; Cicchetti, G.; Bonomo, L.; Larici, A.R. Missed lung cancer: When, where, and why? Diagn Interv. Radiol. 2017, 23, 118–126. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, H. Improvement in detection of pulmonary nodules: Digital image processing and computer-aided diagnosis. Radiographics 2000, 20, 1169–1177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schaefer-Prokop, C.; Neitzel, U.; Venema, H.W.; Uffmann, M.; Prokop, M. Digital chest radiography: An update on modern technology, dose containment and control of image quality. Eur. Radiol. 2008, 18, 1818–1830. [Google Scholar] [CrossRef]
- McKenna, R.J.; Brenner, M.; Fischel, R.J.; Singh, N.; Yoong, B.; Gelb, A.F.; Osann, K.E. Patient selection criteria for lung volume reduction surgery. J. Thorac. Cardiovasc. Surg. 1997, 114, 957–964; discussion 964–967. [Google Scholar] [CrossRef][Green Version]
- Lee, C.W.; Seo, J.B.; Lee, Y.; Chae, E.J.; Kim, N.; Lee, H.J.; Hwang, H.J.; Lim, C.H. A pilot trial on pulmonary emphysema quantification and perfusion mapping in a single-step using contrast-enhanced dual-energy computed tomography. Investig. Radiol. 2012, 47, 92–97. [Google Scholar] [CrossRef]
- Koike, H.; Sueyoshi, E.; Sakamoto, I.; Uetani, M. Quantification of Lung Perfusion Blood Volume by Dual-Energy CT in Patients With and Without Chronic Obstructive Pulmonary Disease. J. Belg. Soc. Radiol. 2015, 99, 62–68. [Google Scholar] [CrossRef][Green Version]
- Lu, G.M.; Zhao, Y.; Zhang, L.J.; Schoepf, U.J. Dual-energy CT of the lung. AJR Am. J. Roentgenol. 2012, 199, S40–S53. [Google Scholar] [CrossRef]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age > 18 years | Incapability of undergoing upright chest radiography |
| Existence of chest examinations with DE and CT | Cardiopulmonary decompensation |
| Description of lung emphysema in radiologic findings or COPD in list of diagnosis | Obscured lung tissue by foreign bodies, e.g., cardiac devices |
| Short time interval between the two imaging modalities | Consolidation, e.g., empyema, encapsulated pneumonia |
| Changes between performed DE and reference CTOccurrence of a pneumothorax Lung volume reduction surgery Endoscopic lung volume reduction, e.g., valves, coils, sealants |
| Patients | Controls | |
|---|---|---|
| Number | 61 | 13 |
| Age (years), mean ± SD | 71.9 ± 8.2 | 70.6 ± 10.8 |
| Time between CT and CR/DE (days), mean ± SD | 41.8 ± 1.4 | 20.7 ± 46.8 |
| Male:Female ratio | 40:21 | 8:5 |
| Assessment Features | Kappa bzw. ICC CR | Kappa bzw. ICC DE |
|---|---|---|
| Presence of emphysema (yes/no) | 0.693 (substantial) | 0.462 (moderate) |
| Subjective emphysema score (none = 1, mild = 2, moderate = 3, severe = 4) | 0.834 (good) | 0.809 (good) |
| Location of maximal emphysema manifestation | 0.306 (fair) | 0.027 (slight) |
| Presence of Emphysema | Location of Maximal Emphysema Manifestation | |||
|---|---|---|---|---|
| Assessment parameter | CR | DE | CR | DE |
| Sensitivity | 96.3% | 90.7% | 50% | 57.4% |
| Specifity | 75% | 83.33% | 100% | 100% |
| NPV | 81.82% | 66.67% | 30.77% | 34.29% |
| PPV | 94.55% | 96.08% | 100% | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller, J.A.; Martini, K.; Eberhard, M.; Mueller, M.A.; De Silvestro, A.A.; Breiding, P.; Frauenfelder, T. Diagnostic Performance of Dual-Energy Subtraction Radiography for the Detection of Pulmonary Emphysema: An Intra-Individual Comparison. Diagnostics 2021, 11, 1849. https://doi.org/10.3390/diagnostics11101849
Mueller JA, Martini K, Eberhard M, Mueller MA, De Silvestro AA, Breiding P, Frauenfelder T. Diagnostic Performance of Dual-Energy Subtraction Radiography for the Detection of Pulmonary Emphysema: An Intra-Individual Comparison. Diagnostics. 2021; 11(10):1849. https://doi.org/10.3390/diagnostics11101849
Chicago/Turabian StyleMueller, Julia A., Katharina Martini, Matthias Eberhard, Mathias A. Mueller, Alessandra A. De Silvestro, Philipp Breiding, and Thomas Frauenfelder. 2021. "Diagnostic Performance of Dual-Energy Subtraction Radiography for the Detection of Pulmonary Emphysema: An Intra-Individual Comparison" Diagnostics 11, no. 10: 1849. https://doi.org/10.3390/diagnostics11101849
APA StyleMueller, J. A., Martini, K., Eberhard, M., Mueller, M. A., De Silvestro, A. A., Breiding, P., & Frauenfelder, T. (2021). Diagnostic Performance of Dual-Energy Subtraction Radiography for the Detection of Pulmonary Emphysema: An Intra-Individual Comparison. Diagnostics, 11(10), 1849. https://doi.org/10.3390/diagnostics11101849








