Personalized First-Line Treatment of Metastatic Pancreatic Neuroendocrine Carcinoma Facilitated by Liquid Biopsy and Computational Decision Support
Abstract
1. Introduction
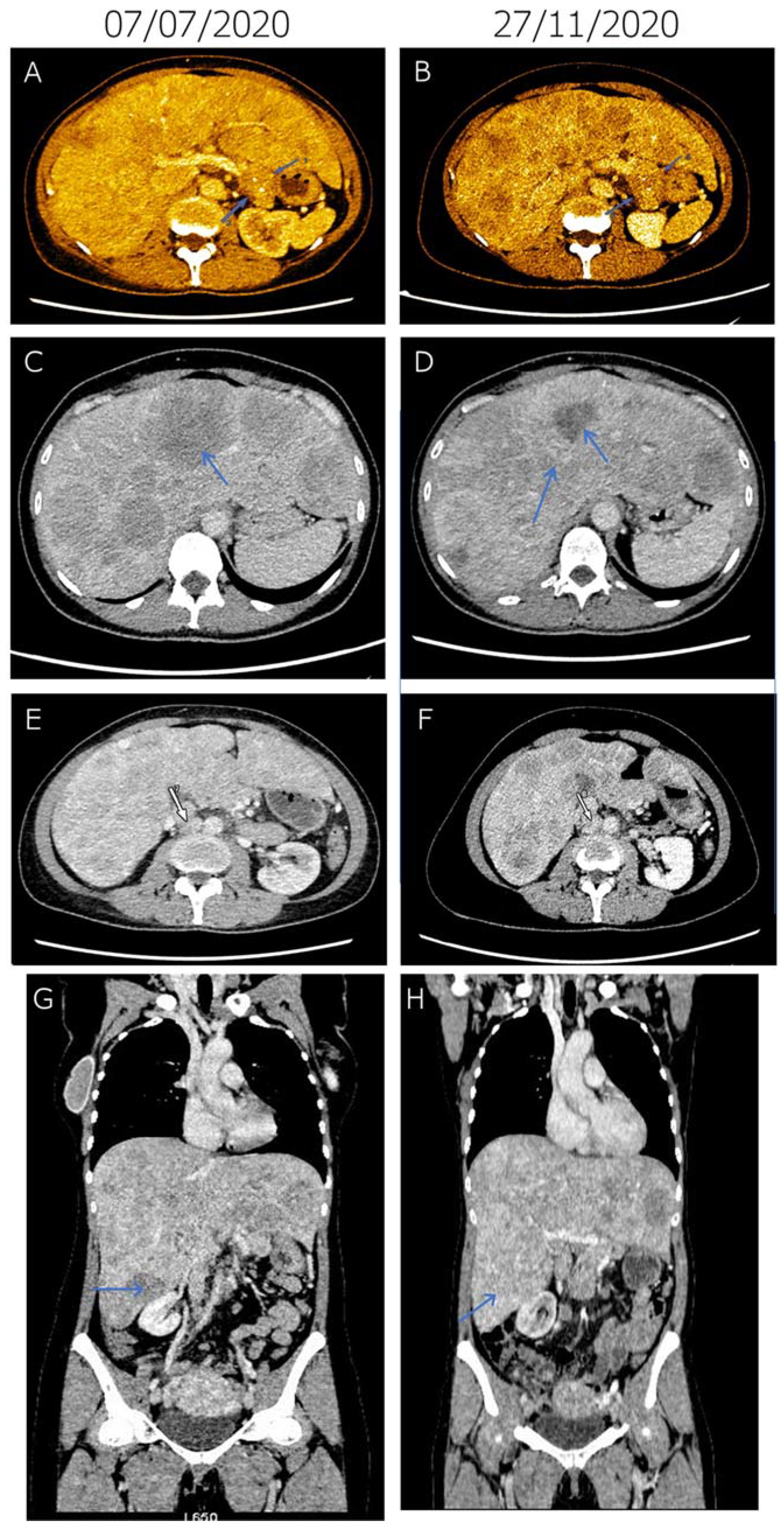
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Young, K.; Iyer, R.; Morganstein, D.; Chau, I.; Cunningham, D.; Starling, N. Pancreatic neuroendocrine tumors: A review. Futur. Oncol. 2015, 11, 853–864. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.; et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.V.; Konda, B.; Fountzilas, C.; Mukherjee, S.; Owen, D.; Attwood, K.; Wang, C.; Ma, C.W.; Minderman, H.; Ba, S.S.; et al. Multicenter phase 2 trial of nintedanib in advanced nonpancreatic neuroendocrine tumors. Cancer 2020, 126, 3689–3697. [Google Scholar] [CrossRef]
- Capdevila, J.; Fazio, N.; Lopez, C.L.; Teule, A.; Valle, J.W.; Tafuto, S.; Custodio, A.B.; Reed, N.; Raderer, M.; Grande, E.; et al. Final results of the TALENT trial (GETNE1509): A prospective multicohort phase II study of lenvatinib in patients (pts) with G1/G2 advanced pancreatic (panNETs) and gastrointestinal (giNETs) neuroendocrine tumors (NETs). J. Clin. Oncol. 2019, 37, 4106. [Google Scholar] [CrossRef]
- Xu, J.; Shen, L.; Bai, C.; Wang, W.; Li, J.; Yu, X.; Li, Z.; Li, E.; Yuan, X.; Chi, Y.; et al. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 1489–1499. [Google Scholar] [CrossRef]
- Tirosh, A.; Kebebew, E. Genetic and epigenetic alterations in pancreatic neuroendocrine tumors. J. Gastrointest. Oncol. 2020, 11, 567–577. [Google Scholar] [CrossRef]
- Koschmann, C.; Lowenstein, P.R.; Castro, M.G. ATRX mutations and glioblastoma: Impaired DNA damage repair, alternative lengthening of telomeres, and genetic instability. Mol. Cell. Oncol. 2016, 3, e1167158. [Google Scholar] [CrossRef]
- Hughes, C.M.; Rozenblatt-Rosen, O.; Milne, T.; Copeland, T.D.; Levine, S.; Lee, J.C.; Hayes, D.N.; Shanmugam, K.S.; Bhattacharjee, A.; Biondi, C.A.; et al. Menin Associates with a Trithorax Family Histone Methyltransferase Complex and with the Hoxc8 Locus. Mol. Cell 2004, 13, 587–597. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr. Molecular basis of the VHL hereditary cancer syndrome. Nat. Rev. Cancer 2002, 2, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, L.; Yuzugullu, H.; Zhao, J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; Yap, T.A.; Meric-Bernstam, F. Targeting the PI3K pathway in cancer: Are we making headway? Nat. Rev. Clin. Oncol. 2018, 15, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Oxnard, G.R.; Paweletz, C.P.; Camidge, D.R.; Heymach, J.V.; Solit, D.B.; Johnson, B.E. Realizing the Potential of Plasma Genotyping in an Age of Genotype-Directed Therapies. J. Natl. Cancer Inst. 2014, 106, dju214. [Google Scholar] [CrossRef][Green Version]
- Rodríguez, J.; Avila, J.; Rolfo, C.; Ruíz-Patiño, A.; Russo, A.; Ricaurte, L.; Ordóñez-Reyes, C.; Arrieta, O.; Zatarain-Barrón, Z.L.; Recondo, G.; et al. When Tissue is an Issue the Liquid Biopsy is Nonissue: A Review. Oncol. Ther. 2021, 9, 89–110. [Google Scholar] [CrossRef]
- Kamyabi, N.; Bernard, V.; Maitra, A. Liquid biopsies in pancreatic cancer. Expert Rev. Anticancer Ther. 2019, 19, 869–878. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.S.; Lee, Y.K.; Norton, J.A.; Jeffrey, S.S. Liquid biopsy in pancreatic ductal adenocarcinoma: Current status of circulating tumor cells and circulating tumorDNA. Mol. Oncol. 2019, 13, 1623–1650. [Google Scholar] [CrossRef]
- Gall, T.M.; Belete, S.; Khanderia, E.; Frampton, A.E.; Jiao, L.R. Circulating Tumor Cells and Cell-Free DNA in Pancreatic Ductal Adenocarcinoma. Am. J. Pathol. 2019, 189, 71–81. [Google Scholar] [CrossRef]
- Rizzo, F.M.; Meyer, T. Liquid Biopsies for Neuroendocrine Tumors: Circulating Tumor Cells, DNA, and MicroRNAs. Endocrinol. Metab. Clin. N. Am. 2018, 47, 471–483. [Google Scholar] [CrossRef]
- Boons, G.; Vandamme, T.; Peeters, M.; Beyens, M.; Driessen, A.; Janssens, K.; Zwaenepoel, K.; Roeyen, G.; Van Camp, G.; De Beeck, K.O. Cell-Free DNA From Metastatic Pancreatic Neuroendocrine Tumor Patients Contains Tumor-Specific Mutations and Copy Number Variations. Front. Oncol. 2018, 8, 467. [Google Scholar] [CrossRef]
- Zakka, K.; Nagy, R.; Drusbosky, L.; Akce, M.; Wu, C.; Alese, O.B.; El-Rayes, B.F.; Kasi, P.M.; Mody, K.; Starr, J.; et al. Blood-based next-generation sequencing analysis of neuroendocrine neoplasms. Oncotarget 2020, 11, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Thieblemont, C.; Alran, S.; Faivre, S. Impact of the COVID-19 Outbreak on the Management of Patients with Cancer. Target. Oncol. 2020, 15, 249–259. [Google Scholar] [CrossRef]
- Petak, I.; Kamal, M.; Dirner, A.; Bieche, I.; Doczi, R.; Mariani, O.; Filotas, P.; Salomon, A.; Vodicska, B.; Servois, V.; et al. A computational method for prioritizing targeted therapies in precision oncology: Performance analysis in the SHIVA01 trial. NPJ Precis. Oncol. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Dogruluk, T.; Tsang, Y.H.; Espitia, M.; Chen, F.; Chen, T.; Chong, Z.; Appadurai, V.; Dogruluk, A.; Eterovic, A.K.; Bonnen, P.E.; et al. Identification of Variant-Specific Functions of PIK3CA by Rapid Phenotyping of Rare Mutations. Cancer Res. 2015, 75, 5341–5354. [Google Scholar] [CrossRef]
- Gymnopoulos, M.; Elsliger, M.-A.; Vogt, P.K. Rare cancer-specific mutations in PIK3CA show gain of function. Proc. Natl. Acad. Sci. USA 2007, 104, 5569–5574. [Google Scholar] [CrossRef]
- Ng, P.K.-S.; Li, J.; Jeong, K.J.; Shao, S.; Chen, H.; Tsang, Y.H.; Sengupta, S.; Wang, Z.; Bhavana, V.H.; Tran, R.; et al. Systematic Functional Annotation of Somatic Mutations in Cancer. Cancer Cell 2018, 33, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Dearth, L.R.; Qian, H.; Wang, T.; Baroni, T.E.; Zeng, J.; Chen, S.W.; Yi, S.Y.; Brachmann, R.K. Inactive full-length p53 mutants lacking dominant wild-type p53 inhibition highlight loss of heterozygosity as an important aspect of p53 status in human cancers. Carcinogenesis 2007, 28, 289–298. [Google Scholar] [CrossRef]
- Jordan, J.J.; Inga, A.; Conway, K.; Edmiston, S.; Carey, L.A.; Wu, L.; Resnick, M.A. Altered-Function p53 Missense Mutations Identified in Breast Cancers Can Have Subtle Effects on Transactivation. Mol. Cancer Res. 2010, 8, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Gonin-Laurent, N.; Gibaud, A.; Huygue, M.; Lefèvre, S.H.; Le Bras, M.; Chauveinc, L.; Sastre-Garau, X.; Doz, F.; Lumbroso, L.; Chevillard, S.; et al. Specific TP53 mutation pattern in radiation-induced sarcomas. Carcinog 2006, 27, 1266–1272. [Google Scholar] [CrossRef]
- Goldschneider, D.; Horvilleur, E.; Plassa, L.-F.; Guillaud-Bataille, M.; Million, K.; Wittmer-Dupret, E.; Danglot, G.; De Thé, H.; Bénard, J.; May, E.; et al. Expression of C-terminal deleted p53 isoforms in neuroblastoma. Nucleic Acids Res. 2006, 34, 5603–5612. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, K.; Reading, J.L.; Lim, E.L.; Xu, H.; Liu, P.; Al-Bakir, M.; Wong, Y.N.S.; Rowan, A.; Funt, S.A.; Merghoub, T.; et al. Escape from nonsense-mediated decay associates with antitumor immunogenicity. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Vaser, R.; Adusumalli, S.; Leng, S.N.; Sikic, M.; Ng, P.C. SIFT missense predictions for genomes. Nat. Protoc. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Inzunza, H.; Chang, H.; Qi, Z.; Hu, B.; Malone, D.; Cogswell, J. Mutations in the Hedgehog Pathway Genes SMO and PTCH1 in Human Gastric Tumors. PLoS ONE 2013, 8, e54415. [Google Scholar] [CrossRef]
- Martínez-Avilés, L.; Besses, C.; Álvarez-Larrán, A.; Torres, E.; Serrano, S.; Bellosillo, B. TET2, ASXL1, IDH1, IDH2, and c-CBL genes in JAK2- and MPL-negative myeloproliferative neoplasms. Ann. Hematol. 2012, 91, 533–541. [Google Scholar] [CrossRef]
- Abdel-Wahab, O.; Mullally, A.; Hedvat, C.; Garcia-Manero, G.; Patel, J.; Wadleigh, M.; Malinge, S.; Yao, J.J.; Kilpivaara, O.; Bhat, R.; et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood 2009, 114, 144–147. [Google Scholar] [CrossRef]
- Nibourel, O.; Kosmider, O.; Cheok, M.; Boissel, N.; Renneville, A.; Philippe, N.; Dombret, H.; Dreyfus, F.; Quesnel, B.; Geffroy, S.; et al. Incidence and prognostic value of TET2 alterations in de novo acute myeloid leukemia achieving complete remission. Blood 2010, 116, 1132–1135. [Google Scholar] [CrossRef]
- Mouliere, F.; Rosenfeld, N. Circulating tumor-derived DNA is shorter than somatic DNA in plasma. Proc. Natl. Acad. Sci. USA 2015, 112, 3178–3179. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Jahr, S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.O.; Hesch, R.D.; Knippers, R. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001, 61, 1659–1665. [Google Scholar] [PubMed]
- Diehl, F.; Li, M.; Dressman, D.; He, Y.; Shen, D.; Szabo, S.; Diaz, L.A., Jr.; Goodman, S.N.; David, K.A.; Juhl, H.; et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 16368–16373. [Google Scholar] [CrossRef]
- Akirov, A.; Larouche, V.; AlShehri, S.; Asa, S.L.; Ezzat, S. Treatment Options for Pancreatic Neuroendocrine Tumors. Cancers 2019, 11, 828. [Google Scholar] [CrossRef]
- Berger, M.F.; Mardis, E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018, 15, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Schwaederle, M.; Zhao, M.; Lee, J.J.; Eggermont, A.M.; Schilsky, R.L.; Mendelsohn, J.; Lazar, V.; Kurzrock, R. Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials. J. Clin. Oncol. 2015, 33, 3817–3825. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulou, M.; Stalla, G.K. Somatostatin receptors: From signaling to clinical practice. Front. Neuroendocr. 2013, 34, 228–252. [Google Scholar] [CrossRef]
| Position | Gene Symbol | Protein Alteration | CDS Alteration | dbSNP ID | Driver AEL Score * | Allele Frequency (%) |
|---|---|---|---|---|---|---|
| chr3:179218286 | PIK3CA | p.P539R | c.1616C>G | 121913285 | 70.81 | 65.00 |
| chr17:7675208 | TP53 | p.C135F | c.404G>T | 587781991 | 53.7 | 44.03 |
| chr1:45332803 | MUTYH | p.Y179C | c.536A>G | 34612342 | 12.35 | 89.92 |
| chr16:2064422 | TSC2 | p.E532* | c.1594G>T | 2.68 | 78.11 | |
| chr16:2065544 | TSC2 | p.P542R | c.1625C>G | 764191178 | 2.68 | 91.11 |
| chr3:37047638 | MLH1 | p.K617N | c.1851G>T | 0.61 | 76.68 | |
| chr4:162111346 | FSTL5 | p.E17D | c.51G>C | 140747357 | 0.39 | 46.97 |
| chr6:33320116 | DAXX | p.E454* | c.1360G>T | 0.34 | 80.74 | |
| chr7:99011389 | TRRAP | p.A3717T | c.11149G>A | 199541716 | 0.25 | 45.65 |
| chr20:53576056 | ZNF217 | p.R903Q | c.2708G>A | 61748378 | 0.24 | 47.70 |
| chr2:140475231 | LRP1B | p.A3178T | c.9532G>A | 72899872 | 0.21 | 89.73 |
| chr5:256368 | SDHA | p.L649fs*4 | c.1945_1946delTT | 112307877 | 0.18 | 13.11 |
| chr12:53215425 | RARG | p.R115C | c.343C>T | 0.05 | 2.23 | |
| chr16:56834405 | NUP93 | p.L567P | c.1700T>C | 774760575 | 0.04 | 10.78 |
| chrX:67711447 | AR | p.G644V | c.1931G>T | 0.04 | 78.93 | |
| chr3:138946005 | FOXL2 | p.G240S | c.718G>A | 767088367 | 0.01 | 11.41 |
| chr1:205661956 | SLC45A3 | p.V377M | c.1129G>A | 150525587 | 0 | 88.35 |
| chr10:17071551 | CUBN | p.N834D | c.2500A>G | 759954219 | 0 | 90.93 |
| chr10:86892221 | BMPR1A | p.Q109K | c.325C>A | 0 | 78.77 | |
| chr14:95455532 | SYNE3 | p.R328G | c.982C>G | 145141808 | 0 | 50.75 |
| chr18:33211925 | CCDC178 | p.V737L | c.2209G>C | 117587736 | 0 | 52.54 |
| chr19:40843915 | CYP2A6 | p.V456I | c.1366G>A | 201305272 | 0 | 27.02 |
| chr19:8946760 | MUC16 | p.P10004S | c.30010C>T | 200869910 | 0 | 40.88 |
| chr19:8958984 | MUC16 | p.S5929F | c.17786C>T | 74872724 | 0 | 44.66 |
| chr3:187729913 | BCL6 | p.E164D | c.492G>T | 61752081 | 0 | 91.27 |
| chr3:49897319 | MST1R | p.R715Q | c.2144G>A | 777611015 | 0 | 87.90 |
| chr4:59457 | ZNF595 | p.I11V | c.31A>G | 6834707 | 0 | 16.78 |
| chr5:14291211 | TRIO | p.E346* | c.1036G>T | 0 | 45.38 | |
| chr9:136475373 | SEC16A | p.Y748S | c.2243A>C | 201466249 | 0 | 48.75 |
| chrX:1193297 | CRLF2 | p.K258R | c.773A>G | 1348007359 | 0 | 15.38 |
| chr12:31097896 | DDX11 | p.Q592E | c.1774C>G | 2911826 | −0.01 | 1.99 |
| chr7:129212264 | SMO | p.R726Q | c.2177G>A | 142495470 | −2.5 | 47.40 |
| chr7:108180375 | NRCAM | p.E900G | c.2699A>G | 34721383 | −5 | 51.18 |
| chrX:45063645 | KDM6A | p.T584M | c.1751C>T | 141353229 | −9.33 | 89.78 |
| chr9:8486142 | PTPRD | p.V892A | c.2675T>C | 151005956 | −9.93 | 9.66 |
| chr17:65538235 | AXIN2 | p.S390G | c.1168A>G | 139871607 | −10 | 48.85 |
| chrX:1196817 | CRLF2 | p.V244M | c.730G>A | 151218732 | −24.22 | 100.00 |
| chr4:105234042 | TET2 | p.L34F | c.100C>T | 111948941 | -53.32 | 49.29 |
| Compound | Associated Driver(s) | Compound AEL Score * |
|---|---|---|
| ALPELISIB | PIK3CA p.P539R | 406.80 |
| COPANLISIB | PIK3CA p.P539R | 397.10 |
| EVEROLIMUS | PIK3CA p.P539R TSC2 p.P542R TSC2 p.E532* | 249.20 |
| SIROLIMUS | PIK3CA p.P539R TSC2 p.P542R TSC2 p.E532* | 200.68 |
| BEVACIZUMAB | TP53 p.C135F | 178.03 |
| TEMSIROLIMUS | PIK3CA p.P539R | 171.96 |
| METFORMIN | PIK3CA p.P539R | 171.15 |
| ASPIRIN | PIK3CA p.P539R | 150.57 |
| SUNITINIB | 114.69 | |
| PAZOPANIB | 113.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkukalek, J.; Dóczi, R.; Dirner, A.; Boldizsár, Á.; Varga, Á.; Déri, J.; Lakatos, D.; Tihanyi, D.; Vodicska, B.; Schwáb, R.; et al. Personalized First-Line Treatment of Metastatic Pancreatic Neuroendocrine Carcinoma Facilitated by Liquid Biopsy and Computational Decision Support. Diagnostics 2021, 11, 1850. https://doi.org/10.3390/diagnostics11101850
Szkukalek J, Dóczi R, Dirner A, Boldizsár Á, Varga Á, Déri J, Lakatos D, Tihanyi D, Vodicska B, Schwáb R, et al. Personalized First-Line Treatment of Metastatic Pancreatic Neuroendocrine Carcinoma Facilitated by Liquid Biopsy and Computational Decision Support. Diagnostics. 2021; 11(10):1850. https://doi.org/10.3390/diagnostics11101850
Chicago/Turabian StyleSzkukalek, Judita, Róbert Dóczi, Anna Dirner, Ákos Boldizsár, Ágnes Varga, Júlia Déri, Dóra Lakatos, Dóra Tihanyi, Barbara Vodicska, Richárd Schwáb, and et al. 2021. "Personalized First-Line Treatment of Metastatic Pancreatic Neuroendocrine Carcinoma Facilitated by Liquid Biopsy and Computational Decision Support" Diagnostics 11, no. 10: 1850. https://doi.org/10.3390/diagnostics11101850
APA StyleSzkukalek, J., Dóczi, R., Dirner, A., Boldizsár, Á., Varga, Á., Déri, J., Lakatos, D., Tihanyi, D., Vodicska, B., Schwáb, R., Pajkos, G., Várkondi, E., Vályi-Nagy, I., Valtinyi, D., Nagy, Z., & Peták, I. (2021). Personalized First-Line Treatment of Metastatic Pancreatic Neuroendocrine Carcinoma Facilitated by Liquid Biopsy and Computational Decision Support. Diagnostics, 11(10), 1850. https://doi.org/10.3390/diagnostics11101850






