The Prognostic Relevance of Poly (ADP-Ribose) Polymerase Expression in Ovarian Cancer Tissue of Wild Type and BRCA-Mutation Carrier Patients
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Population
2.2. Methods
2.3. Data Collection and Statistical Analyses
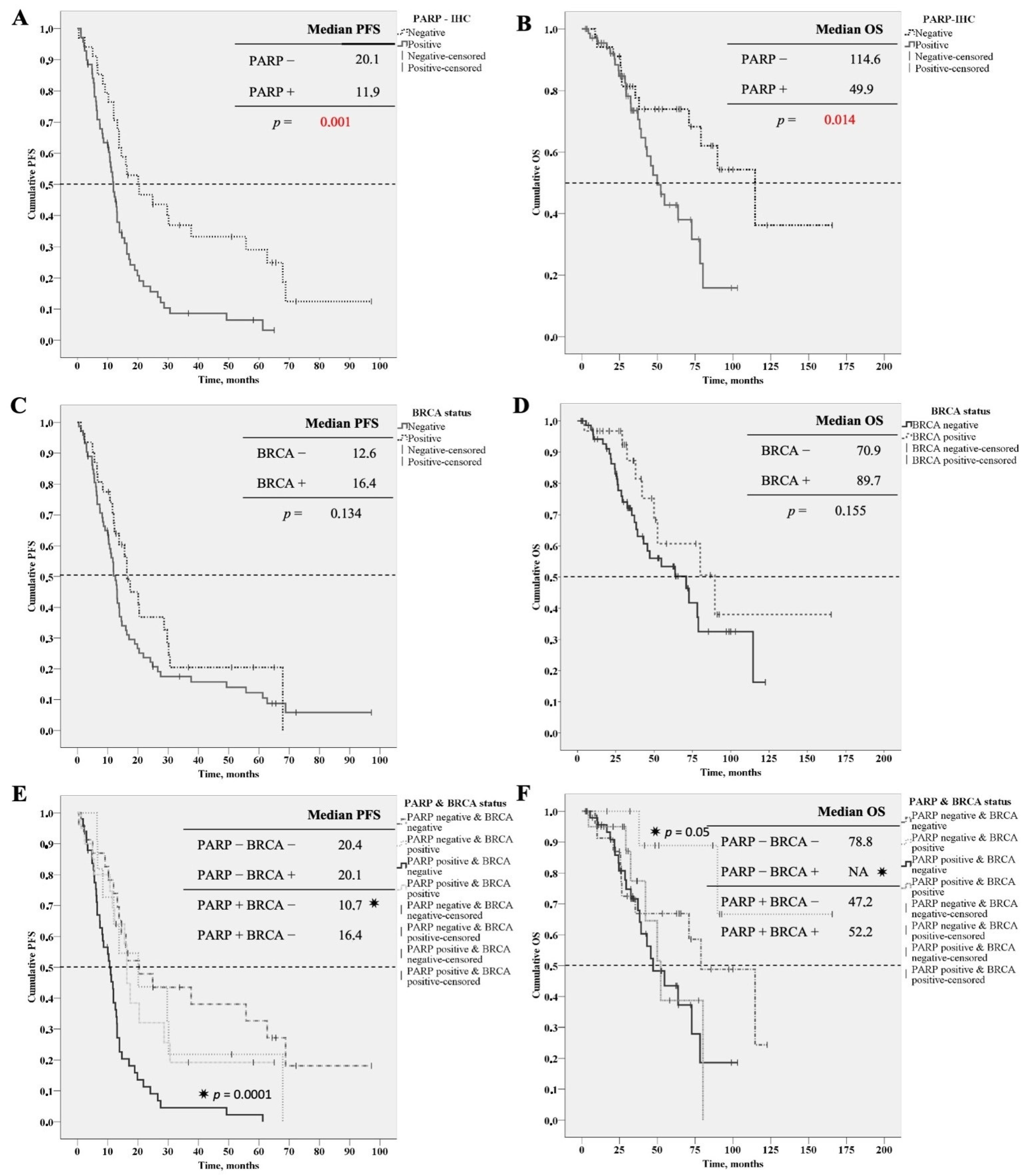
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| BRCA | Breast cancer gene |
| DNA | Deoxyribonucleic acid |
| DSB | Double strand break |
| EOC | Epithelial ovarian cancer |
| ESGO | European Society of Gynecological Oncologists |
| FIGO | International Federation of Gynecology and Obstetrics |
| HGEOC | High grade epithelial ovarian cancer |
| HR | Hazard ratio |
| HRD | Homologous repair deficiency |
| IHC | Immunohistochemistry |
| IQR | Interquartile range |
| MAPK | Mitogen-activated protein kinase |
| MKP | MAPK phosphatase |
| OR | Odds ratio |
| OS | Overall survival |
| PARP | Poly (ADP-ribose) polymerase |
| PDS | Primer debulking surgery |
| PFS | Progression free survival |
| Q3W | Once every 3 weeks |
| R0 | No residual disease after debulking surgery |
| R1 | Residual disease after debulking surgery |
References
- Ataseven, B.; Grimm, C.; Harter, P.; Prader, S.; Traut, A.; Heitz, F.; du Bois, A. Prognostic value of lymph node ratio in patients with advanced epi-thelial ovarian cancer. Gynecol. Oncol. 2014, 135, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Hacker, N.F.; Rao, A. Surgery for advanced epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.A.; Bohlke, K.; Armstrong, D.K.; Bookman, M.A.; Cliby, W.A.; Coleman, R.L.; Dizon, D.S.; Kash, J.J.; Meyer, L.A.; Moore, K.N.; et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Gynecol. Oncol. 2016, 143, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Sammartino, P.; Biacchi, D.; Di Giorgio, A. Letters to the Editor How to improve cytoreductive surgery for ad-vanced ovarian cancer and talk about it in a common language. Gynecol. Oncol. 2012, 127, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J. (Jan) DNA Damage, Aging, and Cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Keijzers, G.; Bakula, D.; Scheibye-Knudsen, M. Monogenic Diseases of DNA Repair. N. Engl. J. Med. 2017, 377, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.C.; Boss, D.S.; Yap, T.A. Inhibition of poly (ADP-ribose) polymerase in tumors from BRCA mutation car-riers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef] [PubMed]
- McLornan, D.P.; List, A.F.; Mufti, G.J. Applying Synthetic Lethality for the Selective Targeting of Cancer. N. Engl. J. Med. 2014, 371, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Marquard, A.M.; Eklund, A.C.; Joshi, T.; Krzystanek, M.; Favero, F.; Wang, Z.C.; Richardson, A.L.; Silver, D.P.; Szallasi, Z.; Birkbak, N.J. Pan-cancer analysis of genomic scar signatures associated with homologous recombination deficiency suggests novel indications for existing cancer drugs. Biomark. Res. 2015, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wiggans, A.J.; Cass, G.K.S.; Bryant, A.; Lawrie, T.A.; Morrison, J. Poly (ADP-ribose) Polymerase (PARP) Inhibitors for the Treatment of Ovarian Cancer (Review); The Cochrane Library: London, UK, 2015. [Google Scholar]
- Molnár, S.; Beke, L.; Méhes, G.; Póka, R. The Prognostic Value of PARP Expression in High-Grade Epithelial Ovarian Cancer. Pathol. Oncol. Res. 2020, 26, 2549–2555. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kho, D.; Zhou, J.-Y.; Davis, R.J.; Wu, G.S. MKP-1 suppresses PARP-1 degradation to mediate cisplatin resistance. Oncogene 2017, 36, 5939–5947. [Google Scholar] [CrossRef] [PubMed]
- Rojo, F.; García-Parra, J.; Zazo, S.; Tusquets, I.; Ferrer-Lozano, J.; Menendez, S.; Eroles, P.; Chamizo, C.; Servitja, S.; Ramírez-Merino, N.; et al. Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann. Oncol. 2012, 23, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.-F.; Li, D.; Yang, Q. Promoter hypomethylation, especially around the E26 transformation-specific motif, and increased expression of poly (ADP-ribose) polymerase 1 in BRCA-mutated serous ovarian cancer. BMC Cancer 2013, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Godoy, H.; Mhawech-Fauceglia, P.; Beck, A.; Miller, A.; Lele, S.; Odunsi, K. Expression of Poly (Adenosine Diphosphate-Ribose) Poly-merase and p53 in Epithelial Ovarian Cancer and Their Role in Prognosis and Disease Outcome. Int. J. Gynecol. Pathol. 2011, 30, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.C.; Bean, S.M.; Nakayama, J.M.; Kondoh, E.; Murphy, S.K.; Berchuck, A. High Poly(Adenosine Diphosphate-Ribose) Polymerase Expression and Poor Survival in Advanced-Stage Serous Ovarian Cancer. Obstet. Gynecol. 2010, 115, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Gan, A.; Green, A.R.; Nolan, C.C.; Martin, S.G.; Deen, S. Poly (adenosine diphosphate-ribose) polymerase expression in BRCA-proficient ovarian high-grade serous carcinoma; association with patient survival. Hum. Pathol. 2013, 44, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall (%) |
|---|---|
| Number of patients | 104 (100%) |
| Mean age (years) | 57.93 (±11.17) |
| Histological type | |
| Serous | 100 (96.15%) |
| other | 4 (3.85%) |
| Grade (2-tier) | |
| High | 104 (100%) |
| Stage | |
| Early (FIGO IIIA») | 15 (14.42%) |
| Advanced (FIGO IIIB«) | 89 (85.58%) |
| Bulky lymph node metastasis | |
| Yes | 33 (31.73%) |
| No | 71 (68.27%) |
| Primer debulking surgery | |
| with no residual disease (R0) | 56 (53.85%) |
| with residual disease (R1) | 48 (46.15%) |
| Median follow-up time | 33.58 months |
| No of relapse | 86 (82.69%) |
| Median PFS | 13.1 months |
| No of death | 40 (38.46%) |
| Median OS | 72.7 months |
| Characteristics | |||
|---|---|---|---|
| Variables | PARP Positive | PARP Negative | p-Value |
| Number of patients (n = 104; 100%) | 70 (67.31%) | 34 (32.69%) | - |
| Mean age (years) | 59.01 ± 10.37 years | 55.71 ± 12.55 years | 0.158 |
| Histological type | |||
| Serous | 68/70 (97.14%) | 32/34 (94.12%) | 0.454 |
| other | 2/70 (2.86%) | 2/34 (6.88%) | |
| Grade (2-tier) | |||
| High | 70/70 (100%) | 34/34 (100%) | 1 |
| Stage | |||
| Early (FIGO IIIA») | 8/70 (11.43%) | 7/34 (20.59%) | 0.41 |
| Advanced (FIGO IIIB«) | 62/70 (88.57%) | 27/34 (79.41%) | |
| Bulky lymph node metastasis | |||
| Yes | 21/70 (30.00%) | 12/34 (35.29%) | 0.59 |
| No | 49/70 (70.00%) | 22/34 (64.71%) | |
| Primer debulking surgery | |||
| with no residual disease (R0) | 36/70 (51.43%) | 20/34 (58.82%) | 0.48 |
| with residual disease (R1) | 34/70 (48.57%) | 14/34 (41.18%) | |
| BRCA status | |||
| positive | 20/70 (28.57%) | 11/34 (32.35%) | 0.694 |
| negative | 50/70 (71.43%) | 23/34 (67.65%) | |
| No of relapse | 60/70 (85.71%) | 26/34 (76.47%) | 0.245 |
| Median PFS | 11.9 months | 20.1 months | 0.001 |
| No of death | 28/70 (40%) | 12/34 (35.29%) | 0.646 |
| Median OS | 49 months | 114 months | 0.014 |
| Reference Column OR = 1 | ||||
|---|---|---|---|---|
| Overall | PARP Negative (n = 34) | PARP Positive (n = 70) | OR (95% CI) | p-Value |
| Relapse | 26 (76.47%) | 60 (85.71%) | 1.85 (0.65–5.21) | 0.25 |
| PFS 12 months> | 8 (23.53%) | 26 (37.14%) | 1.590 (0.62–4.06) | 0.33 |
| Death | 12 (35.29%) | 28 (40.00%) | 1.22 (0.52–2.86) | 0.64 |
| OS 32 months< | 22 (64.70%) | 25 (35.71%) | 0.303 (0.13–0.71) | 0.01> |
| Adjusted for BRCA positive | PARP negative | PARP positive | ||
| (n = 11) | (n = 20) | |||
| Relapse | 9 (81.82%) | 14 (70.00%) | 0.52 (0.09–3.16) | 0.48 |
| PFS 12 months> | 3 (27.27%) | 9 (45.00%) | 2.18 (0.44–10.73) | 0.34 |
| Death | 2 (18.18%) | 7 (35.00%) | 2.42 (0.41–14.46) | 0.33 |
| OS 32 months< | 8 (72.73%) | 7 (35.00%) | 0.20 (0.04–1.01) | 0.05 |
| Adjusted for BRCA negative | PARP negative | PARP positive | ||
| (n = 23) | (n = 50) | |||
| Relapse | 17 (73.91%) | 46 (92.00%) | 4.06 (1.2–16.17) | 0.04 |
| PFS 12 months> | 5 (21.74%) | 35 (70.00%) | 8.40 (2.63–26.82) | 0.01> |
| Death | 10 (43.48%) | 21 (42.00%) | 0.94 (0.35–2.55) | 0.91 |
| OS 32 months< | 14 (60.87%) | 18 (36.00%) | 0.36 (0.13–1.00) | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnár, S.; Vida, B.; Beke, L.; Méhes, G.; Póka, R. The Prognostic Relevance of Poly (ADP-Ribose) Polymerase Expression in Ovarian Cancer Tissue of Wild Type and BRCA-Mutation Carrier Patients. Diagnostics 2021, 11, 144. https://doi.org/10.3390/diagnostics11010144
Molnár S, Vida B, Beke L, Méhes G, Póka R. The Prognostic Relevance of Poly (ADP-Ribose) Polymerase Expression in Ovarian Cancer Tissue of Wild Type and BRCA-Mutation Carrier Patients. Diagnostics. 2021; 11(1):144. https://doi.org/10.3390/diagnostics11010144
Chicago/Turabian StyleMolnár, Szabolcs, Beáta Vida, Lívia Beke, Gábor Méhes, and Róbert Póka. 2021. "The Prognostic Relevance of Poly (ADP-Ribose) Polymerase Expression in Ovarian Cancer Tissue of Wild Type and BRCA-Mutation Carrier Patients" Diagnostics 11, no. 1: 144. https://doi.org/10.3390/diagnostics11010144
APA StyleMolnár, S., Vida, B., Beke, L., Méhes, G., & Póka, R. (2021). The Prognostic Relevance of Poly (ADP-Ribose) Polymerase Expression in Ovarian Cancer Tissue of Wild Type and BRCA-Mutation Carrier Patients. Diagnostics, 11(1), 144. https://doi.org/10.3390/diagnostics11010144







