Cancer Diagnosis by Neural Network Analysis of Data from Semiconductor Sensors
Abstract
1. Introduction
- Support vector machine (SVM);
- k-nearest neighbors algorithm (k-NN);
- Artificial neural network (ANN).
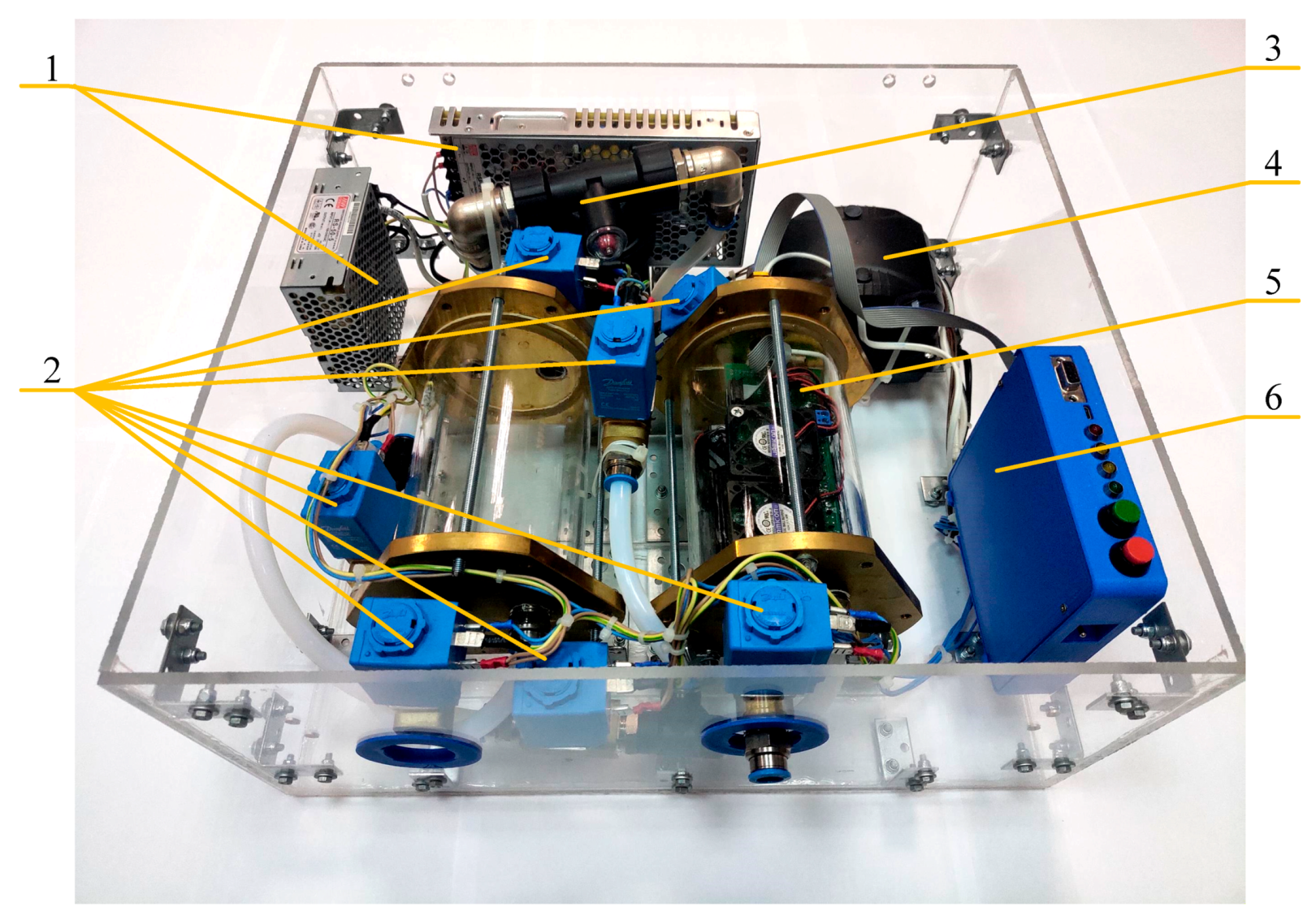
2. Materials and Methods
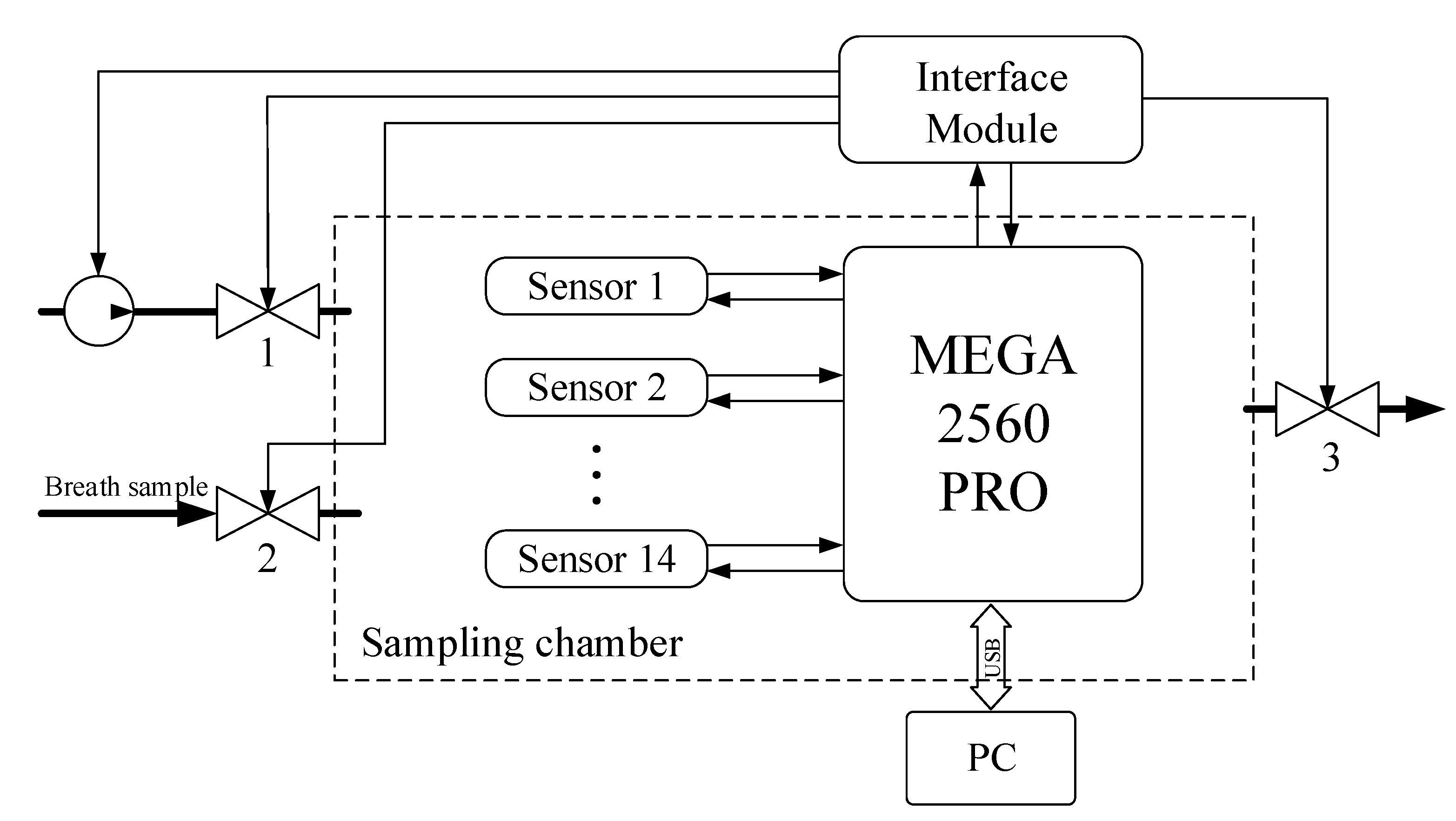
- A half-filled sample bag is connected to the inlet valve. The bag is loaded with the same weight for all measurements. At this moment, the inlet (2) and outlet (3) valves of the sampling chamber are closed.
- In the interface of the data collection program, a button is pressed and the reading and transmission of data at a frequency of 30 Hz to a personal computer begins.
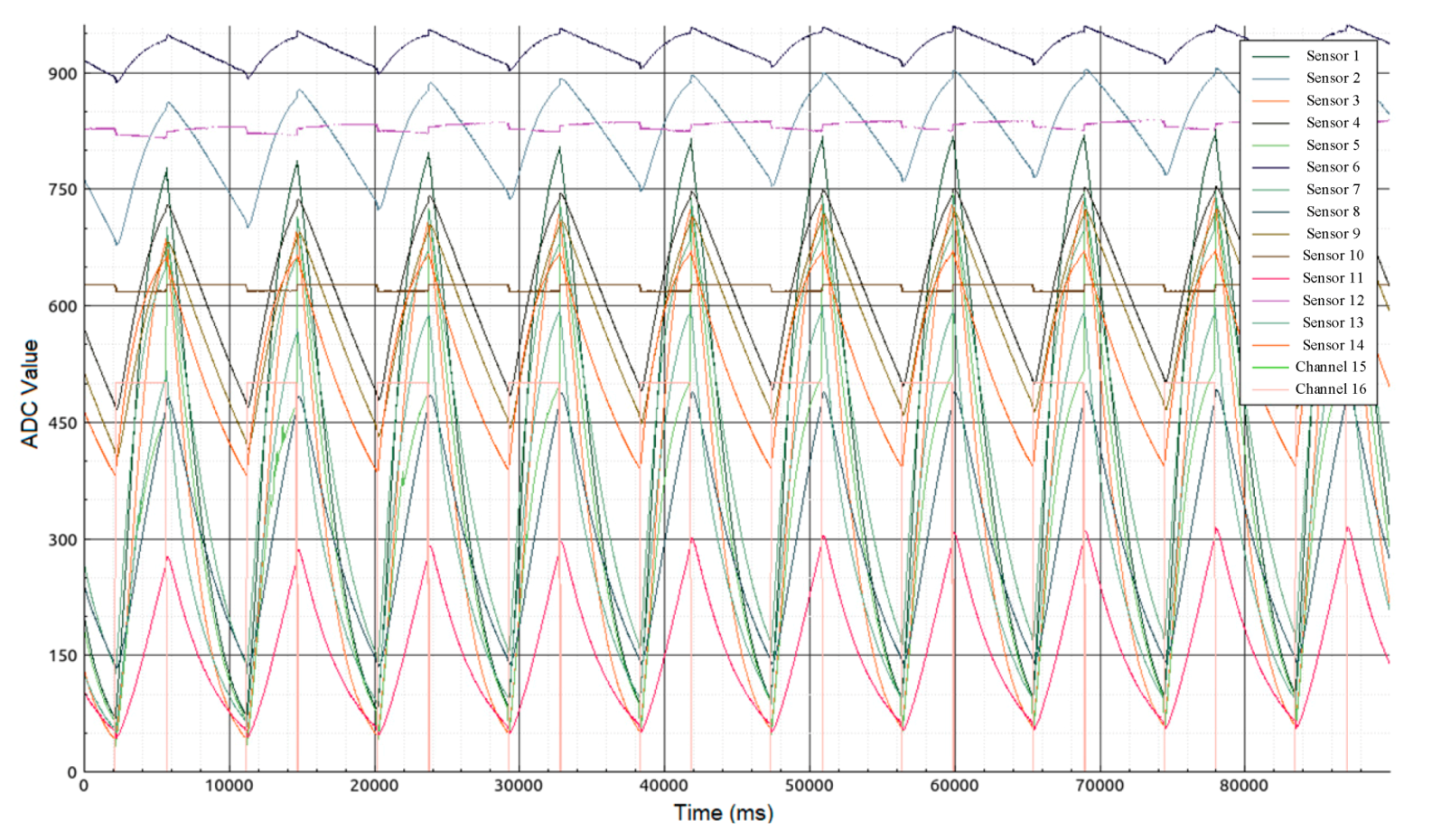
- At the moment of transition from the heating phase to the cooling phase of the sensors (Figure 3—mark 5000 ms), valves (2) and (3) open for exactly one second. The same opening time is used for valves (2) and (3), and the weight of the load on the bag provides the same volume of gas sample (~ 250 mL) introduced into the 1 L sampling chamber.
- After the valves are closed, data collection continues with the sample gas inside the chamber. The process of collecting data continues until the time stamp of 90,000 ms. The total residence time of the sample in the chamber does not exceed 90 s.
- After 90 s, the sample chamber purge is automatically turned on for 120 s. The instrument is then ready to analyze the next sample. These time intervals were selected experimentally and take into account the inertia of the sensors.
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaha, A.R. Training in head and neck surgery and oncology. J. Surg. Oncol. 2008, 97, 717–720. [Google Scholar] [CrossRef] [PubMed]
- McGivney, W.T. The National Comprehensive Cancer Network. Cancer 1998, 82, 2057–2060. [Google Scholar] [CrossRef]
- Harris, A.; Lyu, L.; Wasserman-Winko, T.; George, S.; Johnson, J.T.; Nilsen, M.L. Neck Disability and Swallowing Function in Posttreatment Head and Neck Cancer Patients. Otolaryngol. Neck Surg. 2020, 1–8. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Franklin, W.A.; Gazdar, A.F.; Bunn, P.A. Early detection of lung cancer: Clinical perspectives of recent advances in biology and radiology. Clin. Cancer Res. 2001, 7, 5–22. [Google Scholar]
- Skarysz, A.; Alkhalifah, Y.; Darnley, K.; Eddleston, M.; Hu, Y.; McLaren, D.; Nailon, W.H.; Salman, D.; Sykora, M.; Thomas, C.L.P.; et al. Convolutional neural networks for automated targeted analysis of raw gas chromatography-mass spectrometry data. In Proceedings of the 2018 International Joint Conference on Neural Networks (IJCNN), Rio de Janeiro, Brazil, 8–13 July 2018; pp. 1–8. [Google Scholar]
- Butcher, J.B.; Rutter, A.V.; Wootton, A.J.; Day, C.R.; Sulé-Suso, J. Artificial Neural Network Analysis of Volatile Organic Compounds for the Detection of Lung Cancer. Adv. Intell. Syst. Comput. 2017, 650, 183–190. [Google Scholar] [CrossRef]
- Shimizu, R.; Yanagawa, S.; Monde, Y.; Yamagishi, H.; Hamada, M.; Shimizu, T.; Kuroda, T. Deep learning application trial to lung cancer diagnosis for medical sensor systems. In Proceedings of the 2016 International SoC Design Conference (ISOCC), Jeju, Korea, 23–26 October 2016; pp. 191–192. [Google Scholar]
- Altomare, D.F.; Di Lena, M.; Porcelli, F.; Travaglio, E.; Longobardi, F.; Tutino, M.; DePalma, N.; Tedesco, G.; Sardaro, A.; Memeo, R.; et al. Effects of Curative Colorectal Cancer Surgery on Exhaled Volatile Organic Compounds and Potential Implications in Clinical Follow-up. Ann. Surg. 2015, 262, 862–867. [Google Scholar] [CrossRef]
- Altomare, D.F. Author’s reply: Exhaled volatile organic compounds identify patients with colorectal cancer (Br J Surg 2013; 100: 144–150). BJS 2013, 100, 980. [Google Scholar] [CrossRef]
- Ligor, T.; Pater, Ł.; Buszewski, B. Application of an artificial neural network model for selection of potential lung cancer biomarkers. J. Breath Res. 2015, 9, 027106. [Google Scholar] [CrossRef]
- Rudnicka, J.; Walczak, M.; Kowalkowski, T.; Jezierski, T.; Buszewski, B. Determination of volatile organic compounds as potential markers of lung cancer by gas chromatography–mass spectrometry versus trained dogs. Sens. Actuators B Chem. 2014, 202, 615–621. [Google Scholar] [CrossRef]
- Rudnicka, J.; Kowalkowski, T.; Buszewski, B. Searching for selected VOCs in human breath samples as potential markers of lung cancer. Lung Cancer 2019, 135, 123–129. [Google Scholar] [CrossRef]
- Li, Z.; Askim, J.R.; Suslick, K.S. The Optoelectronic Nose: Colorimetric and Fluorometric Sensor Arrays. Chem. Rev. 2018, 119, 231–292. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.-C.; Hou, C.; Huo, D.; Luo, X.-G.; Bao, M.-Z.; Li, X.; Yang, M.; Fa, H. A novel device based on a fluorescent cross-responsive sensor array for detecting lung cancer related volatile organic compounds. Rev. Sci. Instrum. 2015, 86, 025106. [Google Scholar] [CrossRef]
- Behera, B.; Joshi, R.; Vishnu, G.K.A.; Bhalerao, S.; Pandya, H.J. Electronic nose: A non-invasive technology for breath analysis of diabetes and lung cancer patients. J. Breath Res. 2019, 13, 024001. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Nanda, R.; Chakraborty, T. Clinical Application of Volatile Organic Compound Analysis for Detecting Infectious Diseases. Clin. Microbiol. Rev. 2013, 26, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Blatt, R.; Bonarini, A.; Calabrò, E.; Della Torre, M.; Matteucci, M.; Pastorino, U. Lung Cancer Identification by an Electronic Nose based on an Array of MOS Sensors. In Proceedings of the 2007 International Joint Conference on Neural Networks, Orlando, FL, USA, 12–17 August 2007; pp. 1423–1428. [Google Scholar]
- Rydosz, A. A Negative Correlation Between Blood Glucose and Acetone Measured in Healthy and Type 1 Diabetes Mellitus Patient Breath. J. Diabetes Sci. Technol. 2015, 9, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Lirk, P.; Bodrogi, F.; Raifer, H.; Greiner, K.; Ulmer, H.; Rieder, J. Elective haemodialysis increases exhaled isoprene. Nephrol. Dial. Transplant. 2003, 18, 937–941. [Google Scholar] [CrossRef]
- Španěl, P.; Dryahina, K.; Smith, D. Acetone, ammonia and hydrogen cyanide in exhaled breath of several volunteers aged 4–83 years. J. Breath Res. 2007, 1, 11001. [Google Scholar] [CrossRef]
- Smith, D.; Španěl, P.; Enderby, B.; Lenney, W.; Turner, C.; Davies, S. Isoprene levels in the exhaled breath of 200 healthy pupils within the age range 7–18 years studied using SIFT-MS. J. Breath Res. 2009, 4, 17101. [Google Scholar] [CrossRef]
- Winkler, K.; Herbig, J.; Kohl, I. Real-time metabolic monitoring with proton transfer reaction mass spectrometry. J. Breath Res. 2013, 7, 36006. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Cummin, A.R.; Gagliardi, A.J.; Gleeson, K.; Greenberg, J.; Maxfield, R.A.; Rom, W.N. Detection of Lung Cancer With Volatile Markers in the Breatha. Chest 2003, 123, 2115–2123. [Google Scholar] [CrossRef]
- Kesavaraj, G.; Sukumaran, S. A study on classification techniques in data mining. In Proceedings of the 2013 Fourth International Conference on Computing, Communications and Networking Technologies (ICCCNT), Tiruchengode, India, 4–6 July 2013; pp. 1–7. [Google Scholar]
- Schmekel, B.; Winquist, F.; Vikström, A. Analysis of breath samples for lung cancer survival. Anal. Chim. Acta 2014, 840, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Ozsandikcioglu, U.; Atasoy, A.; Yapici, S. Diagnosis of lung cancer with E-nose. In Proceedings of the 2018 26th Signal Processing and Communications Applications Conference (SIU), Izmir, Turkey, 2–5 May 2018; pp. 1–4. [Google Scholar]
- Herman-Saffar, O.; Boger, Z.; Libson, S.; Lieberman, D.; Gonen, R.; Zeiri, Y. Early non-invasive detection of breast cancer using exhaled breath and urine analysis. Comput. Boil. Med. 2018, 96, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Schuermans, V.N.E.; Li, Z.; Jongen, A.C.H.M.; Wu, Z.; Shi, J.; Ji, J.; Bouvy, N.D. Pilot Study: Detection of Gastric Cancer From Exhaled Air Analyzed With an Electronic Nose in Chinese Patients. Surg. Innov. 2018, 25, 429–434. [Google Scholar] [CrossRef]
- Van De Goor, R.M.G.E.; Van Hooren, M.; Dingemans, A.-M.; Kremer, B.; Kross, K. Training and Validating a Portable Electronic Nose for Lung Cancer Screening. J. Thorac. Oncol. 2018, 13, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, H.; Xie, D.; He, Z.; Pi, X. Lung Cancer Screening Based on Type-different Sensor Arrays. Sci. Rep. 2017, 7, 1969. [Google Scholar] [CrossRef]
- Wang, D.; Yu, K.; Wang, Y.; Hu, Y.; Zhao, C.; Wang, L.; Ying, K.; Wang, P. A hybrid electronic noses’ system based on mos-saw detection units intended for lung cancer diagnosis. J. Innov. Opt. Health Sci. 2012, 5, 1150006. [Google Scholar] [CrossRef]
- Kononov, A.; Korotetsky, B.; Jahatspanian, I.; Gubal, A.; Vasiliev, A.; Arsenjev, A.; Nefedov, A.; Barchuk, A.; Gorbunov, I.; Kozyrev, K.; et al. Online breath analysis using metal oxide semiconductor sensors (electronic nose) for diagnosis of lung cancer. J. Breath Res. 2019, 14, 016004. [Google Scholar] [CrossRef]
- Mohamed, E.I.; Mohamed, M.A.; Abdel-Mageed, S.; Abdel-Mohdy, T.S.; Badawi, M.I.; Darwish, S.H. Volatile organic compounds of biofluids for detecting lung cancer by an electronic nose based on artificial neural network. J. Appl. Biomed. 2019, 17, 67. [Google Scholar] [CrossRef]
- Van Hooren, M.R.A.; Leunis, N.; Brandsma, D.S.; Dingemans, A.-M.C.; Kremer, B.; Kross, K.W. Differentiating head and neck carcinoma from lung carcinoma with an electronic nose: A proof of concept study. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3897–3903. [Google Scholar] [CrossRef]
- D’Amico, A.; Pennazza, G.; Santonico, M.; Martinelli, E.; Roscioni, C.; Galluccio, G.; Paolesse, R.; Di Natale, C. An investigation on electronic nose diagnosis of lung cancer. Lung Cancer 2010, 68, 170–176. [Google Scholar] [CrossRef]
- De Boer, N.K.; De Meij, T.G.; Oort, F.A.; Ben Larbi, I.; Mulder, C.J.; Van Bodegraven, A.A.; Van Der Schee, M.P. The Scent of Colorectal Cancer: Detection by Volatile Organic Compound Analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Van De Goor, R.M.G.E.; Leunis, N.; Van Hooren, M.R.A.; Francisca, E.; Masclee, A.; Kremer, B.; Kross, K.W. Feasibility of electronic nose technology for discriminating between head and neck, bladder, and colon carcinomas. Eur. Arch. Oto-Rhino-Laryngol. 2016, 274, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Leunis, N.; Boumans, M.-L.; Kremer, B.; Din, S.; Stobberingh, E.; Kessels, A.G.H.; Kross, K.W. Application of an electronic nose in the diagnosis of head and neck cancer. Laryngoscope 2013, 124, 1377–1381. [Google Scholar] [CrossRef]
- Waltman, C.G.; van Roermund, J.G.H. Detecting prostate cancer by analyzing exhaled-breath using a portable electronic nose (Aeonose™). Tijdschr. Urol. 2018, 8, 44–49. [Google Scholar] [CrossRef][Green Version]
- Kort, S.; Brusse-Keizer, M.G.J.; Gerritsen, J.-W.; Van Der Palen, J. Data analysis of electronic nose technology in lung cancer: Generating prediction models by means of Aethena. J. Breath Res. 2017, 11, 026006. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, P.M.; Tolba, A.; Al-Makhadmeh, Z.; Jaber, M.M. Automatic detection of lung cancer from biomedical data set using discrete AdaBoost optimized ensemble learning generalized neural networks. Neural Comput. Appl. 2019, 32, 777–790. [Google Scholar] [CrossRef]
- Becker, M.; Zaidi, H. Imaging in head and neck squamous cell carcinoma: The potential role of PET/MRI. Br. J. Radiol. 2014, 87, 20130677. [Google Scholar] [CrossRef]
- Rivera, M.P.; Mehta, A.C.; Wahidi, M.M. Establishing the Diagnosis of Lung Cancer. Chest 2013, 143, e142S–e165S. [Google Scholar] [CrossRef]
| Stage of the Pathological Process (According to TNM System) | Number of Patients with Malignant Pathology by Tumor Location | |||||
|---|---|---|---|---|---|---|
| Morphological Diagnosis Confirmation Method | ||||||
| Lungs | Larynx | Oral Cavity | Oropharynx | Tongue | The Mucous Membrane of the Alveolar Process of the Lower Jaw | |
| Stage T1 | 2 | 1 | ||||
| Stage T2 | 3 | 2 | 3 | 2 | 4 | 1 |
| Stage T3 | 9 | 1 | 2 | |||
| Stage T4 | 4 | 1 | 1 | |||
| № | Sensor | Sensitivity |
|---|---|---|
| 1 | MP503 | Alcohol, Smoke, Isobutane, Methanol |
| 2 | WSP2110 | Toluene, Benzene, Methane |
| 3 | MQ3 | Alcohol |
| 4 | MQ2 | Isobutane, Propane, Methane, Alcohol, Hydrogen, Smoke |
| 5 | MQ7 | CO |
| 6 | MQ131 | O3 |
| 7 | MQ135 | NH3,NOx, Alcohol, Benzene, Smoke, CO2 |
| 8 | MQ8 | Hydrogen |
| 9 | MQ138 | n-Hexane, Benzene, NH3, Alcohol, Smoke, CO |
| 10 | TGS822 | Methane, CO, Isobutane, n-Hexane, Benzene, Ethanol, Acetone |
| 11 | TGS2602 | Ethanol, Toluene, NH3, H2S |
| 12 | TGS2620 | Methane, CO, Isobutane, Hydrogen, Ethanol |
| 13 | TGS2600 | Isobutane, Hydrogen, Ethanol |
| 14 | TGS2603 | Hydrogen, H2S, Ethanol, Methanethiol, Trimethylamine etc. |
| Parameter | Value |
|---|---|
| Accuracy | 81.8% |
| Sensitivity | 90.7% |
| Specificity | 61.4% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernov, V.I.; Choynzonov, E.L.; Kulbakin, D.E.; Obkhodskaya, E.V.; Obkhodskiy, A.V.; Popov, A.S.; Sachkov, V.I.; Sachkova, A.S. Cancer Diagnosis by Neural Network Analysis of Data from Semiconductor Sensors. Diagnostics 2020, 10, 677. https://doi.org/10.3390/diagnostics10090677
Chernov VI, Choynzonov EL, Kulbakin DE, Obkhodskaya EV, Obkhodskiy AV, Popov AS, Sachkov VI, Sachkova AS. Cancer Diagnosis by Neural Network Analysis of Data from Semiconductor Sensors. Diagnostics. 2020; 10(9):677. https://doi.org/10.3390/diagnostics10090677
Chicago/Turabian StyleChernov, Vladimir I., Evgeniy L. Choynzonov, Denis E. Kulbakin, Elena V. Obkhodskaya, Artem V. Obkhodskiy, Aleksandr S. Popov, Victor I. Sachkov, and Anna S. Sachkova. 2020. "Cancer Diagnosis by Neural Network Analysis of Data from Semiconductor Sensors" Diagnostics 10, no. 9: 677. https://doi.org/10.3390/diagnostics10090677
APA StyleChernov, V. I., Choynzonov, E. L., Kulbakin, D. E., Obkhodskaya, E. V., Obkhodskiy, A. V., Popov, A. S., Sachkov, V. I., & Sachkova, A. S. (2020). Cancer Diagnosis by Neural Network Analysis of Data from Semiconductor Sensors. Diagnostics, 10(9), 677. https://doi.org/10.3390/diagnostics10090677






