Influence of 99m-Tc-Nanocolloid Activity Concentration on Sentinel Lymph Node Detection in Endometrial Cancer: A Quantitative SPECT/CT Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. SPECT/CT Imaging and Quantification
2.3. Surgery
2.4. Statistics
3. Results
3.1. General Characteristics of the Populations
3.2. Quantification in SPECT/CT and Multivariate Analysis
3.3. Quantitative SPECT/CT Analysis of Detected SLNs
3.4. Visual Analysis of SPECT/CT
3.5. Analysis of Parameters Influencing SLN Detection During Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA A Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, N.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Koh, W.-J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 170–199. [Google Scholar] [CrossRef] [PubMed]
- Collarino, A.; Sicart, S.V.; Perotti, G.; Olmos, R.A.V. The sentinel node approach in gynaecological malignancies. Clin. Transl. Imaging 2016, 4, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Preti, E.; Landoni, F.; Carinelli, S.; Colombo, A.; Marini, C.; Sessa, C.; Colombo, N. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol 2013, 24 (Suppl. S6), vi33–vi38. [Google Scholar] [CrossRef] [PubMed]
- FIGO Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int. J. Gynaecol. Obstet. 2014, 125, 97–98. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Alektiar, K.; Iasonos, A.; Lev, G.; Sonoda, Y.; Aghajanian, C.; Chi, D.S.; Barakat, R.R. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: A 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol. Oncol. 2006, 103, 714–718. [Google Scholar] [CrossRef]
- Achouri, A.; Huchon, C.; Bats, A.S.; Bensaid, C.; Nos, C.; Lecuru, F. Complications of lymphadenectomy for gynecologic cancer. Eur. J. Surg. Oncol. 2013, 39, 81–86. [Google Scholar] [CrossRef]
- Marnitz, S.; Köhler, C.; Roth, C.; Fuller, J.; Hinkelbein, W.; Schneider, A. Is there a benefit of pretreatment laparoscopic transperitoneal surgical staging in patients with advanced cervical cancer? Gynecol. Oncol. 2005, 99, 536–544. [Google Scholar] [CrossRef]
- De Gournay, E.; Guyomard, A.; Coutant, C.; Boulet, S.; Arveux, P.; Causeret, S.; Gouy, S.; Padeano, M.-M.; Loustalot, C.; Sauzedde, J.-M.; et al. Impact of sentinel node biopsy on long-term quality of life in breast cancer patients. Br. J. Cancer 2013, 109, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Niikura, H.; Okamoto, S.; Otsuki, T.; Yoshinaga, K.; Utsunomiya, H.; Nagase, S.; Takano, T.; Ito, K.; Watanabe, M.; Yaegashi, N. Prospective Study of Sentinel Lymph Node Biopsy Without Further Pelvic Lymphadenectomy in Patients with Sentinel Lymph Node–Negative Cervical Cancer. Int. J. Gynecol. Cancer 2012, 22, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Giammarile, F.; Bozkurt, M.F.; Cibula, D.; Pahisa, J.; Oyen, W.J.; Paredes, P.; Olmos, R.V.; Sicart, S.V. The EANM clinical and technical guidelines for lymphoscintigraphy and sentinel node localization in gynaecological cancers. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1463–1477. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.B.; Fader, A.N.; Tanner, E.J. Sentinel lymph node assessment in endometrial cancer: A systematic review and meta-analysis. Am. J. Obs. Gynecol. 2017, 216, 459–476.e10. [Google Scholar] [CrossRef]
- Kang, S.; Yoo, H.-J.; Hwang, J.H.; Lim, M.C.; Seo, S.-S.; Park, S.-Y. Sentinel lymph node biopsy in endometrial cancer: Meta-analysis of 26 studies. Gynecol. Oncol. 2011, 123, 522–527. [Google Scholar] [CrossRef]
- Hoogendam, J.P.; Veldhuis, W.B.; Bosch, M.A.V.D.; Zweemer, R.P.; Hobbelink, M.G.G.; Verheijen, R.H. 99mTc SPECT/CT Versus Planar Lymphoscintigraphy for Preoperative Sentinel Lymph Node Detection in Cervical Cancer: A Systematic Review and Metaanalysis. J. Nucl. Med. 2015, 56, 675–680. [Google Scholar] [CrossRef]
- Sahbai, S.; Taran, F.-A.; Fiz, F.; Staebler, A.; Becker, S.; Solomayer, E.; Wallwiener, D.; La Fougère, C.; Brucker, S.; Dittmann, H. Pericervical Injection of 99mTc-Nanocolloid Is Superior to Peritumoral Injection for Sentinel Lymph Node Detection of Endometrial Cancer in SPECT/CT. Clin. Nucl. Med. 2016, 41, 927–932. [Google Scholar] [CrossRef]
- Sahbai, S.; Taran, F.-A.; Staebler, A.; Wallwiener, D.; La Fougère, C.; Brucker, S.; Dittmann, H. Sentinel lymph node mapping using SPECT/CT and gamma probe in endometrial cancer: An analysis of parameters affecting detection rate. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1511–1519. [Google Scholar] [CrossRef]
- Hirschmann, M.T.; Wagner, C.R.; Rasch, H.; Henckel, J. Standardized volumetric 3D-analysis of SPECT/CT imaging in orthopaedics: Overcoming the limitations of qualitative 2D analysis. BMC Med Imaging 2012, 12, 5. [Google Scholar] [CrossRef]
- Yonas, H.; Pindzola, R.R.; Meltzer, C.C.; Sasser, H. Qualitative versus quantitative assessment of cerebrovascular reserves. Neurosurgery 1998, 42, 1005–1010. [Google Scholar] [CrossRef]
- Ogasawara, K.; Okuguchi, T.; Sasoh, M.; Kobayashi, M.; Yukawa, H.; Terasaki, K.; Inoue, T.; Ogawa, A. Qualitative versus quantitative assessment of cerebrovascular reactivity to acetazolamide using iodine-123-N-isopropyl-p-iodoamphetamine SPECT in patients with unilateral major cerebral artery occlusive disease. Am. J. Neuroradiol. 2003, 24, 1090–1095. [Google Scholar]
- Bailey, D.L.; Willowson, K.P. Quantitative SPECT/CT: SPECT joins PET as a quantitative imaging modality. Eur. J. Nucl. Med. Mol. Imaging 2013, 41 (Suppl. S1), 17–25. [Google Scholar]
- Gnesin, S.; Ferreira, P.L.; Malterre, J.; Laub, P.; Prior, J.O.; Verdun, F.R. Phantom Validation of Tc-99m Absolute Quantification in a SPECT/CT Commercial Device. Comput. Math. Methods Med. 2016, 2016, 1–6. [Google Scholar]
- Dittmann, H.; Kopp, D.; Kupferschlaeger, J.; Feil, D.; Groezinger, G.; Syha, R.; Weissinger, M.; La Fougère, C. A Prospective Study of Quantitative SPECT/CT for Evaluation of Lung Shunt Fraction before SIRT of Liver Tumors. J. Nucl. Med. 2018, 59, 1366–1372. [Google Scholar]
- Provost, K.; Leblond, A.; Lemire, A.G.; Filion, E.; Bahig, H.; Lord, M. Reproducibility of Lobar Perfusion and Ventilation Quantification Using SPECT/CT Segmentation Software in Lung Cancer Patients. J. Nucl. Med. Technol. 2017, 45, 185–192. [Google Scholar] [CrossRef][Green Version]
- Tanis, P.J.; E Nieweg, O.; Olmos, R.A.V.; Kroon, B.B. Anatomy and physiology of lymphatic drainage of the breast from the perspective of sentinel node biopsy11No competing interests declared. J. Am. Coll. Surg. 2001, 192, 399–409. [Google Scholar]
- Dittmann, H.; Becker, S.; Bares, R. Detection of sentinel lymph nodes after hysteroscopic peritumoral injection of Tc-99m-Nanocolloid in grade 1 endometrial carcinoma. J. Nucl. Med. 2010, 51 (Suppl. S2), 18. [Google Scholar]
- Khoury-Collado, F.; Glaser, G.E.; Zivanovic, O.; Sonoda, Y.; Levine, D.A.; Chi, D.S.; Gemignani, M.L.; Barakat, R.R.; Abu-Rustum, N.R. Improving sentinel lymph node detection rates in endometrial cancer: How many cases are needed? Gynecol. Oncol. 2009, 115, 453–455. [Google Scholar] [CrossRef]
- Kupferschlaeger, J.; Kuenzel, L.; Dittmann, H.; la Fougère, C. Absolute Quantification in SPECT: A Phantom Study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 148–149. [Google Scholar]
- Pelosi, E.; Arena, V.; Baudino, B.; Bello, M.; Giusti, M.; Gargiulo, T.; Palladin, D.; Bisi, G. Pre-operative lymphatic mapping and intra-operative sentinel lymph node detection in early stage endometrial cancer. Nucl. Med. Commun. 2003, 24, 971–975. [Google Scholar]
- Sahbai, S.; Fiz, F.; Taran, F.; Brucker, S.; Wallwiener, D.; Staebler, A.; Dittmann, H.; la Fougere, C. Combined imaging in cervical cancer with hybrid FDG-PET/MRI for primary staging followed by Tc-99m-SPECT/CT for pre-surgical sentinel lymph node mapping. Eur. J. Nucl. Med. Mol. Imaging 2017, 44 (Suppl. S2), 119–956. [Google Scholar]
- Mayoral, M.; Paredes, P.; Domenech, B.; Fuste, P.; Vidal-Sicart, S.; Tapias, A.; Torné, A.; Pahisa, J.; Ordi, J.; Pons, F.; et al. 18F-FDG PET/CT and sentinel lymph node biopsy in the staging of patients with cervical and endometrial cancer. Role of dual-time-point imaging. Rev. Esp. Med. Nucl. E Imagen Mol. 2017, 36, 20–26. [Google Scholar]
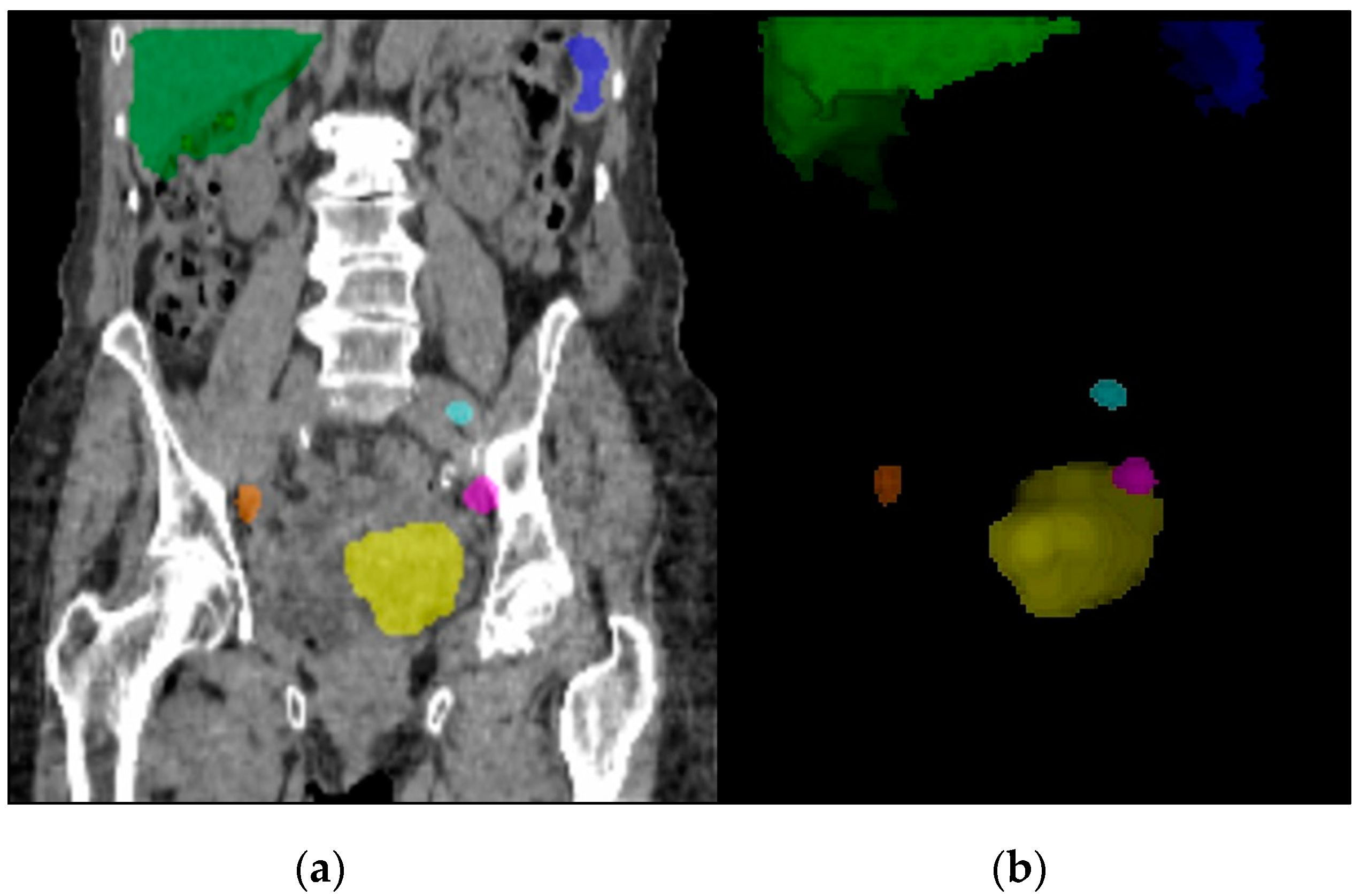
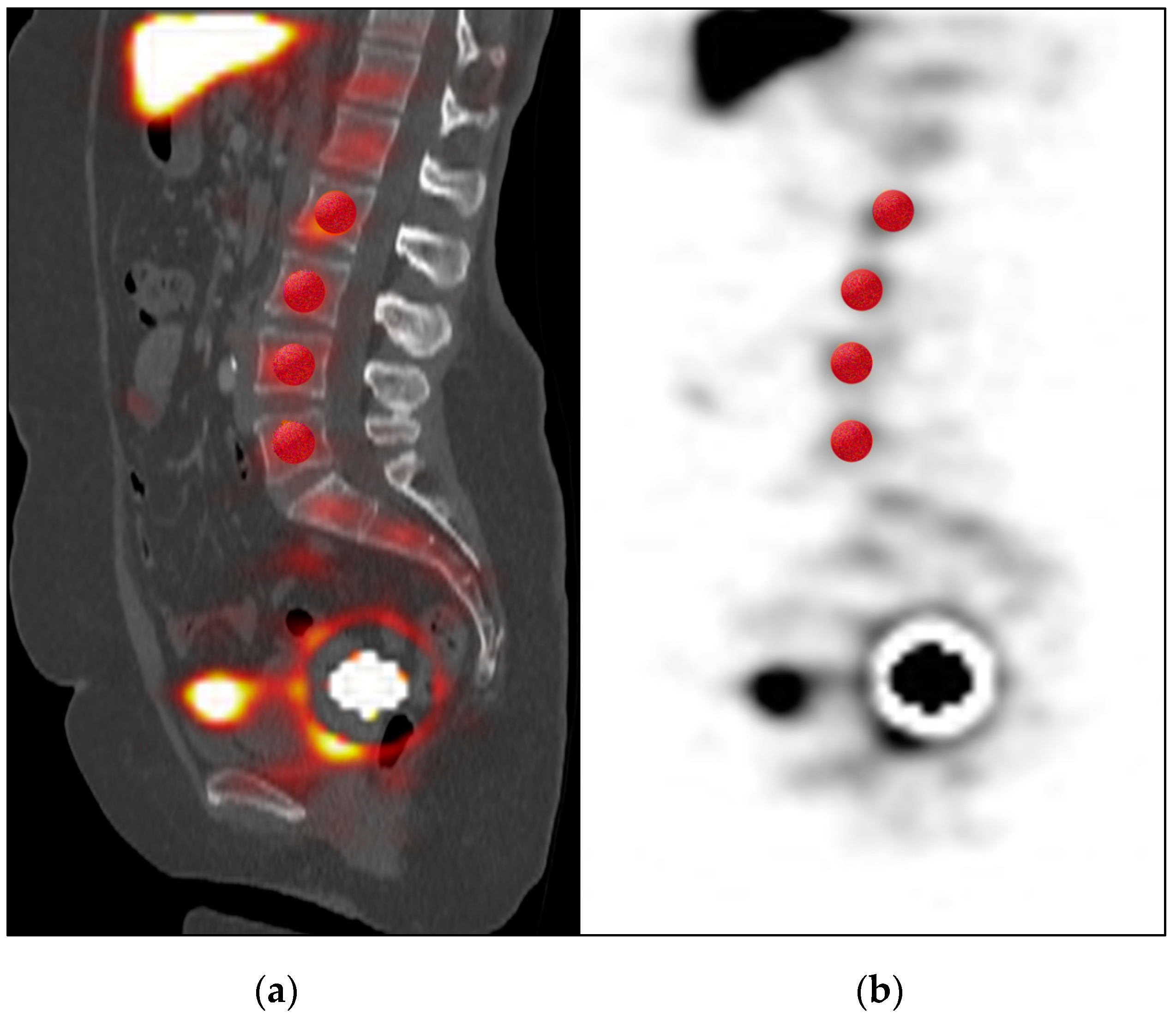
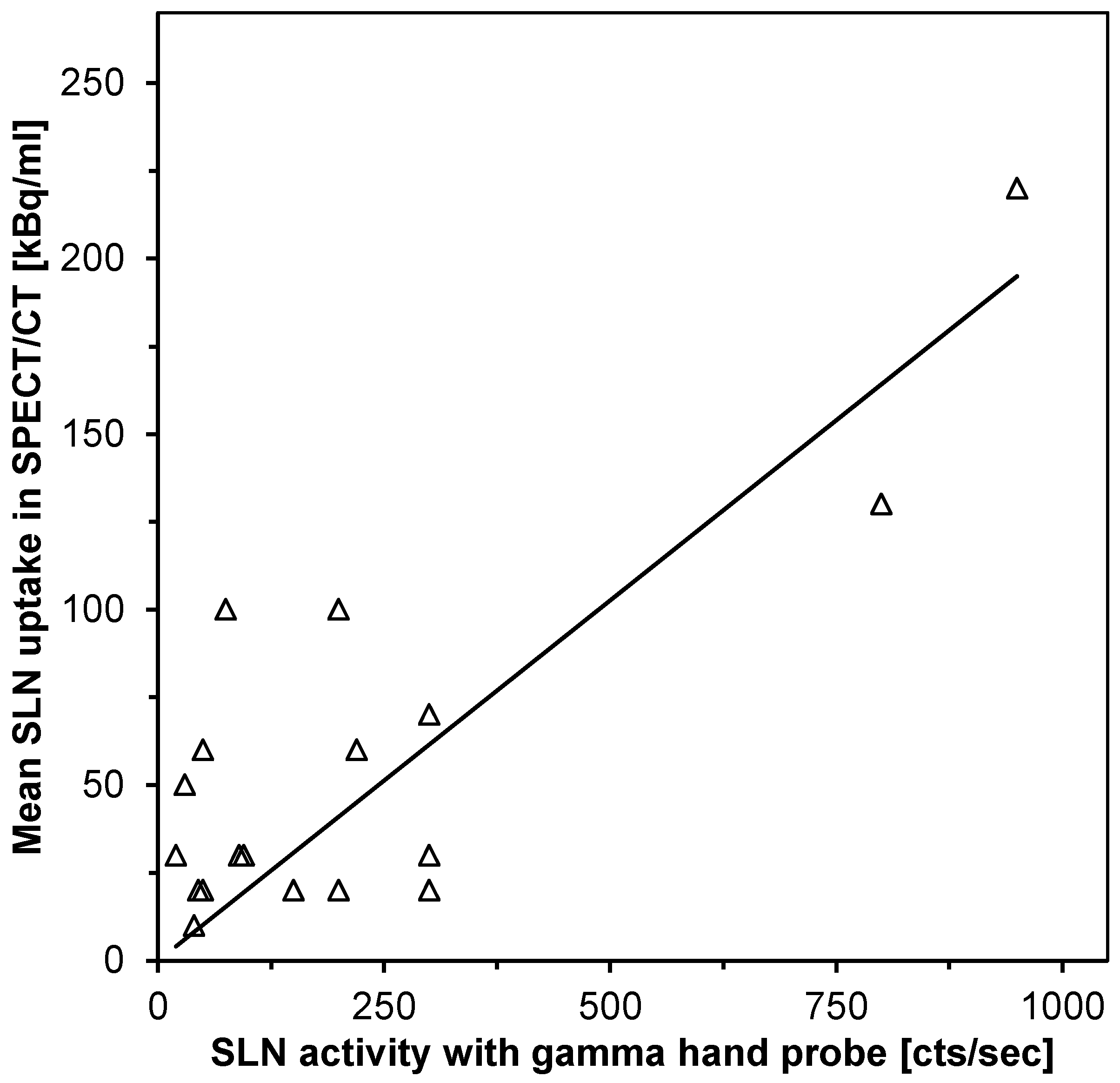
| SPECT/CT Detection | No SLN Detected | SLN Detected |
|---|---|---|
| Number of patients | 20 | 20 |
| Mean age (range) | 63 (36–88) | 63 (35–79) |
| Mean BMI (range) | 29.3 (17–39) | 29.6 (21–42) |
| Mean activity (MBq) | 263 | 260 |
| Mean time p.i. h (range) | 3:46 (2:20–5:41) | 3:36 (2:20–5:51) |
| Histological Type | ||
| Endometrioid | 19 | 18 |
| Serous | 0 | 2 |
| Carcinosarcoma | 1 | 0 |
| Grade | ||
| G1 | 9 | 12 |
| G2 | 9 | 5 |
| G3 | 2 | 3 |
| FIGO | ||
| IA | 14 | 14 |
| IB | 3 | 4 |
| II | 2 | 1 |
| III | 1 | 1 |
| IV | 0 | 0 |
| SPECT/CT Detection | No SLN Detected | SLN Detected | p-Value |
|---|---|---|---|
| Patients, n | 20 | 20 | |
| Quantitative SPECT/CT parameters | |||
| Liver mean uptake (kBq/mL) | 26 | 11 | 0.02 |
| Spleen mean uptake (kBq/mL) | 11 | 5 | 0.02 |
| Bone marrow mean uptake (kBq/mL) | 1.8 | 1.0 | 0.02 |
| Absolute activity at injection site (MBq) | 43 | 73 | 0.04 |
| VOI of the injection site (ml) | 183 | 266 | 0.03 |
| %ID of the injection site %ID of the whole FOV | 30% 62% | 55% 77% | 0.01 0.04 |
| Visual SPECT/CT parameters | |||
| Presence of bone marrow uptake (%) | 35% | 0% | 0.004 |
| Radioactivity in crotch area (%) | 45% | 15% | 0.04 |
| Gamma Hand Probe Detection | No SLN Detected | SLN Detected | p-Value |
|---|---|---|---|
| Patients, n | 17 # | 22 | |
| Quantitative SPECT/CT parameters | |||
| Injection site mean uptake (kBq/mL) | 22 | 35 | 0.001 |
| Absolute activity at injection site (MBq) | 43 | 73 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahbai, S.; Fiz, F.; Taran, F.; Brucker, S.; Wallwiener, D.; Kupferschlaeger, J.; La Fougère, C.; Dittmann, H. Influence of 99m-Tc-Nanocolloid Activity Concentration on Sentinel Lymph Node Detection in Endometrial Cancer: A Quantitative SPECT/CT Study. Diagnostics 2020, 10, 700. https://doi.org/10.3390/diagnostics10090700
Sahbai S, Fiz F, Taran F, Brucker S, Wallwiener D, Kupferschlaeger J, La Fougère C, Dittmann H. Influence of 99m-Tc-Nanocolloid Activity Concentration on Sentinel Lymph Node Detection in Endometrial Cancer: A Quantitative SPECT/CT Study. Diagnostics. 2020; 10(9):700. https://doi.org/10.3390/diagnostics10090700
Chicago/Turabian StyleSahbai, Samine, Francesco Fiz, Florin Taran, Sara Brucker, Diethelm Wallwiener, Juergen Kupferschlaeger, Christian La Fougère, and Helmut Dittmann. 2020. "Influence of 99m-Tc-Nanocolloid Activity Concentration on Sentinel Lymph Node Detection in Endometrial Cancer: A Quantitative SPECT/CT Study" Diagnostics 10, no. 9: 700. https://doi.org/10.3390/diagnostics10090700
APA StyleSahbai, S., Fiz, F., Taran, F., Brucker, S., Wallwiener, D., Kupferschlaeger, J., La Fougère, C., & Dittmann, H. (2020). Influence of 99m-Tc-Nanocolloid Activity Concentration on Sentinel Lymph Node Detection in Endometrial Cancer: A Quantitative SPECT/CT Study. Diagnostics, 10(9), 700. https://doi.org/10.3390/diagnostics10090700





