The Role of Urine F2-isoprostane Concentration in Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Haemorrhage—A Poor Prognostic Factor
Abstract
1. Introduction
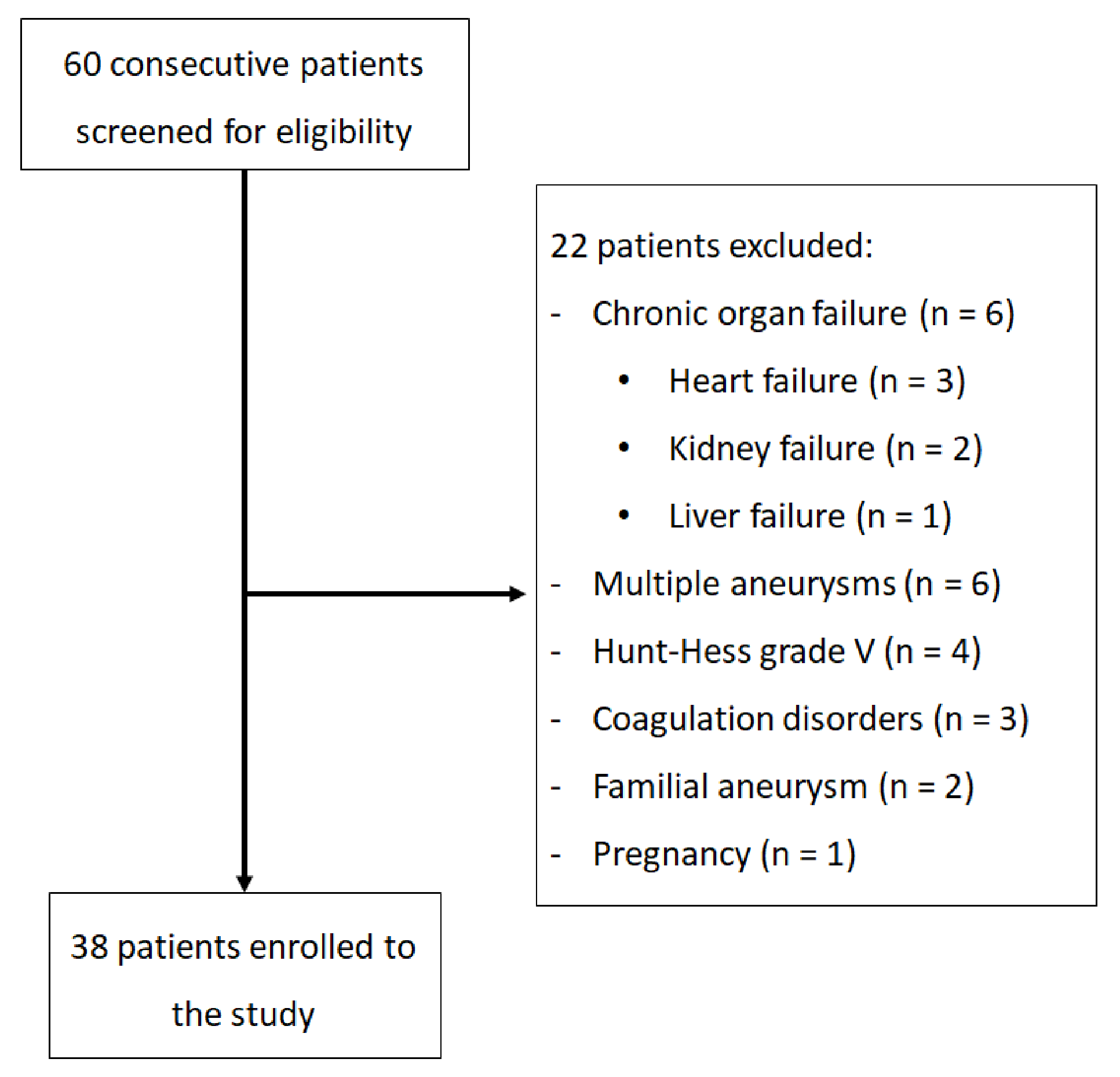
2. Materials and Methods
2.1. Patients
2.2. Clinical Assessment
2.3. Specimen Collection
2.4. Detection of Free form of F2-IsoPs in Urine
2.5. Statistical Analysis
Power and Sample Size Analysis
3. Results
3.1. The Patients’ Clinical Condition
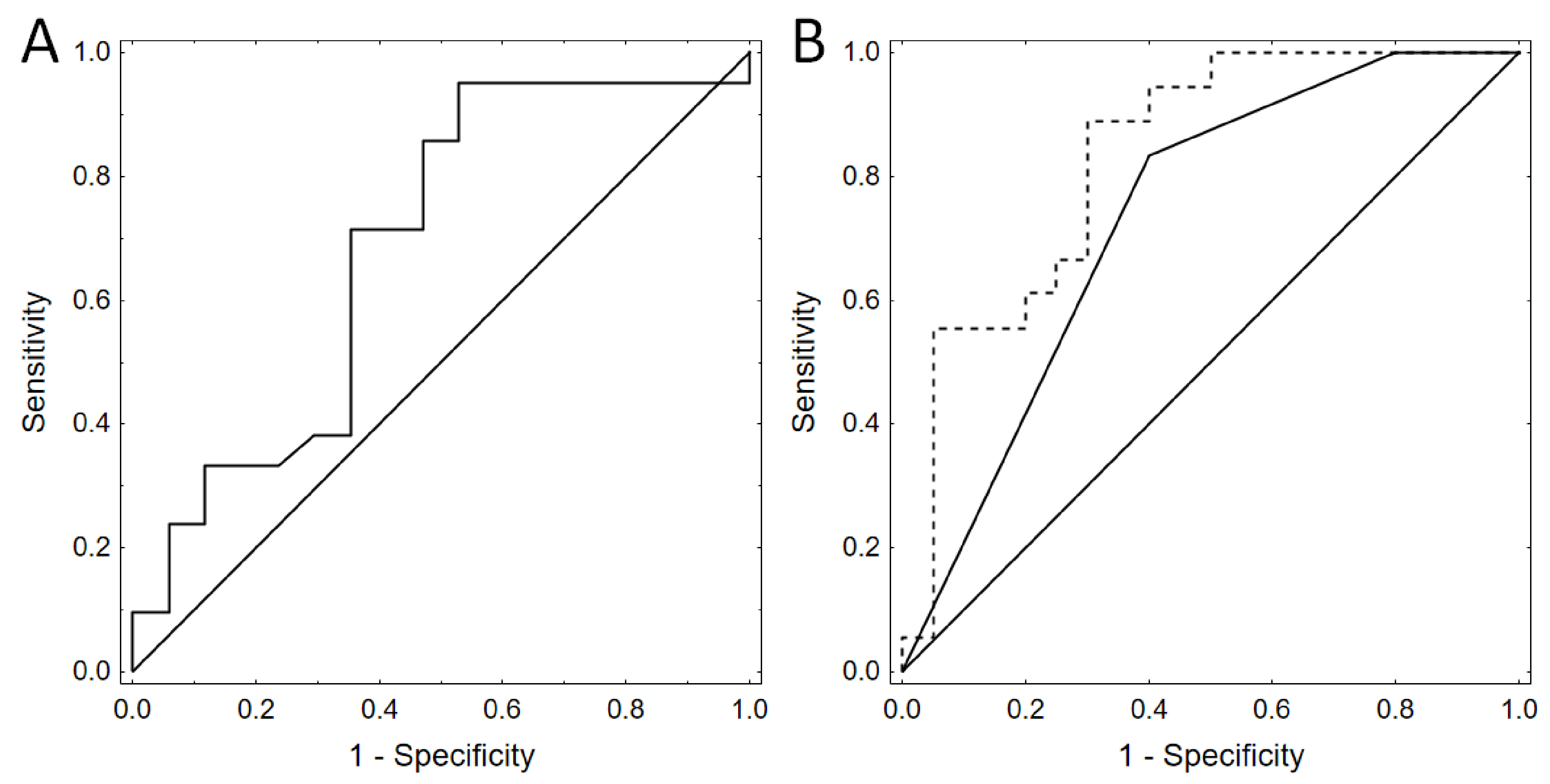
3.2. Urine F2-IsoP Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macdonald, R.L. Cerebral Vasospasm: Advances in Research and Treatment, 1st ed.; Thieme: New York, NY, USA, 2004. [Google Scholar]
- Macdonald, R.L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 2014, 10, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Soehle, M.; Czosnyka, M.; Pickard, J.D.; Kirkpatrick, P.J. Continuous assessment of cerebral autoregulation in subarachnoid hemorrhage. Anesth Analg. 2004, 98, 1133–1139. [Google Scholar] [CrossRef]
- Aldrich, E.F.; Higashida, R.; Hmissi, A.; Le, J.E.; Macdonald, R.L.; Marr, A.; Mayer, S.A.; Roux, S.; Bruder, N. Thick and diffuse cisternal clot independently predicts vasospasm-related morbidity and poor outcome after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Kumar, G.; Shahripour, R.B.; Harrigan, M.R. Vasospasm on transcranial Doppler is predictive of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J. Neurosurg. 2016, 124, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Lubrica, R.J.; Song, M.; Vandse, R.; Boling, W.; Pillai, P. The Role of Transcranial Doppler in Cerebral Vasospasm: A Literature Review. Acta Neurochir. Suppl. 2020, 127, 201–205. [Google Scholar] [PubMed]
- Ayer, R.E.; Zhang, J.H. Oxidative stress in subarachnoid haemorrhage: Significance in acute brain injury and vasospasm. Acta Neurochir. Suppl. 2008, 104, 33–41. [Google Scholar]
- Connolly, E.S.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2020, 43, 1711–1737. [Google Scholar] [CrossRef]
- Abulhasan, Y.B.; Ortiz Jimenez, J.; Teitelbaum, J.; Simoneau, G.; Angle, M.R. Milrinone for refractory cerebral vasospasm with delayed cerebral ischemia. J. Neurosurg. 2020, 27, 1–12. [Google Scholar] [CrossRef]
- de Zwart, L.L.; Meerman, J.H.; Commandeur, J.N.; Vermeulen, N.P. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic. Biol. Med. 1999, 26, 202–226. [Google Scholar] [CrossRef]
- Rokach, J.; Khanapure, S.P.; Hwang, S.W.; Adiyaman, M.; Lawson, J.A.; FitzGerald, G.A. The isoprostanes: A perspective. Prostaglandins 1997, 54, 823–851. [Google Scholar] [CrossRef]
- Janssen, L.J. Isoprostanes and lung vascular pathology. Am. J. Respir. Cell Mol. Biol. 2008, 39, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Barnes, P.; Roberts, L.J. Insights into oxidative stress: The isoprostanes. Curr. Med. Chem. 2007, 14, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, K.; Bieńkowski, M.; Tomasik, B.; Braun, M.; Bobeff, E.J.; Liberski, P.P.; Jaskólski, D.J. Urinary F2-Isoprostane Concentration as a Poor Prognostic Factor After Subarachnoid Hemorrhage. World Neurosurg. 2017, 107, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Philip, I.; Lebret, M.; Chatel, D.; Maclouf, J.; Tedgui, A. Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: A potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation 1998, 28, 1536–1539. [Google Scholar] [CrossRef]
- Yan, Z.; Mas, E.; Mori, T.A.; Croft, K.D.; Barden, A.E. A significant proportion of F2-isoprostanes in human urine are excreted as glucuronide conjugates. Anal. Biochem. 2010, 403, 126–128. [Google Scholar] [CrossRef]
- Fisher, C.M.; Kistler, J.P.; Davis, J.M. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980, 6, 1–9. [Google Scholar] [CrossRef]
- Mayberg, M.R.; Batjer, H.H.; Dacey, R.; Diringer, M.; Haley, E.C.; Heros, R.C.; Sternau, L.L.; Torner, J.; Adams, H.P.; Feinberg, W. Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 1994, 25, 2315–2328. [Google Scholar] [CrossRef]
- Jennett, B.; Bond, M. Assessment of outcome after severe brain damage. Lancet 1975, 1, 480–484. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Core Team, R. A Language and Environment for Statistical Computing. 2013. Available online: https://www.R-project.org/ (accessed on 20 May 2020).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; SAGE Publications: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Marschner I glm2. Fitting Generalized Linear Models. R Package Version 1.1.2.. 2018. Available online: http://CRAN.R-project.org/package1/4glm2 (accessed on 20 March 2020).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open source package for R and Sþ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2009. [Google Scholar]
- Bauer, A.M.; Rasmussen, P.A. Treatment of intracranial vasospasm following subarachnoid hemorrhage. Front. Neurol. 2014, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Treggiari-Venzi, M.M.; Suter, P.M.; Romand, J.A. Review of medical prevention of vasospasm after aneurysmal subarachnoid hemorrhage: A problem of neurointensive care. Neurosurgery 2001, 48, 249–261. [Google Scholar] [PubMed]
- Ferguson, S.; Macdonald, R.L. Predictors of cerebral infarction in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery 2007, 60, 658–667; discussion 667. [Google Scholar] [CrossRef]
- Kumagai, K.; Tomiyama, A.; Takeuchi, S.; Otani, N.; Fujita, M.; Fujii, K.; Wada, K.; Mori, K. New endovascular perforation subarachnoid hemorrhage model for investigating the mechanisms of delayed brain injury. J. Neurosurg. 2019, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Roos, Y.B.; de Haan, R.J.; Beenen, L.F.; Groen, R.J.; Albrecht, K.W.; Vermeulen, M. Complications and outcome in patients with aneurysmal subarachnoid haemorrhage: A prospective hospital based cohort study in the Netherlands. J. Neurol. Neurosurg. Psychiatry 2000, 68, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Stienen, M.N.; Smoll, N.R.; Weisshaupt, R.; Fandino, J.; Hildebrandt, G.; Studerus-Germann, A.; Schatlo, B. Delayed cerebral ischemia predicts neurocognitive impairment following aneurysmal subarachnoid hemorrhage. World Neurosurg. 2014, 82, 599–605. [Google Scholar] [CrossRef]
- Helbok, R.; Madineni, R.C.; Schmidt, M.J.; Kurtz, P.; Fernandez, L.; Ko, S.B.; Choi, A.; Stuart, M.R.; Connolly, E.S.; Lee, K.; et al. Intracerebral monitoring of silent infarcts after subarachnoid hemorrhage. Neurocrit. Care 2011, 14, 162–167. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Ko, S.B.; Helbok, R.; Kurtz, P.; Stuart, R.M.; Presciutti, M.; Fernandez, L.; Lee, K.; Badjatia, N.; Connolly, E.S.; et al. Cerebral perfusion pressure thresholds for brain tissue hypoxia and metabolic crisis after poor-grade subarachnoid hemorrhage. Stroke 2011, 42, 1351–1356. [Google Scholar] [CrossRef]
- Khatibi, K.; Szeder, V.; Blanco, M.B.; Tateshima, S.; Jahan, R.; Duckwiler, G.; Vespa, P. Role of Bedside Multimodality Monitoring in the Detection of Cerebral Vasospasm Following Subarachnoid Hemorrhage. Acta Neurochir. Suppl. 2020, 127, 141–144. [Google Scholar]
- de Oliveira, J.G.; Beck, J.; Ulrich, C.; Rathert, J.; Raabe, A.; Seifert, V. Comparison between clipping and coiling on the incidence of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. Neurosurg. Rev. 2007, 30, 22–30; discussion 21–30. [Google Scholar] [CrossRef]
- Kolias, A.G.; Sen, J.; Belli, A. Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: Putative mechanisms and novel approaches. J. Neurosci. Res. 2009, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Roberts, L.J.; Daniel, V.C.; Awad, J.A.; Mirochnitchenko, O.; Swift, L.L.; Burk, R.F. Comparison of formation of D2/E2- isoprostanes and F2-isoprostanes in vitro and in vivo-effects of oxygen tension and glutathione. Arch Biochem. Biophy. 1998, 353, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Montine, T.J.; Markesbery, W.R.; Morrow, J.D.; Roberts, L.J., 2nd. Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer’s disease. Ann. Neurol. 1998, 44, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Clark, C.M.; Lee, V.M.; Trojanowski, J.Q.; Rokach, J.; FitzGerald, G.A. Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: Correlation of a noninvasive index of lipid peroxidation with disease severity. Ann. Neurol. 2000, 48, 809–812. [Google Scholar] [CrossRef]
- Montine, T.J.; Beal, M.F.; Robertson, D.; Cudkowicz, M.E.; Biaggioni, I.; O’Donnell, H.; Zackert, W.E.; Roberts, L.J.; Morrow, J.D. Cerebrospinal fluid F2-isoprostanes are elevated in Huntington’s disease. Neurology 1999, 52, 1104–1105. [Google Scholar] [CrossRef]
- Seet, R.C.; Lee, C.Y.; Lim, E.C.; Tan, J.J.; Quek, A.M.; Chong, W.L.; Looi, W.F.; Huang, S.H.; Wang, H.; Chan, Y.H.; et al. Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radic. Biol. Med. 2010, 48, 560–566. [Google Scholar] [CrossRef]
- D’Amico, E.; Factor-Litvak, P.; Santella, R.M.; Mitsumoto, H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2013, 65, 509–527. [Google Scholar] [CrossRef]
- Li, G.; Millard, S.P.; Peskind, E.R.; Zhang, J.; Yu, C.E.; Leverenz, J.B.; Mayer, C.; Shofer, J.S.; Raskind, M.A.; Quinn, J.F.; et al. Cross-sectional and longitudinal relationships between cerebrospinal fluid biomarkers and cognitive function in people without cognitive impairment from across the adult life span. JAMA Neurol. 2014, 71, 742–751. [Google Scholar] [CrossRef]
- Lin, C.L.; Hsu, Y.T.; Lin, T.K.; Morrow, J.D.; Hsu, J.C.; Hsu, Y.H.; Hsieh, T.C.; Tsay, P.K.; Yen, H.C. Increased levels of F2-isoprostanes following aneurysmal subarachnoid hemorrhage in humans. Free Radic. Biol. Med. 2006, 15, 1466–1473. [Google Scholar] [CrossRef]
- Roberts, L.J.; Morrow, J.D. The generation and actions of isoprostanes. Biochim. Biophys. Acta 1997, 1345, 121–135. [Google Scholar] [CrossRef]
- Morrow, J.D.; Harris, T.M.; Roberts, L.J. Noncyclooxygenase oxidative formation of a series of novel prostaglandins: Analytical ramifications for measurement of eicosanoids. Anal. Biochem. 1990, 184, 1–10. [Google Scholar] [CrossRef]
- Taylor, A.W.; Bruno, R.S.; Traber, M.G. Women and smokers have elevated urinary F(2)- isoprostane metabolites: A novel extraction and LC-MS methodology. Lipids 2008, 43, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, P.; Lombardi, D. Brain damage following subarachnoid hemorrhage: The imbalance between anti-oxidant systems and lipid peroxidative processes. J. Neurosurg. Sci. 1992, 36, 1–10. [Google Scholar] [PubMed]
- Tian, W.N.; Braunstein, L.D.; Pang, J.; Stuhlmeier, K.M.; Xi, Q.C.; Tian, X.; Stanton, R.C. Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J. Biol. Chem. 1998, 273, 10609–10617. [Google Scholar] [CrossRef] [PubMed]
- Bessard, J.; Cracowski, J.L.; Stanke-Labesque, F.; Bessard, G. Determination of isoprostaglandin F2alpha type III in human urine by gas chromatography-electronic impact mass spectrometry. Comparison with enzyme immunoassay. J. Chromatogr. B Biomed. Sci. Appl. 2001, 754, 333–343. [Google Scholar] [CrossRef]
- Dahl, J.H.; van Breemen, R.B. Rapid quantitative analysis of 8-iso-prostaglandin- F(2alpha) using liquid chromatography-tandem mass spectrometry and comparison with an enzyme immunoassay method. Anal. Biochem. 2001, 404, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, J.; Barden, A.; Mori, T.A.; Burke, V.; Croft, K.D.; Beilin, L.J.; Puddey, I.B. Measurement of urinary F2-isoprostanes as markers of in vivo lipid peroxidation—A comparison of enzyme immunoassay with gas chromatography/mass spectrometry. Anal. Biochem 1999, 272, 209–215. [Google Scholar] [CrossRef]
- Mikeladze, K.G.; Okishev, D.N.; Belousova, O.B.; Konovalov, A.N.; Pilipenko, Y.V.; Ageev, I.S.; Kaftanov, A.N.; Shekhtman, O.D.; Kurdyumova, N.V.; Tabasaransky, T.F.; et al. Intra-arterial Administration of Verapamil for the Prevention and Treatment of Cerebral Angiospasm. Acta Neurochir. Suppl. 2020, 127, 179–183. [Google Scholar]

| Study Group | Control Group | |
|---|---|---|
| Inclusion criteria |
|
|
| Exclusion criteria |
|
|
| Admission * | Discharge † | 1 Month ‡ | 12 Months § | F2-IsoPs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Age (years) | Sex | H/H | Fisher | GOS | GOS | GOS | GOS | mRS | DCI (day) | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
| 1 | 73 | M | 2 | 4 | 3 | 1 | 1 | 1 | 6 | Yes | 13.3 | 14.1 | 27.7 | 20.2 | 17 |
| 2 | 64 | M | 3 | 4 | 2 | 2 | 2 | 2 | 5 | Yes | 20.2 | 22.5 | 23.8 | 7.4 | 0.7 |
| 3 | 60 | F | 3 | 2 | 3 | 3 | 3 | 3 | 4 | Yes | 11.5 | 14.1 | 9.8 | 7.4 | 10.2 |
| 4 | 48 | M | 3 | 2 | 3 | 3 | 3 | 3 | 4 | No | 19.8 | 7.4 | 6.1 | 11.7 | 8 |
| 5 | 82 | F | 1 | 2 | 3 | 3 | 3 | 3 | 3 | No | 8.7 | 9.3 | 12.3 | 13.7 | 25.2 |
| 6 | 57 | F | 3 | 3 | 3 | 4 | 4 | 4 | 2 | No | 29.2 | 39.4 | 31.5 | 23.3 | 17.4 |
| 7 | 66 | F | 3 | 2 | 4 | 4 | 4 | 4 | 2 | No | N/A | 16.3 | 7.0 | 5.6 | 23.4 |
| 8 | 45 | F | 2 | 4 | 4 | 4 | 4 | 5 | 1 | No | 24.1 | 13.1 | 15.5 | 13.1 | N/A |
| 9 | 36 | M | 3 | 3 | 2 | 3 | 4 | 4 | 3 | No | 17 | 12.9 | 13.4 | 9.9 | 17.6 |
| 10 | 65 | F | 2 | 4 | 4 | 4 | 4 | 4 | 2 | No | N/A | 5.6 | 6.2 | 8.5 | 4.2 |
| 11 | 61 | F | 2 | 4 | 4 | 4 | 4 | 4 | 2 | No | 20.5 | 16.9 | 21.8 | 19.6 | 24.7 |
| 12 | 59 | M | 3 | 3 | 3 | 4 | 4 | 4 | 2 | No | 20.7 | 12 | 8.4 | 8.8 | 10.4 |
| 13 | 60 | M | 3 | 4 | 3 | 2 | 2 | 2 | 5 | Yes | 12.7 | 11.9 | 14 | 13.2 | 18.3 |
| 14 | 27 | F | 1 | 1 | 4 | 4 | 4 | 4 | 2 | No | 12.2 | 11 | 8.6 | 19.3 | N/A |
| 15 | 87 | F | 3 | 4 | 2 | 2 | 2 | 2 | 5 | Yes | 8.7 | 8 | 16.8 | 5.8 | 9.5 |
| 16 | 61 | F | 3 | 4 | 3 | 4 | 4 | 4 | 2 | Yes | 86.5 | 16 | 17.4 | 16.2 | 19.9 |
| 17 | 73 | F | 3 | 3 | 3 | 3 | 4 | 4 | 3 | Yes | 16.3 | 14.9 | 13.8 | 9.3 | 6 |
| 18 | 65 | F | 3 | 4 | 3 | 3 | 4 | 4 | 3 | Yes | 24.5 | 30.5 | 21.5 | 13.6 | 18.8 |
| 19 | 39 | M | 1 | 2 | 4 | 4 | 4 | 4 | 2 | No | 26.9 | 14.9 | 8.1 | 7.7 | 6.2 |
| 20 | 47 | M | 2 | 4 | 3 | 4 | 4 | 4 | 2 | Yes | 17.5 | 20 | 17.5 | 20.8 | 22 |
| 21 | 52 | M | 3 | 4 | 3 | 4 | 4 | 4 | 2 | Yes | 19.8 | 23.1 | 16.8 | 14 | 14.3 |
| 22 | 35 | F | 3 | 4 | 3 | 3 | 4 | 4 | 3 | Yes | 38.4 | 26.2 | 26.8 | 26.8 | 34.5 |
| 23 | 68 | M | 3 | 4 | 3 | 2 | 2 | 2 | 5 | Yes | 31.2 | 37 | 40.7 | 36.5 | 22.9 |
| 24 | 60 | F | 3 | 4 | 2 | 2 | 1 | 1 | 6 | Yes | 12.3 | 36.2 | 14.5 | 19 | 11.6 |
| 25 | 60 | F | 3 | 3 | 3 | 3 | 4 | 4 | 3 | Yes | 11.8 | 12 | 11.1 | 16.7 | 9.9 |
| 26 | 90 | M | 2 | 3 | 4 | 3 | 1 | 1 | 6 | Yes | 11.5 | 9.9 | 9.9 | 9.3 | 11.4 |
| 27 | 79 | F | 3 | 3 | 3 | 4 | 4 | 4 | 2 | Yes | 15.1 | 9.6 | 8.8 | 12.7 | N/A |
| 28 | 62 | F | 2 | 2 | 2 | 2 | 1 | 1 | 6 | No | 11.6 | 13 | 11 | 10.6 | 13.7 |
| 29 | 48 | M | 3 | 4 | 3 | 3 | 3 | 3 | 4 | No | 9.5 | 10.5 | 10.9 | 9.6 | 9.2 |
| 30 | 47 | M | 3 | 4 | 3 | 1 | 1 | 1 | 6 | Yes | N/A | 12.6 | 21.7 | 15.1 | N/A |
| 31 | 86 | F | 3 | 3 | 3 | 3 | 4 | 4 | 3 | No | 9.8 | N/A | 12.7 | 10.3 | 9.5 |
| 32 | 65 | F | 1 | 1 | 4 | 5 | 5 | 5 | 0 | No | 15.5 | 11 | 10.2 | 13 | 9.8 |
| 33 | 68 | F | 3 | 3 | 4 | 3 | 4 | 4 | 3 | Yes | 8.5 | 9 | 14.2 | 11.5 | 9.5 |
| 34 | 80 | F | 3 | 2 | 2 | 1 | 1 | 1 | 6 | No | 32.7 | 37.4 | 32.7 | 20.8 | N/A |
| 35 | 46 | F | 2 | 3 | 4 | 4 | 5 | 5 | 1 | No | 8.5 | 9.7 | 11.5 | 7.8 | 6.8 |
| 36 | 42 | F | 2 | 2 | 4 | 4 | 5 | 5 | 0 | No | 10.7 | 30.5 | 8 | 7.4 | 10.3 |
| 37 | 44 | F | 2 | 1 | 4 | 4 | 5 | 5 | 1 | No | 6.8 | 9.3 | 11.8 | 9.6 | 8.9 |
| 38 | 43 | M | 2 | 2 | 4 | 4 | 4 | 4 | 2 | No | 8.7 | 6 | 7.4 | 4.9 | 6 |
| Day 3 | Mean | Peak | |
|---|---|---|---|
| SAH (N = 38) | 15.3 ± 6.2 | 15.5 ± 1.6 | 18.6 ± 8.6 |
| Female sex (N = 24) | 14.8 ± 5.4 | 15.8 ± 2 | 19.2 ± 11 |
| Male sex (N = 14) | 16.1 ± 7.3 | 15 ± 1.5 | 17.6 ± 5 |
| ≤60 years old (N = 16) | 14 ± 5.7 | 14.8 ± 1.7 | 17.9 ± 7 |
| ≥60 years old (N = 22) | 16.2 ± 6.5 | 15.9 ± 1.7 | 19.1 ± 10 |
| SAH—CVS (N = 18) | 18 ± 5.9 | 17.5 ± 1.9 | 21.1 ± 11.2 |
| SAH—no CVS (N = 20) | 12.7 ± 5.1 | 13.6 ± 1.6 | 16.2 ± 6.7 |
| GOS 12 months = 4/5 (N = 24) | 13.7 ± 4.9 | 15.3 ± 2.2 | 20.4 ± 9.7 |
| GOS 12 months = 1/2/3 (N = 14) | 17.9 ± 8 | 15.7 ± 1.6 | 17.9 ± 8 |
| mRS 12 months = 0/1/2 (N = 17) | 12.7 ± 5.1 | 21.5 ± 2.7 | 21.5 ± 10.7 |
| mRS 12 months = 3/4/5/6 (N = 21) | 17.4 ± 6.9 | 15.8 ± 1.4 | 17.5 ± 8.5 |
| Anterior circulation bleeding (N = 33) | 14.8 ± 6 | 15.2 ± 1.6 | 17.2 ± 8.1 |
| Posterior circulation bleeding (N = 5) | 18.2 ± 5.2 | 17.3 ± 4.4 | 27.5 ± 23.5 |
| H/H = 1/2 (N = 15) | 12.4 ± 4.3 | 12.9 ± 0.4 | 14 ± 4.9 |
| H/H = 3 (N = 23) | 17.1 ± 6.8 | 17.2 ± 3.2 | 21.7 ± 10.6 |
| Fisher = 1/2 (N = 12) | 11 ± 3.9 | 12.8 ± 1.7 | 15 ± 6.3 |
| Fisher = 3/4 (N = 26) | 17.2 ± 5.9 | 16.7 ± 1.8 | 20.3 ± 9.3 |
| Controls (N = 13) | mean 7.1 ± 0.9 | ||
| Acute hydrocephalus group (N = 8) | mean 11.7 ± 0.5 | ||
| Intracerebral hemorrhage group (N = 5) | mean 11.8 ± 0.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewski, K.; Popęda, M.; Tomasik, B.; Bieńkowski, M.; Bobeff, E.J.; Stefańczyk, L.; Tybor, K.; Hupało, M.; Jaskólski, D.J. The Role of Urine F2-isoprostane Concentration in Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Haemorrhage—A Poor Prognostic Factor. Diagnostics 2021, 11, 5. https://doi.org/10.3390/diagnostics11010005
Wiśniewski K, Popęda M, Tomasik B, Bieńkowski M, Bobeff EJ, Stefańczyk L, Tybor K, Hupało M, Jaskólski DJ. The Role of Urine F2-isoprostane Concentration in Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Haemorrhage—A Poor Prognostic Factor. Diagnostics. 2021; 11(1):5. https://doi.org/10.3390/diagnostics11010005
Chicago/Turabian StyleWiśniewski, Karol, Marta Popęda, Bartłomiej Tomasik, Michał Bieńkowski, Ernest J. Bobeff, Ludomir Stefańczyk, Krzysztof Tybor, Marlena Hupało, and Dariusz J. Jaskólski. 2021. "The Role of Urine F2-isoprostane Concentration in Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Haemorrhage—A Poor Prognostic Factor" Diagnostics 11, no. 1: 5. https://doi.org/10.3390/diagnostics11010005
APA StyleWiśniewski, K., Popęda, M., Tomasik, B., Bieńkowski, M., Bobeff, E. J., Stefańczyk, L., Tybor, K., Hupało, M., & Jaskólski, D. J. (2021). The Role of Urine F2-isoprostane Concentration in Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Haemorrhage—A Poor Prognostic Factor. Diagnostics, 11(1), 5. https://doi.org/10.3390/diagnostics11010005






