Histopathological Ratios to Predict Gleason Score Agreement between Biopsy and Radical Prostatectomy
Abstract
:1. Introduction
2. Materials and Methods
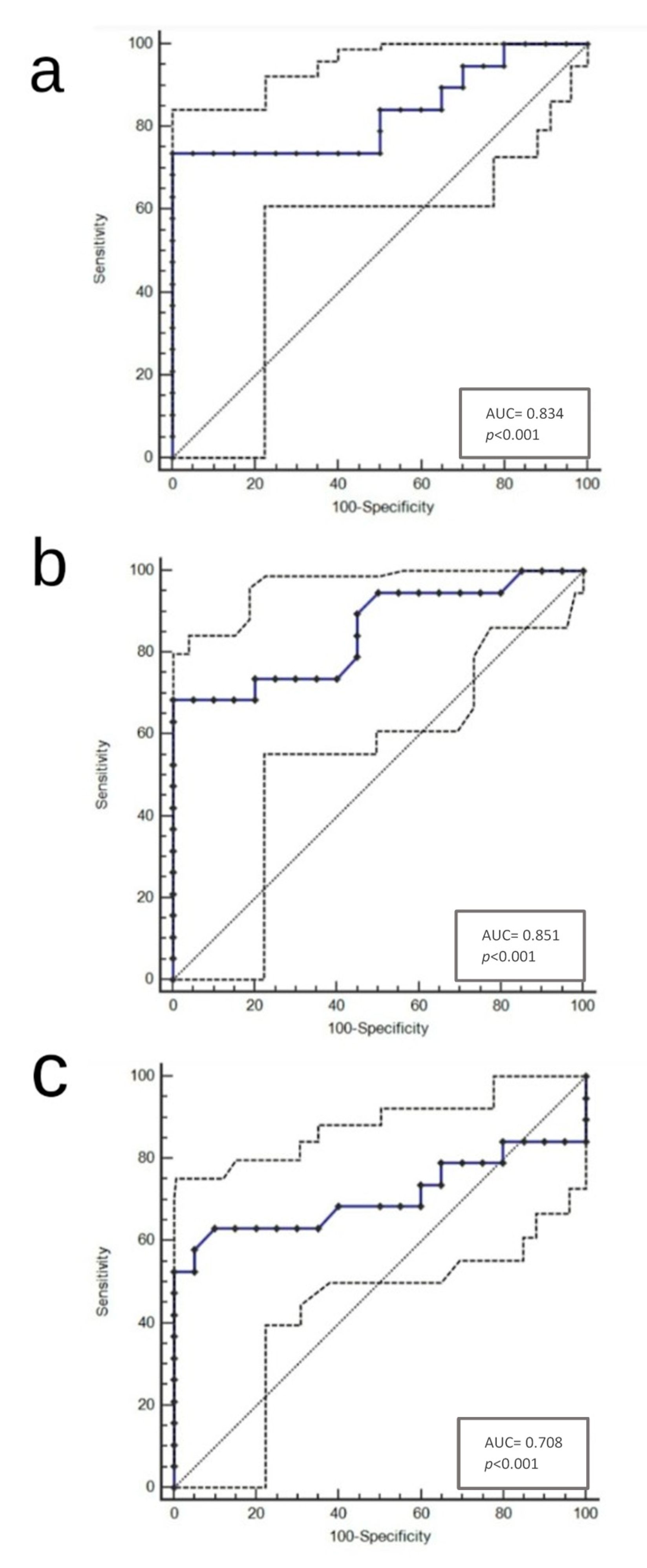
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J.I.; Feng, Z.; Trock, B.J.; Pierorazio, P.M. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: Incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur. Urol. 2012, 61, 1019–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggener, S.E.; Scardino, P.T.; Walsh, P.C.; Han, M.; Partin, A.W.; Trock, B.J.; Feng, Z.; Wood, D.P.; Eastham, J.A.; Yossepowitch, O.; et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J. Urol. 2011, 185, 869–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading Committee. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Pierorazio, P.M.; Walsh, P.C.; Partin, A.W.; Epstein, J.I. Prognostic Gleason grade grouping: Data based on the modified Gleason scoring system. BJU Int. 2013, 111, 753–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Radtke, J.P.; Schwab, C.; Wolf, M.B.; Freitag, M.T.; Alt, C.D.; Kesch, C.; Popeneciu, I.V.; Huettenbrink, C.; Gasch, C.; Klein, T.; et al. Multiparametric Magnetic Resonance Imaging (MRI) and MRI-Transrectal Ultrasound Fusion Biopsy for Index Tumor Detection: Correlation with Radical Prostatectomy Specimen. Eur. Urol. 2016, 70, 846–853. [Google Scholar] [CrossRef]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Fütterer, J.J. European Society of Urogenital Radiology. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef] [Green Version]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Vargas, H.A.; Hötker, A.M.; Goldman, D.A.; Moskowitz, C.S.; Gondo, T.; Matsumoto, K.; Ehdaie, B.; Woo, S.; Fine, S.W.; Reuter, V.E.; et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: Critical evaluation using whole-mount pathology as standard of reference. Eur. Radiol. 2016, 26, 1606–1612. [Google Scholar] [CrossRef] [Green Version]
- Scialpi, M.; Aisa, M.C.; D’Andrea, A.; Martorana, E. Simplified Prostate Imaging Reporting and Data System for Biparametric Prostate MRI: A Proposal. AJR Am. J. Roentgenol. 2018, 211, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Venderink, W.; van Luijtelaar, A.; Bomers, J.G.R.; van der Leest, M.; Hulsbergen-van de Kaa, C.; Barentsz, J.O.; Sedelaar, J.P.M.; Fütterer, J.J. Results of Targeted Biopsy in Men with Magnetic Resonance Imaging Lesions Classified Equivocal, Likely or Highly Likely to Be Clinically Significant Prostate Cancer. Eur. Urol. 2018, 73, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Hanley, R.S.; Kurteva, T.; Ruthazer, R.; Silverman, M.L.; Sorcini, A.; Hamawy, K.; Roth, R.A.; Tuerk, I.; Libertino, J.A. Comparing the Gleason prostate biopsy and Gleason prostatectomy grading system: The Lahey Clinic Medical Center experience and an international meta-analysis. Eur. Urol. 2008, 54, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Le, J.D.; Stephenson, S.; Brugger, M.; Lu, D.Y.; Lieu, P.; Sonn, G.A.; Natarajan, S.; Dorey, F.J.; Huang, J.; Margolis, D.J.; et al. Magnetic resonance imaging-ultrasound fusion biopsy for prediction of final prostate pathology. J. Urol. 2014, 192, 1367–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alchin, D.R.; Murphy, D.; Lawrentschuk, N. Risk factors for Gleason Score upgrading following radical prostatectomy. Minerva Urol. Nefrol. 2017, 69, 459–465. [Google Scholar]
- Bulbul, M.A.; El-Hout, Y.; Haddad, M.; Tawil, A.; Houjaij, A.; Bou Diab, N.; Darwish, O. Pathological correlation between needle biopsy and radical prostatectomy specimen in patients with localized prostate cancer. Can. Urol. Assoc. J. 2007, 1, 264–266. [Google Scholar] [CrossRef] [Green Version]
- Cecchini, S.; Castellani, D.; Fabbietti, P.; Mazzucchelli, R.; Montironi, R.; Cecarini, M.; Carnevali, F.; Pierangeli, T.; Dellabella, M.; Ravasi, E. Combination of Multiparametric Magnetic Resonance Imaging With Elastic-fusion Biopsy Has a High Sensitivity in Detecting Clinically Significant Prostate Cancer in Daily Practice. Clin. Genitourin. Cancer 2020, 8, e501–e509. [Google Scholar] [CrossRef]
- Le, J.D.; Tan, N.; Shkolyar, E.; Lu, D.Y.; Kwan, L.; Marks, L.S.; Huang, J.; Margolis, D.J.; Raman, S.S.; Reiter, R.E. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: Correlation with whole-mount histopathology. Eur. Urol. 2015, 67, 569–576. [Google Scholar] [CrossRef]
- Liu, W.; Laitinen, S.; Khan, S.; Vihinen, M.; Kowalski, J.; Yu, G.; Chen, L.; Ewing, C.M.; Eisenberger, M.A.; Carducci, M.A.; et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat. Med. 2009, 15, 559–565, Erratum in: Nat. Med. 2009, 15, 819. [Google Scholar] [CrossRef] [Green Version]
- Mehra, R.; Tomlins, S.A.; Yu, J.; Cao, X.; Wang, L.; Menon, A.; Rubin, M.A.; Pienta, K.J.; Shah, R.B.; Chinnaiyan, A.M. Characterization of TMPRSS2-ETS gene aberrations in androgen-independent metastatic prostate cancer. Cancer Res. 2008, 15, 3584–3590. [Google Scholar] [CrossRef] [Green Version]
- Mottet, N.; Bellmunt, J.; Briers, E.; Bolla, M.; Bourke, L.; Cornford, P.; De Santis, M.; Henry, A.M.; Joniau, S.; Lam, T.B.; et al. EAU-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. European Association of Urology. Available online: http://uroweb.org/guideline/prostate-cancer.2017 (accessed on 29 August 2020).
- Fuganti, P.E.; Tobias-Machado, M.; Pinto, M.A.; Simardi, L.H.; Wroclawski, E.R. Twelve core prostate biopsy versus six systematic sextant biopsies. Braz. J. Urol. 2002, 28, 207–213. [Google Scholar]
- Stabile, A.; Dell’Oglio, P.; De Cobelli, F.; Esposito, A.; Gandaglia, G.; Fossati, N.; Brembilla, G.; Cristel, G.; Cardone, G.; Deho’, F.; et al. Association Between Prostate Imaging Reporting and Data System (PI-RADS) Score for the Index Lesion and Multifocal, Clinically Significant Prostate Cancer. Eur. Urol. Oncol. 2018, 1, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Qin, Y.; Chen, Y.; Pan, J.; Xu, C.; Wu, D.; Chao, W.Y.; Wei, J.T.; Tomlins, S.A.; Wang, X.; et al. Interstitial assessment of aggressive prostate cancer by physiochemical photoacoustics: An ex vivo study with intact human prostates. Med. Phys. 2018, 45, 4125–4132. [Google Scholar] [CrossRef] [PubMed]
- Ergün, M.; İslamoğlu, E.; Yalçınkaya, S.; Tokgöz, H.; Savaş, M. Does length of prostate biopsy cores have an impact on diagnosis of prostate cancer? Turk. J. Urol. 2016, 42, 130–133. [Google Scholar] [CrossRef]
- Ahmet, C.; Hasan, G. Comparison of prostate biopsy pathology and radical prostatectomy pathologies. Dicle Med. J. 2019, 46, 133–139. [Google Scholar]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Montironi, R.; Mazzucchelli, R.; van der Kwast, T. Morphological assessment of radical prostatectomy specimens. A protocol with clinical relevance. Virchows Arch. 2003, 442, 211–217. [Google Scholar] [CrossRef]
- Divrik, R.T.; Eroglu, A.; Sahin, A.; Zorlu, F.; Ozen, H. Increasing the number of biopsies increases the concordance of Gleason scores of needle biopsies and prostatectomy specimens. Urol. Oncol. 2007, 25, 376–382. [Google Scholar] [CrossRef]
- Yang, C.W.; Lin, T.P.; Huang, Y.H.; Chung, H.J.; Kuo, J.Y.; Huang, W.J.; Wu, H.H.; Chang, Y.H.; Lin, A.T.; Chen, K.K. Does extended prostate needle biopsy improve the concordance of Gleason scores between biopsy and prostatectomy in the Taiwanese population? J. Chin. Med. Assoc. 2012, 75, 97–101. [Google Scholar] [CrossRef]
- Chung, M.S.; Lee, S.H.; Lee, D.H.; Chung, B.H. Is small prostate volume a predictor of Gleason score upgrading after radical prostatectomy? Yonsei Med. J. 2013, 54, 902–906. [Google Scholar] [CrossRef]
- Mehta, V.; Rycyna, K.; Baesens, B.M.; Barkan, G.A.; Paner, G.P.; Flanigan, R.C.; Wojcik, E.M.; Venkataraman, G. Predictors of Gleason Score (GS) upgrading on subsequent prostatectomy: A single Institution study in a cohort of patients with GS 6. Int. J. Clin. Exp. Pathol. 2012, 5, 496–502. [Google Scholar] [PubMed]
- Porpiglia, F.; De Luca, S.; Passera, R.; Manfredi, M.; Mele, F.; Bollito, E.; DEPascale, A.; Cossu, M.; Aimar, R.; Veltri, A. Multiparametric-Magnetic Resonance/Ultrasound Fusion Targeted Prostate Biopsy Improves Agreement Between Biopsy and Radical Prostatectomy Gleason Score. Anticancer Res. 2016, 36, 4833–4839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tourinho-Barbosa, R.R.; de la Rosette, J.; Sanchez-Salas, R. Prostate cancer multifocality, the index lesion, and the microenvironment. Curr. Opin. Urol. 2018, 28, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Baco, E.; Ukimura, O.; Rud, E.; Vlatkovic, L.; Svindland, A.; Aron, M.; Palmer, S.; Matsugasumi, T.; Marien, A.; Bernhard, J.C.; et al. Magnetic resonance imaging-transectal ultrasound image-fusion biopsies accurately characterize the index tumor: Correlation with stepsectioned radical prostatectomy specimens in 135 patients. Eur. Urol. 2015, 67, 787–794. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients | 115 (100%) |
|---|---|
| Mean age in years ± SD (range) | 65.1 ± 7.15 (49–77) |
| Mean PSA at biopsy (ng/mL) ± SD (range) | 12.5 ± 4.9 (6.5–27) |
| Multifocal disease | 79/115 (68.7%) |
| Mean number of nodules ± SD (range) | 2.2 ± 1 (1–4) |
| Mean prostate weight (g) ± SD (range) | 53.5 ± 25.75 (26.1–155) |
| Mean prostate volume (cm3) ± SD (range) | 43.56 ± 29.4 (11.5–137.4) |
| Mean TV (cm3) ± SD (range) | 0.865 ± 0.99 (0.016–3.545) |
| Mean BTA (cm3) ± SD (range) | 0.238 ± 0.88 (0.00574–4.345) |
| Mean BTV (cm3) ± SD (range) | 0.00698 ± 0.0076 (0.000416–0.03) |
| Pathological stage, n (%) | pT2a/b: 20 (17–4) |
| pT2c: 70 (60.9) | |
| pT3a: 20 (17.4) | |
| pT3b: 5 (4.3) | |
| Lymph node status, n (%) | pN0: 50 (43.5) |
| pN1: 0 (0) | |
| pNx: 65 (56.5) | |
| Biopsy Gleason score, n (%) | ISUP 1 (3 + 3): 85 (74) |
| ISUP 2 (3 + 4): 15 (13) | |
| ISUP 3 (4 + 3): 10 (9) | |
| ISUP 4 (4 + 4, 3 + 5, 5 + 3): 5 (4) | |
| Radical prostatectomy Gleason score, n (%) | ISUP 1 (3 + 3): 70 (61) |
| ISUP 2 (3 + 4): 15 (13) | |
| ISUP 3 (4 + 3): 10 (9) | |
| ISUP 4 (4 + 4, 3 + 5, 5 + 3): 20 (17) |
| Ratios | Cut-Off | Upgrade (n = 20) | No Upgrade (n = 95) | p-Value a |
|---|---|---|---|---|
| BTA/TV | >0.05 ≤0.05 | 7 13 | 70 25 | p = 0.0015 |
| BTV/TV | ≥0.0034 <0.0034 | 12 8 | 90 5 | p = 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorentino, V.; Martini, M.; Dell’Aquila, M.; Musarra, T.; Orticelli, E.; Larocca, L.M.; Rossi, E.; Totaro, A.; Pinto, F.; Lenci, N.; et al. Histopathological Ratios to Predict Gleason Score Agreement between Biopsy and Radical Prostatectomy. Diagnostics 2021, 11, 10. https://doi.org/10.3390/diagnostics11010010
Fiorentino V, Martini M, Dell’Aquila M, Musarra T, Orticelli E, Larocca LM, Rossi E, Totaro A, Pinto F, Lenci N, et al. Histopathological Ratios to Predict Gleason Score Agreement between Biopsy and Radical Prostatectomy. Diagnostics. 2021; 11(1):10. https://doi.org/10.3390/diagnostics11010010
Chicago/Turabian StyleFiorentino, Vincenzo, Maurizio Martini, Marco Dell’Aquila, Teresa Musarra, Ersilia Orticelli, Luigi Maria Larocca, Ernesto Rossi, Angelo Totaro, Francesco Pinto, Niccolò Lenci, and et al. 2021. "Histopathological Ratios to Predict Gleason Score Agreement between Biopsy and Radical Prostatectomy" Diagnostics 11, no. 1: 10. https://doi.org/10.3390/diagnostics11010010
APA StyleFiorentino, V., Martini, M., Dell’Aquila, M., Musarra, T., Orticelli, E., Larocca, L. M., Rossi, E., Totaro, A., Pinto, F., Lenci, N., Di Paola, V., Manfredi, R., Bassi, P. F., & Pierconti, F. (2021). Histopathological Ratios to Predict Gleason Score Agreement between Biopsy and Radical Prostatectomy. Diagnostics, 11(1), 10. https://doi.org/10.3390/diagnostics11010010








