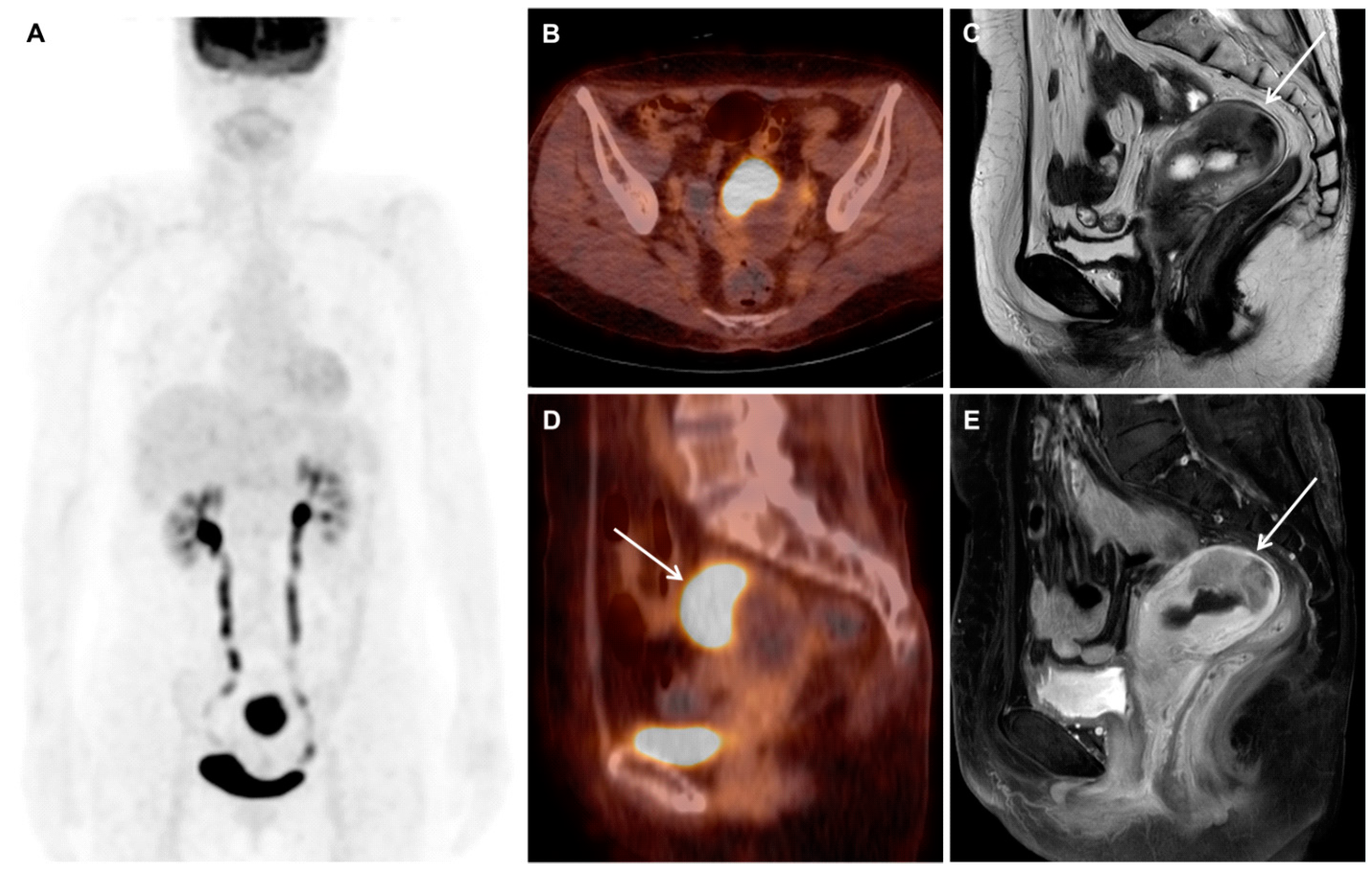
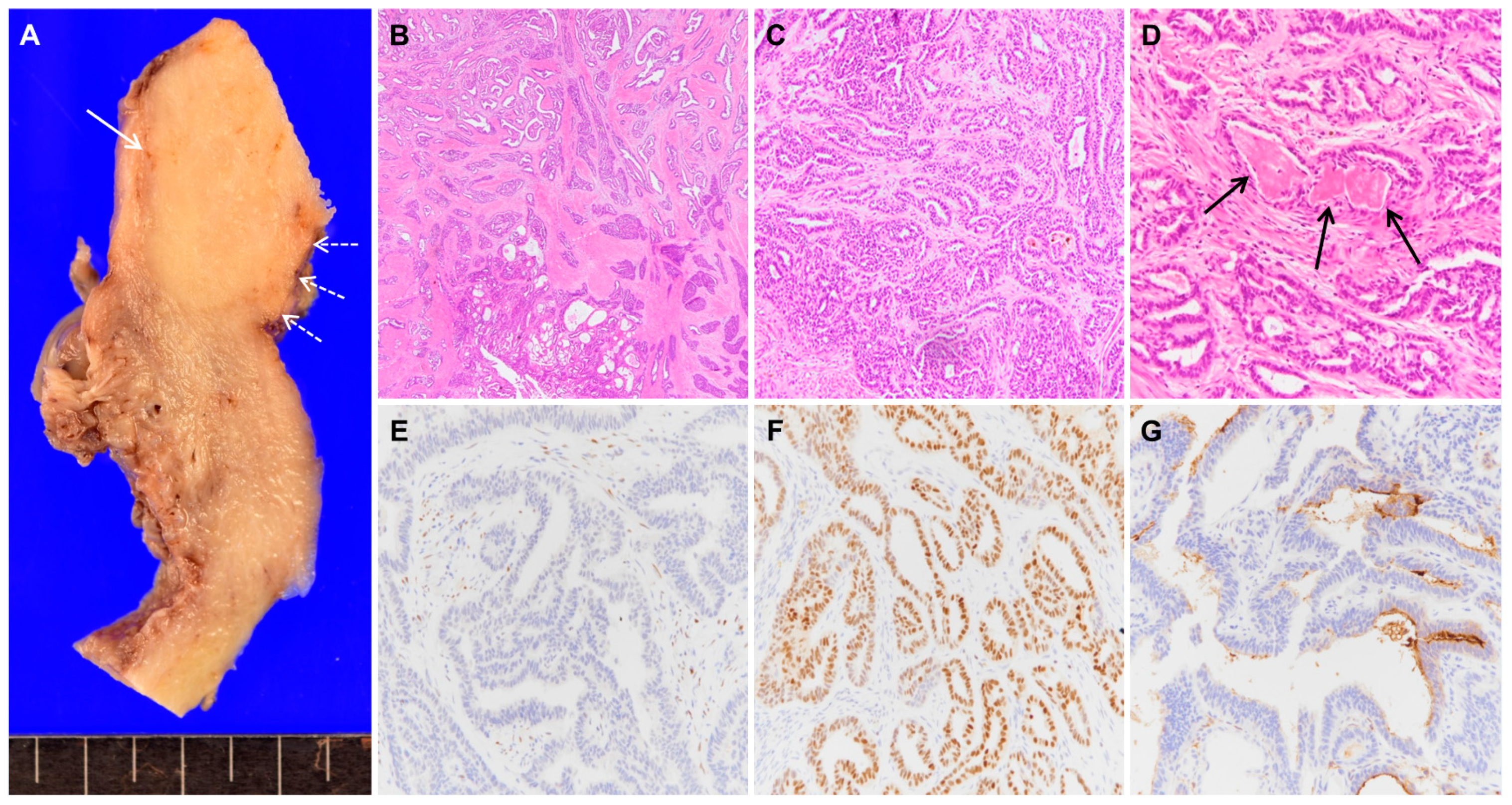
Mesonephric Adenocarcinoma of the Uterine Fundus Exhibiting High 18F-FDG Uptake
Abstract
Author Contributions
Funding
Conflicts of Interest
Consent for Publication
References
- Na, K.; Kim, H.S. Clinicopathologic and molecular characteristics of mesonephric adenocarcinoma arising from the uterine body. Am. J. Surg. Pathol. 2019, 43, 12–25. [Google Scholar] [CrossRef]
- Pors, J.; Cheng, A.; Leo, J.M.; Kinloch, M.A.; Gilks, B.; Hoang, L. A comparison of GATA3, TTF1, CD10, and calretinin in identifying mesonephric and mesonephric-like carcinomas of the gynecologic tract. Am. J. Surg. Pathol. 2018, 42, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Howitt, B.E.; Nucci, M.R. Mesonephric proliferations of the female genital tract. Pathology 2018, 50, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Watanabe, Y.; Ogawa, M.; Tamura, H.; Deguchi, T.; Ikeda, K.; Fujitani, M.; Shioji, M.; Tsujie, T.; Doi, R.; et al. Mesonephric adenocarcinoma of the uterine corpus with intracystic growth completely confined to the myometrium: A case report and literature review. Diagn. Pathol. 2017, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Shintani, D.; Katoh, T.; Hamada, M.; Ito, K.; Kozawa, E.; Hasegawa, K.; Yasuda, M. Coexistence of endometrial mesonephric-like adenocarcinoma and endometrioid carcinoma suggests a Müllerian duct lineage: A case report. Diagn. Pathol. 2019, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- McFarland, M.; Quick, C.M.; McCluggage, W.G. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: Report of a series of mesonephric-like adenocarcinomas. Histopathology 2016, 68, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Euscher, E.D.; Bassett, R.; Duose, D.Y.; Lan, C.; Wistuba, I.; Ramondetta, L.; Ramalingam, P.; Malpica, A. Mesonephric-like carcinoma of the endometrium: A subset of endometrial carcinoma with an aggressive behavior. Am. J. Surg. Pathol. 2020, 44, 429–443. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.K.; Won, K.Y.; Kim, C. Mesonephric Adenocarcinoma of the Uterine Fundus Exhibiting High 18F-FDG Uptake. Diagnostics 2020, 10, 729. https://doi.org/10.3390/diagnostics10090729
Kim HK, Won KY, Kim C. Mesonephric Adenocarcinoma of the Uterine Fundus Exhibiting High 18F-FDG Uptake. Diagnostics. 2020; 10(9):729. https://doi.org/10.3390/diagnostics10090729
Chicago/Turabian StyleKim, Hyung Kyung, Kyu Yeoun Won, and Chanwoo Kim. 2020. "Mesonephric Adenocarcinoma of the Uterine Fundus Exhibiting High 18F-FDG Uptake" Diagnostics 10, no. 9: 729. https://doi.org/10.3390/diagnostics10090729
APA StyleKim, H. K., Won, K. Y., & Kim, C. (2020). Mesonephric Adenocarcinoma of the Uterine Fundus Exhibiting High 18F-FDG Uptake. Diagnostics, 10(9), 729. https://doi.org/10.3390/diagnostics10090729




